Cardiac Computed Tomography for the Assessment of Myocardial Bridging: A Scoping Review of the Emerging Role of Artificial Intelligence and Machine Learning
Abstract
1. Introduction
2. CCTA and Myocardial Bridging
3. Artificial Intelligence and Machine Learning in CCTA
- Artificial neural networks (ANNs) are computational models inspired by biological neurons that can learn complex patterns; in cardiac CT, they may be used to identify subtle morphological signs of myocardial bridging.
- Support vector machines (SVMs) are algorithms that find the optimal boundary separating different classes of data; they are useful in distinguishing stenosed/bridged coronary segments based on extracted image features.
- Decision trees—flowchart-like models that split data based on decision rules; they can be applied to classify stenosis/bridging severity based on vessel compression metrics.
- Random forest (RF) is a combination of decision trees that improves accuracy and model stability by aggregating predictions of multiple decision trees at the cost of higher processing time—it can be used to integrate multiple CT-derived parameters for diagnosis.
- A Naive Bayes classifier is a probabilistic method based on Bayes’ theorem, assuming independence between features, which may help in rapid classification.
- K-nearest neighbor (k-NN) is an algorithm that classifies data points based on the majority label of their closest neighbors/similarity to known examples; it is useful for matching new CT images with a library of annotated MB cases.
- Convolutional neural networks (CNNs) are used for processing grid-like data, including images, making them relevant to cardiac CT analysis. CNNs can segment coronary arteries and detect vessel compression.
- Recurrent neural networks (RNNs) are designed to handle sequential or time-series data; they are potentially useful for analyzing dynamic CT datasets or cinematic reconstructions.
- Deep neural networks (DNNs) are multi-layered models that can learn highly complex and non-linear relationships, for example, integrating CT imaging data with clinical parameters for a more comprehensive assessment of MB.
4. Artificial Intelligence and Machine Learning in Myocardial Bridging
- AI-enhanced segmentation and reconstruction.
- Computational fluid dynamics (CFD), which predicts blood flow dynamics and hemodynamic effects.
- CT-derived fractional flow reserve (CT-FFR), a ML technique which is particularly exciting and has already been established as a tool for assessing the functional significance of stenotic coronary lesions as an alternative to invasive FFR.
4.1. AI-Enhanced Segmentation and Reconstruction
4.2. Computational Fluid Dynamics (CFD)
4.3. CT-Derived Fractional Flow Reserve (CT-FFR)
5. Challenges in Practical Implementation
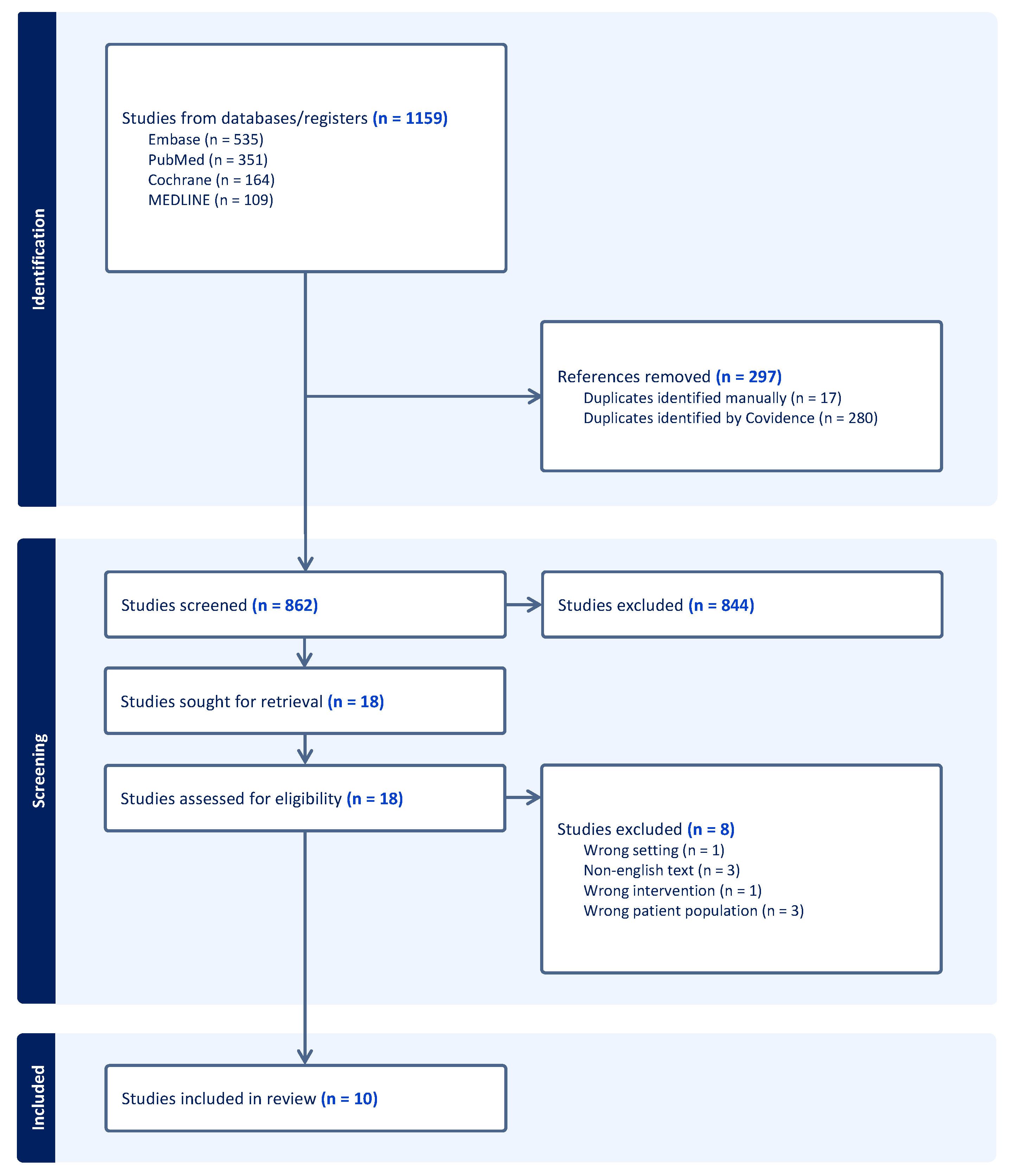
6. Methods
7. Results
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogers, I.S.; Tremmel, J.A.; Schnittger, I. Myocardial bridges: Overview of diagnosis and management. Congenit. Heart Dis. 2017, 12, 619–623. [Google Scholar] [CrossRef]
- Poláček, P. Relation of myocardial bridges and loops on the coronary arteries to coronary occlusions. Am. Heart J. 1961, 61, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Asuwa, N.; Masuda, S.; Ishikawa, Y. The effects of a myocardial bridge on coronary atherosclerosis and ischaemia. J. Pathol. 1998, 185, 4–9. [Google Scholar] [CrossRef]
- Möhlenkamp, S.; Hort, W.; Ge, J.; Erbel, R. Update on myocardial bridging. Circulation 2002, 106, 2616–2622. [Google Scholar] [CrossRef]
- Kawawa, Y.; Ishiwaka, Y.; Gomi, T.; Nagamoto, M.; Terada, H.; Ishii, T.; Kohda, E. Detection of myocardial bridge and evaluation of its anatomical properties by coronary multislice spiral computed tomography. Eur. J. Radiol. 2007, 61, 130–138. [Google Scholar] [CrossRef]
- Rubinshtein, R.; Gaspar, T.; Lewis, B.S.; Prasad, A.; Peled, N.; Halon, D.A. Long-term prognosis and outcome in patients with a chest pain syndrome and myocardial bridging: A 64-slice coronary computed tomography angiography study. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 579–585. [Google Scholar] [CrossRef]
- Feld, H.; Guadanino, V.; Hollander, G.; Greengart, A.; Lichstein, E.; Shani, J. Exercise-induced ventricular tachycardia in association with a myocardial bridge. Chest 1991, 99, 1295–1296. [Google Scholar] [CrossRef]
- Nakanishi, R.; Rajani, R.; Ishikawa, Y.; Ishii, T.; Berman, D.S. Myocardial bridging on coronary CTA: An innocent bystander or a culprit in myocardial infarction? J. Cardiovasc. Comput. Tomogr. 2012, 6, 3–13. [Google Scholar] [CrossRef]
- Ge, J.; Erbel, R.; Rupprecht, H.J.; Koch, L.; Kearney, P.; Görge, G.; Haude, M.; Meyer, J. Comparison of intravascular ultrasound and angiography in the assessment of myocardial bridging. Circulation 1994, 89, 1725–1732. [Google Scholar] [CrossRef]
- Tonino, P.A.; De Bruyne, B.; Pijls, N.H.; Siebert, U.; Ikeno, F.; van’t, V.M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; Oldroyd, K.G.; et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009, 3, 214–224. [Google Scholar] [CrossRef]
- Zimmermann, F.M.; Ferrara, A.; Johnson, N.P.; van Nunen, L.X.; Escaned, J.; Albertsson, P.; Erbel, R.; Legrand, V.; Gwon, H.C.; Remkes, W.S.; et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur. Heart J. 2015, 36, 3182–3188. [Google Scholar] [CrossRef]
- Escaned, J.; Cortés, J.; Flores, A.; Goicolea, J.; Alfonso, F.; Hernández, R.; Fernández-Ortiz, A.; Sabaté, M.; Bañuelos, C.; Macaya, C.; et al. Importance of diastolic fractional flow reserve and dobutamine challenge in physiologic assessment of myocardial bridging. J. Am. Coll. Cardiol. 2003, 42, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P.; Uribe, C.; Raghuram, A. Coronary Myocardial Bridge Updates: Anatomy, Pathophysiology, Clinical Manifestations, Diagnosis, and Treatment Options. Tex. Heart Inst. J. 2025, 52, e238300. [Google Scholar] [CrossRef] [PubMed]
- Ekeke, C.N.; Noble, S.; Mazzaferri, E.; Crestanello, J.A. Myocardial bridging over the left anterior descending: Myotomy, bypass, or both? J. Thorac. Cardiovasc. Surg. 2015, 149, e57–e58. [Google Scholar] [CrossRef][Green Version]
- Tandar, A.; Whisenant, B.K.; Michaels, A.D. Stent fracture following stenting of a myocardial bridge: Report of two cases. Catheter. Cardiovasc. Interv. 2008, 71, 191–196. [Google Scholar] [CrossRef]
- Kunamneni, P.B.; Rajdev, S.; Krishnan, P.; Moreno, P.R.; Kim, M.C.; Sharma, S.K.; Kini, A.S. Outcome of intracoronary stenting after failed maximal medical therapy in patients with symptomatic myocardial bridge. Catheter. Cardiovasc. Interv. 2008, 71, 185–190. [Google Scholar] [CrossRef]
- Bamberg, F.; Marcus, R.P.; Becker, A.; Hildebrandt, K.; Bauner, K.; Schwarz, F.; Greif, M.; von Ziegler, F.; Bischoff, B.; Becker, H.C.; et al. Dynamic myocardial CT perfusion imaging for evaluation of myocardial ischemia as determined by MR imaging. JACC Cardiovasc. Imaging 2014, 7, 267–277. [Google Scholar] [CrossRef]
- Ho, K.T.; Chua, K.C.; Klotz, E.; Panknin, C. Stress and rest dynamic myocardial perfusion imaging by evaluation of complete time-attenuation curves with dual-source CT. JACC Cardiovasc. Imaging 2010, 3, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dou, G.; He, B.; Jin, Q.; Chen, Z.; Jing, J.; Di Carli, M.F.; Chen, Y.; Blankstein, R. Stress Myocardial Blood Flow Ratio by Dynamic CT Perfusion Identifies Hemodynamically Significant CAD. JACC Cardiovasc. Imaging 2020, 13, 966–976. [Google Scholar] [CrossRef]
- Li, Y.; Dai, X.; Lu, Z.; Shen, C.; Zhang, J. Diagnostic performance of quantitative, semi-quantitative, and visual analysis of dynamic CT myocardial perfusion imaging: A validation study with invasive fractional flow reserve. Eur. Radiol. 2021, 31, 525–534. [Google Scholar] [CrossRef]
- Schicchi, N.; Fogante, M.; Paolini, E.; Cela, F.; Pirani, P.E.; Perna, G.P. Stress-rest dynamic-CT myocardial perfusion imaging in the management of myocardial bridging: A ‘one-stop shop’ exam. J. Cardiol. Cases 2023, 28, 229–232. [Google Scholar] [CrossRef]
- Williams, M.C.; Weir-Mccall, J.R.; Baldassarre, L.A.; De Cecco, C.N.; Choi, A.D.; Dey, D.; Dweck, M.R.; Isgum, I.; Kolossvary, M.; Leipsic, J.; et al. Artificial intelligence and machine learning for cardiovascular computed tomography (CCT): A white paper of the society of cardiovascular computed tomography (SCCT). J. Cardiovasc. Comput. Tomogr. 2024, 18, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Slart, R.H.J.A.; Williams, M.C.; Juarez-Orozco, L.E.; Rischpler, C.; Dweck, M.R.; Glaudemans, A.W.J.M.; Gimelli, A.; Georgoulias, P.; Gheysens, O. Position paper of the EACVI and EANM on artificial intelligence applications in multimodality cardiovascular imaging using SPECT/CT, PET/CT, and cardiac CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, B.; Liu, X.; Huang, X.; Wang, X.; Mao, L.; Xiao, Z. Feasibility of artificial intelligence its current status, clinical applications, and future direction in cardiovascular disease. Curr. Probl. Cardiol. 2024, 49, 102349. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Huang, L.; Qu, M.; Chen, B.; Wang, G. Artificial Intelligence in Coronary CT Angiography: Current Status and Future Prospects. Front. Cardiovasc. Med. 2022, 9, 896366. [Google Scholar] [CrossRef]
- Lopez-Jimenez, F.; Attia, Z.; Arruda-Olson, A.M.; Carter, R.; Chareonthaitawee, P.; Jouni, H.; Kapa, S.; Lerman, A.; Luong, C.; Medina-Inojosa, J.R.; et al. Artificial Intelligence in Cardiology: Present and Future. Mayo Clin. Proc. 2020, 95, 1015–1039. [Google Scholar] [CrossRef]
- Krittanawong, C.; Zhang, H.J.; Wang, Z.; Aydar, M.; Kitai, T. Artificial Intelligence in Precision Cardiovascular Medicine. J. Am. Coll. Cardiol. 2017, 69, 2657–2664. [Google Scholar] [CrossRef]
- Tolu-Akinnawo, O.Z.; Ezekwueme, F.; Omolayo, O.; Batheja, S.; Awoyemi, T. Advancements in Artificial Intelligence in Noninvasive Cardiac Imaging: A Comprehensive Review. Clin. Cardiol. 2025, 48, e70087. [Google Scholar] [CrossRef]
- Kwan, A.C.; Salto, G.; Cheng, S.; Ouyang, D. Artificial Intelligence in Computer Vision: Cardiac MRI and Multimodality Imaging Segmentation. Curr. Cardiovasc. Risk Rep. 2021, 15, 18. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, Z.; Li, B.; Firmin, D.; Yang, G. Recent Advances in Fibrosis and Scar Segmentation From Cardiac MRI: A State-of-the-Art Review and Future Perspectives. Front. Physiol. 2021, 12, 709230. [Google Scholar] [CrossRef]
- Alnasser, T.N.; Abdulaal, L.; Maiter, A.; Sharkey, M.; Dwivedi, K.; Salehi, M.; Garg, P.; Swift, A.J.; Alabed, S. Advancements in cardiac structures segmentation: A comprehensive systematic review of deep learning in CT imaging. Front. Cardiovasc. Med. 2024, 11, 1323461. [Google Scholar] [CrossRef]
- Liang, J.; Sun, Y.; Ye, Z.; Sun, Y.; Xu, L.; Zhou, Z.; Thomsen, B.; Li, J.; Sun, Z.; Fan, Z.; et al. Second-generation motion correction algorithm improves diagnostic accuracy of single-beat coronary CT angiography in patients with increased heart rate. Eur. Radiol. 2019, 29, 4215–4227. [Google Scholar] [CrossRef]
- Sun, J.; Okerlund, D.; Cao, Y.; Li, H.; Zhu, Y.; Li, J.; Peng, Y. Further improving image quality of cardiovascular computed tomography angiography for children with high heart rates using second-generation motion correction algorithm. J. Comput. Assist. Tomogr. 2020, 44, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Melo, D.P.; Ouyang, D.; Slomka, P.J.; Williams, M.C.; Dey, D. Current and Future Applications of Artificial Intelligence in Cardiac CT. Curr. Cardiol. Rep. 2023, 25, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, J.; Wang, W.; Qi, Y.; Lyu, J.; Zhang, X.; Li, T.; Lou, X. Predictors of discordance between CT-derived fractional flow reserve (CT-FFR) and △CT-FFR in deep coronary myocardial bridging. Clin. Imaging 2024, 114, 110264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tian, X.; Li, M.Y.; Zheng, W.S.; Yu, Y.; Zhang, H.W.; Pan, T.; Gao, B.L.; Li, C.Y. Quantitative computed tomography angiography evaluation of the coronary fractional flow reserve in patients with left anterior descending artery myocardial bridging. Clin. Physiol. Funct. Imaging 2024, 44, 251–259. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Z.; Hong, N.; Chen, L. Effect of a second-generation motion correction algorithm on image quality and measurement reproducibility of coronary CT angiography in patients with a myocardial bridge and mural coronary artery. Clin. Radiol. 2024, 79, e462–e467. [Google Scholar] [CrossRef]
- Zhou, F.; Zhou, F.; Wang, Y.N.; Schoepf, U.J.; Tesche, C.; Tang, C.X.; Zhou, C.S.; Xu, L.; Hou, Y.; Zheng, M.W.; et al. Diagnostic Performance of Machine Learning Based CT-FFR in Detecting Ischemia in Myocardial Bridging and Concomitant Proximal Atherosclerotic Disease. Can. J. Cardiol. 2019, 35, 1523–1533. [Google Scholar] [CrossRef]
- Gijsen, F.; Katagiri, Y.; Barlis, P.; Bourantas, C.; Collet, C.; Coskun, U.; Daemen, J.; Dijkstra, J.; Edelman, E.; Evans, P.; et al. Expert recommendations on the assessment of wall shear stress in human coronary arteries: Existing methodologies, technical considerations, and clinical applications. Eur. Heart J. 2019, 40, 3421–3433. [Google Scholar] [CrossRef]
- Stone, P.H.; Maehara, A.; Coskun, A.U.; Maynard, C.C.; Zaromytidou, M.; Siasos, G.; Andreou, I.; Fotiadis, D.; Stefanou, K.; Papafaklis, M.; et al. Role of Low Endothelial Shear Stress and Plaque Characteristics in the Prediction of Nonculprit Major Adverse Cardiac Events. JACC Cardiovasc. Imaging 2018, 11, 462–471. [Google Scholar] [CrossRef]
- Kumar, A.; Thompson, E.W.; Lefieux, A.; Molony, D.S.; Davis, E.L.; Chand, N.; Fournier, S.; Lee, H.S.; Suh, J.; Sato, K.; et al. High. Coronary Shear. Stress. in Patients With Coronary Artery Disease Predicts Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 1926–1935. [Google Scholar] [CrossRef] [PubMed]
- Candreva, A.; De Nisco, G.; Rizzini, M.L.; D’Ascenzo, F.; De Ferrari, G.M.; Gallo, D.; Morbiducci, U.; Chiastra, C. Current and Future Applications of Computational Fluid Dynamics in Coronary Artery Disease. Rev. Cardiovasc. Med. 2022, 23, 377. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, K.; Kozłowski, M.; Orciuch, W.; Makowski, Ł. Computational Fluid Dynamics Simulations of Mitral Paravalvular Leaks in Human Heart. Materials 2021, 14, 7354. [Google Scholar] [CrossRef] [PubMed]
- Fezzi, S.; Ding, D.; Scarsini, R.; Huang, J.; Del Sole, P.A.; Zhao, Q.; Pesarini, G.; Simpkin, A.; Wijns, W.; Ribichini, F.; et al. Integrated Assessment of Computational Coronary Physiology From a Single Angiographic View in Patients Undergoing TAVI. Circ. Cardiovasc. Interv. 2023, 16, E013185. [Google Scholar] [CrossRef]
- Javadzadegan, A.; Moshfegh, A.; Fulker, D.; Barber, T.; Qian, Y.; Kritharides, L.; Yong, A.S.C. Development of a Computational Fluid Dynamics Model for Myocardial Bridging. J. Biomech. Eng. 2018, 140, 091010. [Google Scholar] [CrossRef]
- Sharma, P.; Itu, L.; Zheng, X.; Kamen, A.; Bernhardt, D.; Suciu, C.; Comaniciu, D. A framework for personalization of coronary flow computations during rest and hyperemia. In Proceedings of the 34th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; Volume 2012, pp. 6665–6668. [Google Scholar]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/ SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, E368–E454. [Google Scholar]
- Bittl, J.A.; Bangalore, S.; DiMaio, J.M.; Grant, M.C.; Lawton, J.S.; Tamis Holland, J.E. Putting the 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization Into Practice. JACC Case Rep. 2022, 4, 31–35. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M. ‘Ten commandments’ for the 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 79–80. [Google Scholar] [CrossRef]
- Yang, S.; Chung, J.; Lesina, K.; Doh, J.H.; Jegere, S.; Erglis, A.; Leipsic, J.A.; Fearon, W.F.; Narula, J.; Koo, B.K. Long-term prognostic implications of CT angiography-derived fractional flow reserve: Results from the DISCOVER-FLOW study. J. Cardiovasc. Comput. Tomogr. 2024, 18, 251–258. [Google Scholar] [CrossRef]
- Nørgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: The NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J. Am. Coll. Cardiol. 2014, 63, 1145–1155. [Google Scholar] [CrossRef]
- Rajiah, P.; Cummings, K.W.; Williamson, E.; Young, P.M. CT Fractional Flow Reserve: A Practical Guide to Application, Interpretation, and Problem Solving. RadioGraphics 2022, 42, 340–358. [Google Scholar] [CrossRef]
- Tesche, C.; De Cecco, C.N.; Albrecht, M.H.; Duguay, T.M.; Bayer, R.R., 2nd; Litwin, S.E.; Steinberg, D.H.; Schoepf, U.J. Coronary CT Angiography–derived Fractional Flow. Reserve. Radiol. 2017, 285, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Seetharam, K.; Brito, D.; Farjo, P.D.; Sengupta, P.P. The Role of Artificial Intelligence in Cardiovascular Imaging: State of the Art Review. Front. Cardiovasc. Med. 2020, 7, 618849. [Google Scholar] [CrossRef] [PubMed]
- Martens, B.; Michiels, V.; Argacha, J.F.; Cosyns, B. Normalization of FFRCT after surgical unroofing of a myocardial bridge: A case report. Eur. Heart J. Case Rep. 2024, 8, ytae005. [Google Scholar] [CrossRef]
- Zhou, F.; Tang, C.X.; Schoepf, U.J.; Tesche, C.; Bauer, M.J.; Jacobs, B.E.; Zhou, C.S.; Yan, J.; Lu, M.J.; Lu, G.M.; et al. Fractional flow reserve derived from CCTA may have a prognostic role in myocardial bridging. Eur. Radiol. 2019, 29, 3017–3026. [Google Scholar] [CrossRef]
- Zhou, F.; Tang, C.X.; Schoepf, U.J.; Tesche, C.; Rollins, J.D.; Liu, H.; Zhou, C.S.; Yan, J.; Lu, M.J.; Lu, G.M.; et al. Machine Learning Using. CT-FFR Predicts Proximal Atherosclerotic Plaque Formation Associated With LAD Myocardial Bridging. JACC Cardiovasc. Imaging 2019, 12, 1591–1593. [Google Scholar] [CrossRef]
- Jubran, A.; Schnittger, I.; Tremmel, J.; Pargaonkar, V.; Rogers, I.; Becker, H.C.; Yang, S.; Mastrodicasa, D.; Willemink, M.; Fleischmann, D.; et al. Computed Tomographic Angiography-Based Fractional Flow. Reserve Compared With Catheter-Based Dobutamine-Stress. Diastolic Fractional Flow. Reserve in Symptomatic Patients With a Myocardial Bridge and No Obstructive Coronary Artery Disease. Circ. Cardiovasc. Imaging 2020, 13, e009576. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, L.; Dai, X.; Zhang, J. CT Fractional Flow Reserve for the Diagnosis of Myocardial Bridging-Related Ischemia: A Study Using Dynamic CT Myocardial Perfusion Imaging as a Reference Standard. Korean J. Radiol. 2021, 22, 1964–1973. [Google Scholar] [CrossRef]
- Chen, Y.C.; Zheng, J.; Zhou, F.; Tao, X.W.; Chen, Q.; Feng, Y.; Su, Y.Y.; Zhang, Y.; Liu, T.; Zhou, C.S.; et al. Coronary CTA-based vascular radiomics predicts atherosclerosis development proximal to LAD myocardial bridging. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1462–1471. [Google Scholar] [CrossRef]
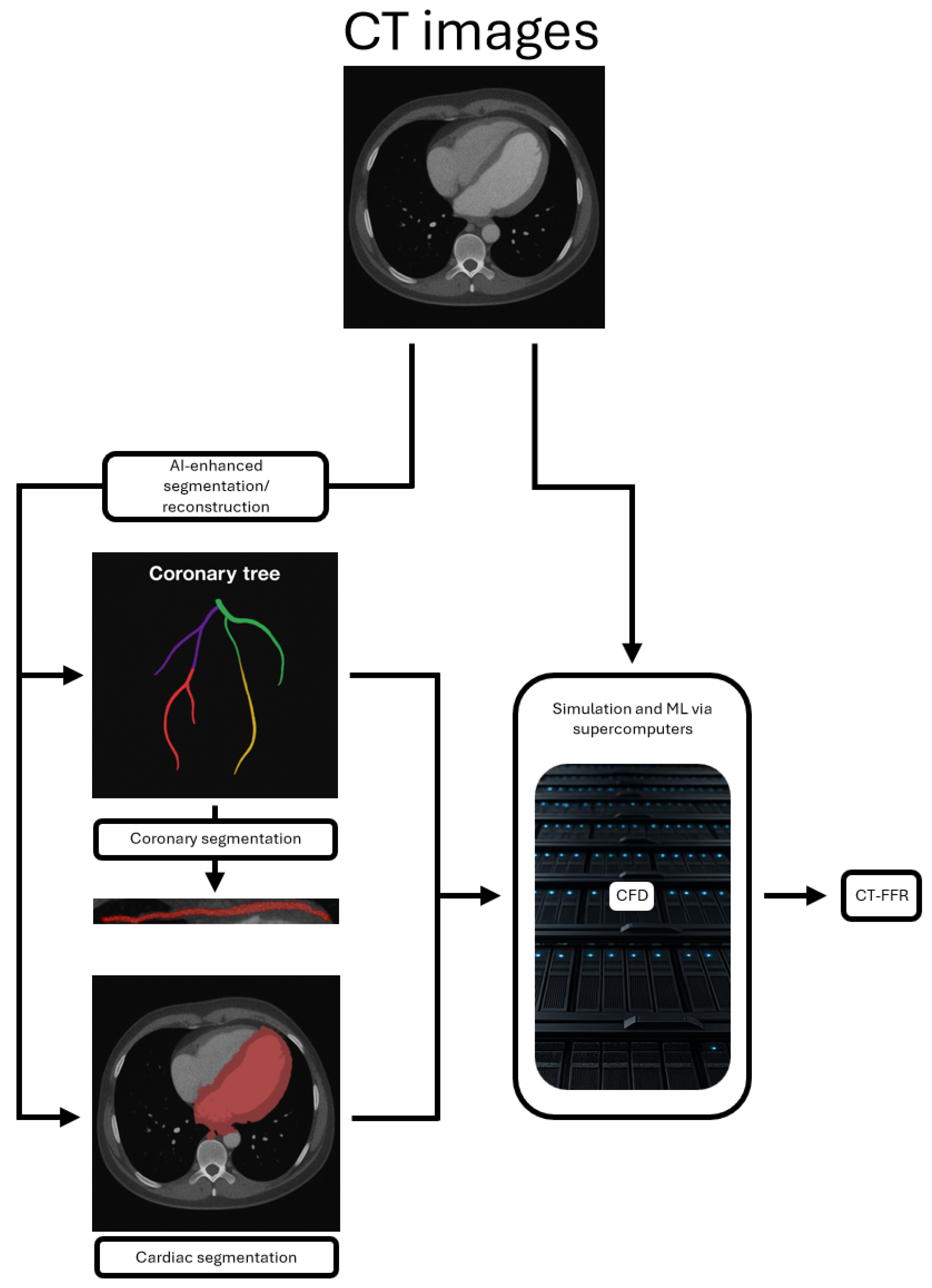
| Purpose | Type | Requirements | Functional or Anatomical | |
|---|---|---|---|---|
| AI-enhanced segmentation and reconstruction | Analysis of images to identify regions of interest, enhance image quality, and assess ischemia | Imaging + AI-based analysis | CT, MRI, or US images | Functional, anatomical |
| CFD | Simulate and analyze blood flow dynamics within vessels or the heart | Imaging + simulation | CT scan with contrast agent, computational modeling | Functional |
| CT-FFR | Assessment of the functional significance of stenosis by simulating blood flow | Imaging + simulation + ML-based analysis | CT scan with contrast agent, computational modeling | Functional |
| Study | Journal | Country | Study Type | Study Aim | Population | Total Participants | Age | % Male | AI/ML Technique | Purpose of AI | Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Martens 2024 [55] | European Heart Journal—Case Reports | Belgium | Case report | To report on a case showing improvement in CT-FFR following treatment of MB | A patient with LAD MB and abnormal CT-FFR, which normalized after treatment with surgical unroofing of the MB | 1 | 55 | 100 | ML-based CT-FFR (HeartFlow) | Computation of CT-FFR | Normalization of CT-FFR from 0.76 pre-surgery to 0.92 post-surgery |
| Zhou 2019 [56] | European Radiology | China | Retrospective case–control | To evaluate the feasibility of CT-FFR derivation from CCTA in patients with MB, its relationship with MB anatomical features, and clinical relevance | Patients with LAD MB on CCTA with no atherosclerosis as compared with controls | 161; 120 cases; 40 controls | Cases: 52.4 ± 11 Controls: 54.3 ± 11.5 | Cases: 68 Controls: 54 | ML-based CT-FFR (cFFR v3.0.0, Siemens Healthineers, Erlangen, Germany) | Computation of CT-FFR | MB is associated with abnormal CT-FFR values. MB length and systolic stenosis are the main contributors to abnormal CT-FFR values, with a combination of the two showing moderate predictive performance. Patients with abnormal FFR were less likely to be asymptomatic and more likely to have typical anginal chest pain |
| Zhou 2019 [57] | JACC: Cardiovascular Imaging | China | Retrospective cohort | To investigate the role of CT-FFR in predicting proximal plaque formation associated with MB in the LAD using ML approaches | Patients with MB in the LAD and no atherosclerosis on baseline CCTA who underwent follow-up CCTA with a minimum interval of 3 months | 188 | 55 ± 6 | 68.6 | ML-based CT-FFR (cFFR v3.0.0, Siemens Healthineers); ML-based prediction model using LASSO algorithms | Computation of CT-FFR; ML models for prediction of plaque formation | CT-FFR distal to LAD MB and ΔCT-FFR significantly differed between patients with CT MB LAD who developed plaque and those who did not. ML algorithms further identified CT-FFR and ΔCT-FFR as the strongest predictors of plaque formation proximal to MB LAD |
| Zhou 2019 [38] | Canadian Journal of Cardiology | China | Retrospective cohort | To study the diagnostic performance of ML-based CT-FFR to detect functional ischemia in MB with iFFR as the reference standard | Patients who underwent CCTA for the evaluation of suspected or known CAD and were found to have LAD MB who then underwent ICA within 60 days of CCTA | 104 | 61.2 ± 9.1 | 72.1 | ML-based CT-FFR (cFFR v3.2.0, Siemens Healthineers) | Automatic generation of centerline and luminal contours of coronary arteries; computation of CT-FFR | CT-FFR has high diagnostic performance for functional ischemia in vessels with MB and concomitant proximal atherosclerotic disease compared with iFFR, regardless of length and depth of MB, with a low PPV for lesions of <70% stenosis |
| Jubran 2020 [58] | Circulation: Cardiovascular Imaging | United States | Retrospective cohort | To compare CT-FFR, dobutamine-stress dFFR, iFFR, and IVUS in assessing the hemodynamic significance of MB | Patients with angina who had been found to have an MB in the LAD with ≤50% coronary artery stenosis by ICA and had undergone CCTA | CT-FFR: 49 dFFR: 43 iFFR: 28 IVUS: 46 | 47.5 ± 13.7 | 39 | ML-based CT-FFR (cFFR v3.1.2, Siemens Healthineers) | Computation of CT-FFR | CT-FFR values measured in LAD MB were lower than arteries without MB. CT-FFR values did not correlate with dobutamine-stress dFFR, iFFR, or LAD systolic compression on IVUS. CT-FFR values were higher than dFFR and lower than iFFR. There was non-concordance between CT-FFR and dFFR, iFFR, or the degree of systolic compression measured by IVUS |
| Yu 2021 [59] | Korean Journal of Radiology | China | Cross-sectional | To investigate the diagnostic performance of CT-FFR for MB-related ischemia using dynamic CT-MPI as a reference standard | Symptomatic patients with LAD MB and no obstructive stenosis on CCTA who also underwent CT-MPI | 75 | 62.7 ± 13.2 | 64 | ML-based CT-FFR (cFFR; version 3.0, Siemens Healthineers) | Computation of CT-FFR | ΔCT-FFRsystolic shows high sensitivity and NPV and reliably excludes MB ischemia. All CT-FFR measurements had low PPV, and other methodologies are needed to confirm positive CT-FFR results |
| Zhang 2024 [37] | Clinical Radiology | China | Retrospective observational comparative study (within-subject) | To determine the effect of second-generation motion correction (MC2) on image quality and measurement reproducibility of CCTA images in patients with MB and mural coronary artery (MB-MCA) compared to standard (STD) images without motion correction and with first-generation motion correction (MC1) | Patients with known or suspected coronary artery disease who underwent CCTA and had MB-MCA in the LAD | 66 | 62 ± 11 | 45 | Deep learning image reconstruction algorithm (DLIR, GE Healthcare). First-generation motion correction algorithm (MC1, GE Healthcare). Second-generation motion correction algorithm (MC2, GE Healthcare) | Image reconstruction and motion correction | MC2 reduced motion artifacts and resulted in significant improvements of image quality, diagnostic confidence, and measurement reproducibility for both MB and MCA in systolic and diastolic phases |
| Zhang 2024 [36] | Clinical Physiology and Functional Imaging | China | Retrospective case–control | To quantitatively investigate the effect of MB in the LAD on CT-FFR | Patients with confirmed LAD MB on CCTA with or without atherosclerosis in the LAD as compared with controls | 404; 300 cases; 104 controls | Cases: 54 ± 6 Controls: 54 ± 7 | Cases: 56 Controls: 24 | ML-based CT-FFR (Shukun, ct-FFR, V1.17) | Automatic extraction of coronary artery tree; automatic segmentation, reconstruction and diagnosis of coronary artery stenosis; computation of CT-FFR | No differences in CT-FFR values between systolic and diastolic phases. MB (with or without atherosclerosis) is associated with greater ΔCT-FFR and lower CT-FFR compared with controls without MB. CT-FFR is significantly lower in MB with atherosclerosis than in MB without atherosclerosis. In MB with atherosclerosis, LAD stenosis severity is an independent risk factor significantly affecting CT-FFR values, and abnormal CT-FFR (<0.80) is associated with more severe LAD stenosis. There was no significant difference detected in terms of clinical or anatomical features between the abnormal and normal CT-FFR groups |
| Chen 2024 [60] | European Heart Journal—Cardiovascular Imaging | China | Retrospective cohort and validation study | To develop and validate CCTA-based radiomics models in predicting proximal plaque development in LAD MB | Patients with MB and no atherosclerotic plaque proximal to the MB segment on baseline CCTA | 295 | 55 ± 10 | 66 | ML models | Predictive modelling and analysis | The proximal MB cross-sectional radiomics model (pMB CS) is able to predict proximal atherosclerotic plaque development associated with LAD MB in an external validation set (AUC 0.75; p < 0.001) and can be integrated with a clinical model to further improve performance (AUC 0.76; p < 0.001) |
| Sun 2024 [35] | Clinical Imaging | China | Cross-sectional | To compare the performance between CT-FFR and ΔCT-FFR in patients with deep LAD MB and explore predictors of discordance between the two measurements | Patients with deep LAD MB on CCTA and <50% stenosis of the LAD and/or left main stem | 175 | 60 ± 7 | 71.4 | Deep learning image reconstruction (TrueFidelity, GE Healthcare); ML-based CT-FFR (uAI Portal; United Imaging Intelligence) | Image reconstruction; automatic labeling of plaque and MB; computation of CT-FFR | A total of 30.9% of patients had discordance of CT-FFR and ΔCT-FFR, with 94.4% of patients leaning towards CT-FFR positivity with a negative ΔCT-FFR. Proximal atherosclerosis and distance from the MB to the aorta were independent risk factors for discordance. Anatomic features (length and depth) of the MB were correlated with ΔCT-FFR rather than CT-FFR, suggesting that ΔCT-FFR is a more specific tool for MB evaluation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Suleiman, A.; Russo, F.; Della Valle, L.; Ausiello, D.; Bukowska-Olech, E.; Iannibelli, V.; Al Droubi, M.O.; Sannino, G.; Bernardi, M.; Spadafora, L. Cardiac Computed Tomography for the Assessment of Myocardial Bridging: A Scoping Review of the Emerging Role of Artificial Intelligence and Machine Learning. J. Cardiovasc. Dev. Dis. 2025, 12, 350. https://doi.org/10.3390/jcdd12090350
Abu Suleiman A, Russo F, Della Valle L, Ausiello D, Bukowska-Olech E, Iannibelli V, Al Droubi MO, Sannino G, Bernardi M, Spadafora L. Cardiac Computed Tomography for the Assessment of Myocardial Bridging: A Scoping Review of the Emerging Role of Artificial Intelligence and Machine Learning. Journal of Cardiovascular Development and Disease. 2025; 12(9):350. https://doi.org/10.3390/jcdd12090350
Chicago/Turabian StyleAbu Suleiman, Amro, Federico Russo, Luigi Della Valle, Davide Ausiello, Ewelina Bukowska-Olech, Vincenzo Iannibelli, M. Omar Al Droubi, Gabriella Sannino, Marco Bernardi, and Luigi Spadafora. 2025. "Cardiac Computed Tomography for the Assessment of Myocardial Bridging: A Scoping Review of the Emerging Role of Artificial Intelligence and Machine Learning" Journal of Cardiovascular Development and Disease 12, no. 9: 350. https://doi.org/10.3390/jcdd12090350
APA StyleAbu Suleiman, A., Russo, F., Della Valle, L., Ausiello, D., Bukowska-Olech, E., Iannibelli, V., Al Droubi, M. O., Sannino, G., Bernardi, M., & Spadafora, L. (2025). Cardiac Computed Tomography for the Assessment of Myocardial Bridging: A Scoping Review of the Emerging Role of Artificial Intelligence and Machine Learning. Journal of Cardiovascular Development and Disease, 12(9), 350. https://doi.org/10.3390/jcdd12090350







