Personalized Treatment of Patients with Coronary Artery Disease: The Value and Limitations of Predictive Models
Abstract
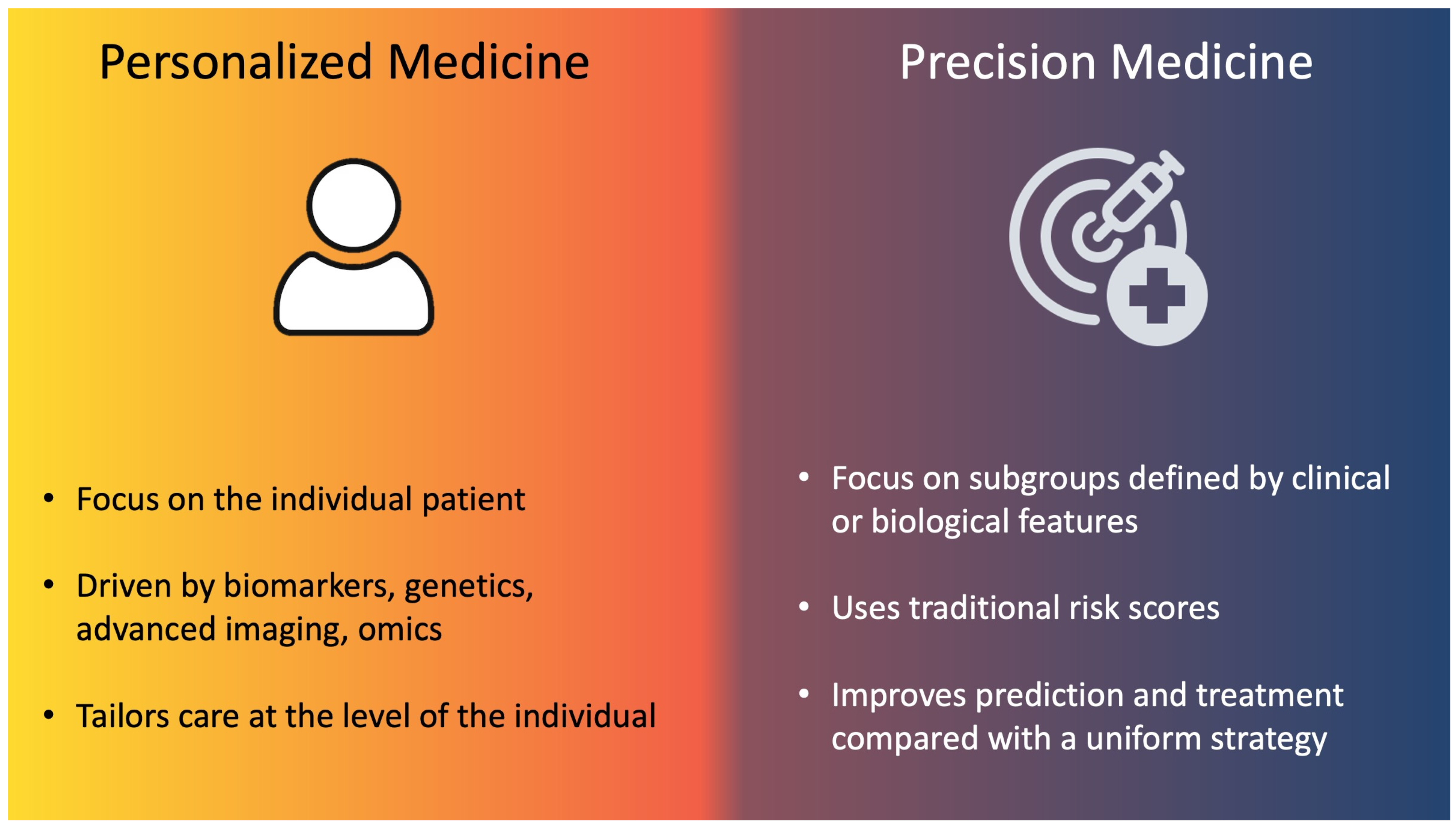
1. Introduction
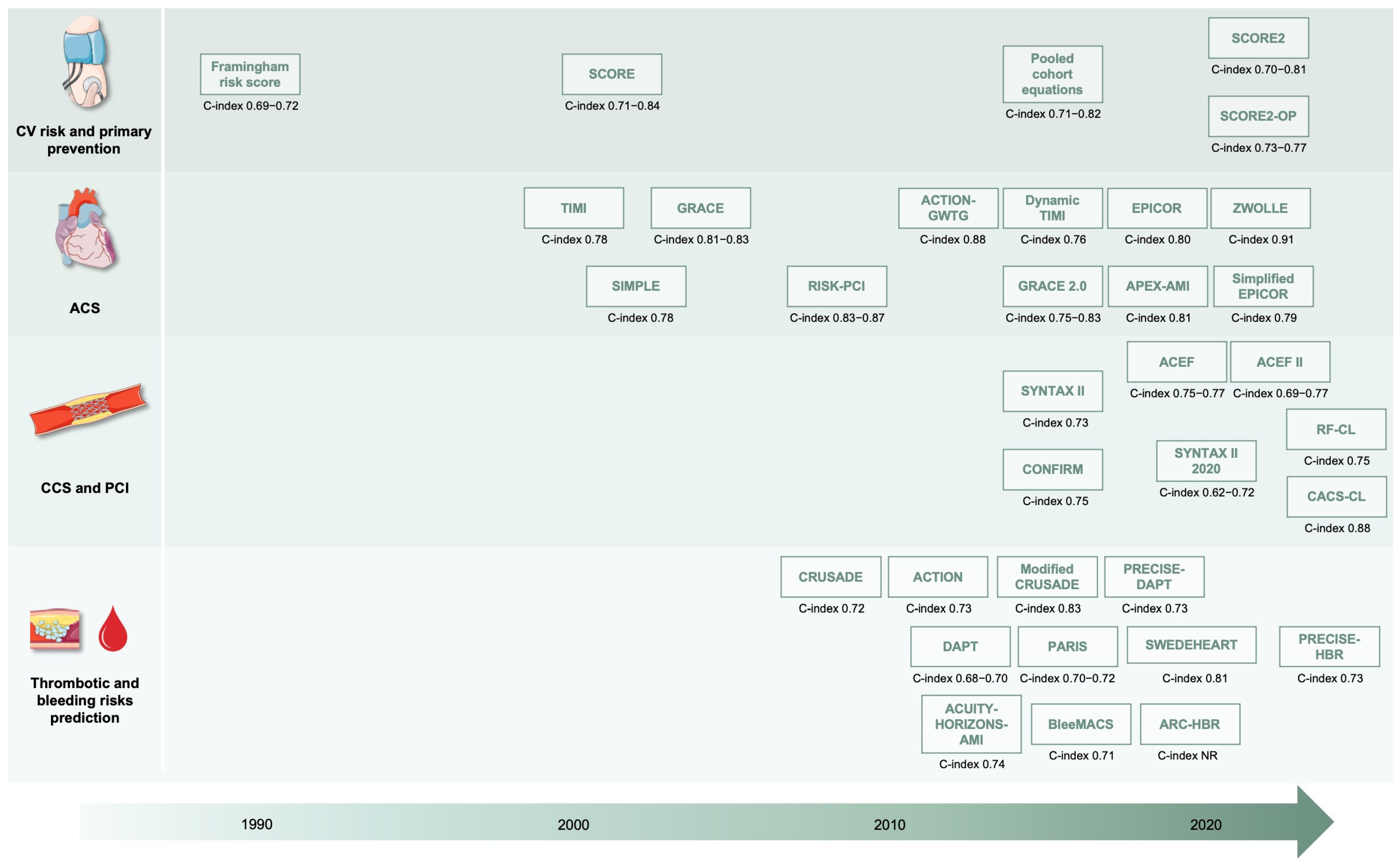
2. Risk Stratification and Scores
2.1. Cardiovascular Risk Assessment and Primary Prevention
2.2. Acute Coronary Syndrome
2.3. Chronic Coronary Syndromes and Percutaneous Coronary Intervention
2.4. Bleeding and Antiplatelet Therapy Modulation
2.5. Artificial Intelligence for Risk Prediction
3. Strengths and Limitations of Predictive Models
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vaduganathan, M.; Mensah George, A.; Turco Justine, V.; Fuster, V.; Roth Gregory, A. The Global Burden of Cardiovascular Diseases and Risk. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Timmis, A.; Aboyans, V.; Vardas, P.; Townsend, N.; Torbica, A.; Kavousi, M.; Boriani, G.; Huculeci, R.; Kazakiewicz, D.; Scherr, D.; et al. European Society of Cardiology: The 2023 Atlas of Cardiovascular Disease Statistics. Eur. Heart J. 2024, 45, 4019–4062. [Google Scholar] [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Hay, S.I.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1260–1344. [Google Scholar] [CrossRef]
- Kazi, D.S.; Elkind, M.S.V.; Deutsch, A.; Dowd, W.N.; Heidenreich, P.; Khavjou, O.; Mark, D.; Mussolino, M.E.; Ovbiagele, B.; Patel, S.S.; et al. Forecasting the Economic Burden of Cardiovascular Disease and Stroke in the United States Through 2050: A Presidential Advisory From the American Heart Association. Circulation 2024, 150, e89–e101. [Google Scholar] [CrossRef]
- Amin, A.P.; Cohen, D.J. Cost-effectiveness of alternative approaches to the management of chronic obstructive coronary artery disease. Rev. Cardiovasc. Med. 2009, 10 (Suppl. S2), S3–S13. [Google Scholar] [CrossRef]
- Scudeler, T.L.; Rezende, P.C.; Hueb, W. The cost-effectiveness of strategies in coronary artery disease. Expert Rev. Pharmacoecon. Outcomes Res. 2014, 14, 805–813. [Google Scholar] [CrossRef]
- Emery, C.; Torreton, E.; Briere, J.B.; Evers, T.; Fagnani, F. Economic burden of coronary artery disease or peripheral artery disease in patients at high risk of ischemic events in the French setting: A claims database analysis. J. Med. Econ. 2020, 23, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, W.S. Diagnosing coronary artery disease and cost of care. J. Cardiovasc. Comput. Tomogr. 2023, 17, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Finocchiaro, S.; Angiolillo, D.J.; Capodanno, D. Advances in the available pharmacotherapy for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Expert Opin. Pharmacother. 2023, 24, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Faro, D.C.; Laudani, C.; Agnello, F.G.; Ammirabile, N.; Finocchiaro, S.; Legnazzi, M.; Mauro, M.S.; Mazzone, P.M.; Occhipinti, G.; Rochira, C.; et al. Complete Percutaneous Coronary Revascularization in Acute Coronary Syndromes with Multivessel Coronary Disease: A Systematic Review. JACC Cardiovasc. Interv. 2023, 16, 2347–2364. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes: Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2025, 85, 2135–2237. [Google Scholar] [CrossRef]
- Spagnolo, M.; Occhipinti, G.; Laudani, C.; Greco, A.; Capodanno, D. Periprocedural Myocardial Infarction and Injury. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 433–445. [Google Scholar] [CrossRef]
- Giugni, F.R.; Berry, J.D.; Khera, A.; Shah, A.M.; de Lemos, J.A. Precision Medicine for Cardiovascular Prevention and Population Health: A Bridge Too Far? Circulation 2024, 150, 1720–1731. [Google Scholar] [CrossRef]
- Roberts, R.; Stewart, A.F. Personalized genomic medicine: A future prerequisite for the prevention of coronary artery disease. Am. Heart Hosp. J. 2006, 4, 222–227. [Google Scholar] [CrossRef]
- Bertsimas, D.; Orfanoudaki, A.; Weiner, R.B. Personalized treatment for coronary artery disease patients: A machine learning approach. Health Care Manag. Sci. 2020, 23, 482–506. [Google Scholar] [CrossRef] [PubMed]
- Tada, H. Personalized Medicine beyond Low-Density Lipoprotein Cholesterol to Combat Residual Risk for Coronary Artery Disease. J. Atheroscler. Thromb. 2021, 28, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Talmor-Barkan, Y.; Bar, N.; Shaul, A.A.; Shahaf, N.; Godneva, A.; Bussi, Y.; Lotan-Pompan, M.; Weinberger, A.; Shechter, A.; Chezar-Azerrad, C.; et al. Metabolomic and microbiome profiling reveals personalized risk factors for coronary artery disease. Nat. Med. 2022, 28, 295–302. [Google Scholar] [CrossRef]
- Moons, K.G.; Kengne, A.P.; Woodward, M.; Royston, P.; Vergouwe, Y.; Altman, D.G.; Grobbee, D.E. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart 2012, 98, 683–690. [Google Scholar] [CrossRef]
- Moons, K.G.; Kengne, A.P.; Grobbee, D.E.; Royston, P.; Vergouwe, Y.; Altman, D.G.; Woodward, M. Risk prediction models: II. External validation, model updating, and impact assessment. Heart 2012, 98, 691–698. [Google Scholar] [CrossRef]
- Deng, W.; Wang, D.; Wan, Y.; Lai, S.; Ding, Y.; Wang, X. Prediction models for major adverse cardiovascular events after percutaneous coronary intervention: A systematic review. Front. Cardiovasc. Med. 2024, 10, 1287434. [Google Scholar] [CrossRef]
- Yun, H.; Noh, N.I.; Lee, E.Y. Genetic risk scores used in cardiovascular disease prediction models: A systematic review. Rev. Cardiovasc. Med. 2022, 23, 8. [Google Scholar] [CrossRef]
- Talha, I.; Elkhoudri, N.; Hilali, A. Major Limitations of Cardiovascular Risk Scores. Cardiovasc. Ther. 2024, 2024, 4133365. [Google Scholar] [CrossRef]
- Rocha, E. Cardiovascular risk scores: Usefulness and limitations. Rev. Port. Cardiol. 2016, 35, 15–18. [Google Scholar] [CrossRef]
- Paredes, S.; Rocha, T.; Mendes, D.; Carvalho, P.; Henriques, J.; Morais, J.; Ferreira, J.; Mendes, M. New approaches for improving cardiovascular risk assessment. Rev. Port. Cardiol. 2016, 35, 5–13. [Google Scholar] [CrossRef]
- Lau, E.; Wu, J.C. Omics, Big Data, and Precision Medicine in Cardiovascular Sciences. Circ. Res. 2018, 122, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Hasegawa, K.; Agewall, S. Toward personalized medicine for cardiovascular pharmacotherapy. Eur. Heart J. 2022, 43, 4719–4721. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Johnson, A.D.; Benjamin, E.J.; Levy, D.; Vasan, R.S. 70-year legacy of the Framingham Heart Study. Nat. Rev. Cardiol. 2019, 16, 687–698. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- deFilippi, C.R.; Christenson, R.H.; Gottdiener, J.S.; Kop, W.J.; Seliger, S.L. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J. Am. Coll. Cardiol. 2010, 55, 441–450. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef]
- Pencina, M.J.; Fine, J.P.; D’Agostino, R.B., Sr. Discrimination slope and integrated discrimination improvement—Properties, relationships and impact of calibration. Stat. Med. 2017, 36, 4482–4490. [Google Scholar] [CrossRef]
- Walsh, C.G.; Sharman, K.; Hripcsak, G. Beyond discrimination: A comparison of calibration methods and clinical usefulness of predictive models of readmission risk. J. Biomed. Inf. Inform. 2017, 76, 9–18. [Google Scholar] [CrossRef]
- Wang, J. Calibration slope versus discrimination slope: Shoes on the wrong feet. J. Clin. Epidemiol. 2020, 125, 161–162. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Shahian, D.M.; O’Brien, S.M.; Badhwar, V. Assessing the Performance of Risk Models with Discrimination and Calibration. Ann. Thorac. Surg. 2023, 115, 282–286. [Google Scholar] [CrossRef]
- Krikella, T.; Dubin, J.A. A Personalized Predictive Model That Jointly Optimizes Discrimination and Calibration. Stat. Med. 2025, 44, e70077. [Google Scholar] [CrossRef]
- Blaha, M.J. The Critical Importance of Risk Score Calibration: Time for Transformative Approach to Risk Score Validation? J. Am. Coll. Cardiol. 2016, 67, 2131–2134. [Google Scholar] [CrossRef]
- Wu, J.; Giles, C.; Dakic, A.; Beyene, H.B.; Huynh, K.; Wang, T.; Meikle, T.; Olshansky, G.; Salim, A.; Duong, T.; et al. Lipidomic Risk Score to Enhance Cardiovascular Risk Stratification for Primary Prevention. J. Am. Coll. Cardiol. 2024, 84, 434–446. [Google Scholar] [CrossRef]
- Qureshi, W.T.; Rana, J.S.; Yeboah, J.; Bin Nasir, U.; Al-Mallah, M.H. Risk Stratification for Primary Prevention of Coronary Artery Disease: Roles of C-Reactive Protein and Coronary Artery Calcium. Curr. Cardiol. Rep. 2015, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R. Genetic Risk Stratification: Tipping Point for Global Primary Prevention of Coronary Artery Disease. Circulation 2018, 137, 2554–2556. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; De Caterina, R. Aspirin for primary prevention of cardiovascular disease: Advice for a decisional strategy based on risk stratification. Anatol. J. Cardiol. 2020, 23, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Barkas, F.; Sener, Y.Z.; Golforoush, P.A.; Kheirkhah, A.; Rodriguez-Sanchez, E.; Novak, J.; Apellaniz-Ruiz, M.; Akyea, R.K.; Bianconi, V.; Ceasovschih, A.; et al. Advancements in risk stratification and management strategies in primary cardiovascular prevention. Atherosclerosis 2024, 395, 117579. [Google Scholar] [CrossRef]
- Anderson, K.M.; Wilson, P.W.; Odell, P.M.; Kannel, W.B. An updated coronary risk profile. A statement for health professionals. Circulation 1991, 83, 356–362. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Mainous, A.G., 3rd; Koopman, R.J.; Diaz, V.A.; Everett, C.J.; Wilson, P.W.; Tilley, B.C. A coronary heart disease risk score based on patient-reported information. Am. J. Cardiol. 2007, 99, 1236–1241. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Conroy, R.M.; Pyörälä, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetière, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef] [PubMed]
- De Backer, G.; Ambrosioni, E.; Borch-Johnsen, K.; Brotons, C.; Cifkova, R.; Dallongeville, J.; Ebrahim, S.; Faergeman, O.; Graham, I.; Mancia, G.; et al. European guidelines on cardiovascular disease prevention in clinical practice: Third Joint Task Force of European and other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of eight societies and by invited experts). Eur. Heart J. 2003, 24, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.B.; Falk, E. Limitations of the SCORE-guided European guidelines on cardiovascular disease prevention. Eur. Heart J. 2017, 38, 2259–2263. [Google Scholar] [CrossRef]
- SCORE2 Working Group and ESC Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef]
- van Trier, T.J.; Snaterse, M.; Boekholdt, S.M.; Scholte Op Reimer, W.J.M.; Hageman, S.H.J.; Visseren, F.L.J.; Dorresteijn, J.A.N.; Peters, R.J.G.; Jørstad, H.T. Validation of Systematic Coronary Risk Evaluation 2 (SCORE2) and SCORE2-Older Persons in the EPIC-Norfolk prospective population cohort. Eur. J. Prev. Cardiol. 2024, 31, 182–189. [Google Scholar] [CrossRef]
- Alfaraj, S.A.; Kist, J.M.; Groenwold, R.H.H.; Spruit, M.; Mook-Kanamori, D.; Vos, R.C. External validation of SCORE2-Diabetes in the Netherlands across various Socioeconomic levels in native-Dutch and non-Dutch populations. Eur. J. Prev. Cardiol. 2025, 32, 555–563. [Google Scholar] [CrossRef]
- Belahnech, Y.; Ródenas-Alesina, E.; Muñoz, M.; Verdu-Rotellar, J.M.; Sao-Avilés, A.; Urio-Garmendia, G.; Osorio, D.; Salas, K.; Pantoja, E.; Ribera, A.; et al. Systematic Coronary Risk Evaluation 2 for Older Persons (SCORE2-OP): 10 years risk validation, clinical utility and potential improvement. Eur. J. Prev. Cardiol. 2025, 32, 527–536. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B., Sr.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2935–2959. [Google Scholar] [CrossRef]
- Yadlowsky, S.; Hayward, R.A.; Sussman, J.B.; McClelland, R.L.; Min, Y.I.; Basu, S. Clinical Implications of Revised Pooled Cohort Equations for Estimating Atherosclerotic Cardiovascular Disease Risk. Ann. Intern. Med. 2018, 169, 20–29. [Google Scholar] [CrossRef]
- Greco, A.; Finocchiaro, S.; Spagnolo, M.; Faro, D.C.; Mauro, M.S.; Raffo, C.; Sangiorgio, G.; Imbesi, A.; Laudani, C.; Mazzone, P.; et al. Lipoprotein (a) as a pharmacological target: Premises, promises and prospects. Circulation 2025, 151, 400–415. [Google Scholar] [CrossRef] [PubMed]
- Myers, P.D.; Huang, W.; Anderson, F.; Stultz, C.M. Choosing Clinical Variables for Risk Stratification Post-Acute Coronary Syndrome. Sci. Rep. 2019, 9, 14631. [Google Scholar] [CrossRef] [PubMed]
- Shavadia, J.; Armstrong, P.W. Risk stratification in non-ST elevation acute coronary syndromes: Searching for the right formula. Eur. Heart J. 2016, 37, 3111–3113. [Google Scholar] [CrossRef] [PubMed]
- Myers, P.D.; Scirica, B.M.; Stultz, C.M. Machine Learning Improves Risk Stratification After Acute Coronary Syndrome. Sci. Rep. 2017, 7, 12692. [Google Scholar] [CrossRef]
- Kolovou, G.D.; Katsiki, N.; Mavrogeni, S. Risk Scores After Acute Coronary Syndrome. Angiology 2017, 68, 185–188. [Google Scholar] [CrossRef]
- Nagesh, C.M.; Roy, A. Role of biomarkers in risk stratification of acute coronary syndrome. Indian J. Med. Res. 2010, 132, 627–633. [Google Scholar] [CrossRef]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; Pieper, K.S.; Eagle, K.A.; Cannon, C.P.; Van De Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef]
- Eagle, K.A.; Lim, M.J.; Dabbous, O.H.; Pieper, K.S.; Goldberg, R.J.; Van de Werf, F.; Goodman, S.G.; Granger, C.B.; Steg, P.G.; Gore, J.M.; et al. A Validated Prediction Model for All Forms of Acute Coronary Syndrome. JAMA 2004, 291, 2727–2733. [Google Scholar] [CrossRef]
- Fox, K.A.A.; Dabbous, O.H.; Goldberg, R.J.; Pieper, K.S.; Eagle, K.A.; Van de Werf, F.; Avezum, Á.; Goodman, S.G.; Flather, M.D.; Anderson, F.A.; et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE). BMJ 2006, 333, 1091. [Google Scholar] [CrossRef]
- Fox, K.A.A.; FitzGerald, G.; Puymirat, E.; Huang, W.; Carruthers, K.; Simon, T.; Coste, P.; Monsegu, J.; Gabriel Steg, P.; Danchin, N.; et al. Should patients with acute coronary disease be stratified for management according to their risk? Derivation, external validation and outcomes using the updated GRACE risk score. BMJ Open 2014, 4, e004425. [Google Scholar] [CrossRef]
- Morrow, D.A.; Antman, E.M.; Charlesworth, A.; Cairns, R.; Murphy, S.A.; de Lemos, J.A.; Giugliano, R.P.; McCabe, C.H.; Braunwald, E. TIMI Risk Score for ST-Elevation Myocardial Infarction: A Convenient, Bedside, Clinical Score for Risk Assessment at Presentation. Circulation 2000, 102, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.T.; Morrow, D.A.; Braunwald, E.; Sloan, S.; Contant, C.; Murphy, S.; Antman, E.M. Dynamic TIMI Risk Score for STEMI. J. Am. Heart Assoc. 2013, 2, e003269. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.A.; Antman, E.M.; Giugliano, R.P.; Cairns, R.; Charlesworth, A.; Murphy, S.A.; de Lemos, J.A.; McCabe, C.H.; Braunwald, E. A simple risk index for rapid initial triage of patients with ST-elevation myocardial infarction: An InTIME II substudy. Lancet 2001, 358, 1571–1575. [Google Scholar] [CrossRef]
- McNamara, R.L.; Kennedy, K.F.; Cohen, D.J.; Diercks, D.B.; Moscucci, M.; Ramee, S.; Wang, T.Y.; Connolly, T.; Spertus, J.A. Predicting In-Hospital Mortality in Patients with Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 68, 626–635. [Google Scholar] [CrossRef]
- De Luca, G.; Suryapranata, H.; van ‘t Hof, A.W.J.; de Boer, M.-J.; Hoorntje, J.C.A.; Dambrink, J.-H.E.; Gosselink, A.T.M.; Ottervanger, J.P.; Zijlstra, F. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: Implications for early discharge. Circulation 2004, 109, 2737–2743. [Google Scholar] [CrossRef]
- Mrdovic, I.; Savic, L.; Perunicic, J.; Asanin, M.; Lasica, R.; Marinkovic, J.; Vasiljevic, Z.; Ostojic, M. Development and validation of a risk scoring model to predict net adverse cardiovascular outcomes after primary percutaneous coronary intervention in patients pretreated with 600 mg clopidogrel: Rationale and design of the RISK-PCI study. J. Interv. Cardiol. 2009, 22, 320–328. [Google Scholar] [CrossRef]
- Pocock, S.J.; Huo, Y.; Van de Werf, F.; Newsome, S.; Chin, C.T.; Vega, A.M.; Medina, J.; Bueno, H. Predicting two-year mortality from discharge after acute coronary syndrome: An internationally-based risk score. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 727–737. [Google Scholar] [CrossRef]
- Stebbins, A.; Mehta, R.H.; Armstrong, P.W.; Lee, K.L.; Hamm, C.; Van de Werf, F.; James, S.; Toftegaard-Nielsen, T.; Seabra-Gomes, R.; White, H.D.; et al. A model for predicting mortality in acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: Results from the Assessment of Pexelizumab in Acute Myocardial Infarction Trial. Circ. Cardiovasc. Interv. 2010, 3, 414–422. [Google Scholar] [CrossRef]
- Agnello, F.; Mauro, M.S.; Rochira, C.; Landolina, D.; Finocchiaro, S.; Greco, A.; Ammirabile, N.; Raffo, C.; Mazzone, P.M.; Spagnolo, M.; et al. PCSK9 inhibitors: Current status and emerging frontiers in lipid control. Expert. Rev. Cardiovasc. Ther. 2023, 22, 41–58. [Google Scholar] [CrossRef]
- Laudani, C.; Occhipinti, G.; Greco, A.; Spagnolo, M.; Giacoppo, D.; Capodanno, D. Completeness, Timing, and Guidance of Percutaneous Coronary Intervention for Myocardial Infarction and Multivessel Disease: A Systematic Review and Network Meta-Analysis. EuroIntervention 2025, 21, e203–e216. [Google Scholar] [CrossRef]
- de Filippo, O.; Bruno, F.; Pinxterhuis, T.H.; Gąsior, M.; Perl, L.; Gaido, L.; Tuttolomondo, D.; Greco, A.; Verardi, R.; Lo Martire, G.; et al. Predictors of target lesion failure after treatment of left main, bifurcation, or chronic total occlusion lesions with ultrathin-strut drug-eluting coronary stents in the ULTRA registry. Catheter. Cardiovasc. Interv. 2023, 102, 221–232. [Google Scholar] [CrossRef]
- Laudani, C.; Occhipinti, G.; Greco, A.; Giacoppo, D.; Spagnolo, M.; Capodanno, D.A. Pairwise and Network Meta-analysis of Anti-inflammatory Strategies After Myocardial Infarction: The TITIAN study. Eur. Heart J. Cardiovasc. Pharmacother. 2025, 11, 218–229. [Google Scholar] [CrossRef]
- Imbesi, A.; Greco, A.; Spagnolo, M.; Laudani, C.; Raffo, C.; Finocchiaro, S.; Mazzone, P.M.; Landolina, D.; Mauro, M.S.; Cutore, L.; et al. Targeting inflammation in acute myocardial infarction. J. Am. Coll. Cardiol. 2025, 72, 199–201. [Google Scholar]
- Sianos, G.; Morel, M.-A.; Kappetein, A.P.; Morice, M.-C.; Colombo, A.; Dawkins, K.; van den Brand, M.; Van Dyck, N.; Russell, M.E.; Mohr, F.W.; et al. The SYNTAX Score: An angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005, 1, 219–227. [Google Scholar] [PubMed]
- Farooq, V.; van Klaveren, D.; Steyerberg, E.W.; Meliga, E.; Vergouwe, Y.; Chieffo, A.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.; et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: Development and validation of SYNTAX score II. Lancet 2013, 381, 639–650. [Google Scholar] [CrossRef]
- Angleitner, P.; Abfalterer, H.; Kaider, A.; Manville, E.; Bichler, M.; Graber, M.; Pölzl, L.; Zimpfer, D.; Sandner, S.; Bonaros, N. External validation of SYNTAX score II in a real-world cohort undergoing coronary artery bypass grafting. J. Cardiothorac. Surg. 2025, 20, 324. [Google Scholar] [CrossRef]
- Takahashi, K.; Serruys, P.W.; Fuster, V.; Farkouh, M.E.; Spertus, J.A.; Cohen, D.J.; Park, S.-J.; Park, D.-W.; Ahn, J.-M.; Kappetein, A.P.; et al. Redevelopment and validation of the SYNTAX score II to individualise decision making between percutaneous and surgical revascularisation in patients with complex coronary artery disease: Secondary analysis of the multicentre randomised controlled SYNTAXES. Lancet 2020, 396, 1399–1412. [Google Scholar] [CrossRef]
- Hadamitzky, M.; Achenbach, S.; Al-Mallah, M.; Berman, D.; Budoff, M.; Cademartiri, F.; Callister, T.; Chang, H.-J.; Cheng, V.; Chinnaiyan, K.; et al. Optimized prognostic score for coronary computed tomographic angiography: Results from the CONFIRM registry (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry). J. Am. Coll. Cardiol. 2013, 62, 468–476. [Google Scholar] [CrossRef]
- Chichareon, P.; Modolo, R.; van Klaveren, D.; Takahashi, K.; Kogame, N.; Chang, C.-C.; Katagiri, Y.; Tomaniak, M.; Asano, T.; Spitzer, E.; et al. Predictive ability of ACEF and ACEF II score in patients undergoing percutaneous coronary intervention in the GLOBAL LEADERS study. Int. J. Cardiol. 2019, 286, 43–50. [Google Scholar] [CrossRef]
- Rasmussen, L.D.; Karim, S.R.; Westra, J.; Nissen, L.; Dahl, J.N.; Brix, G.S.; Knuuti, J.; Schmidt, S.E.; Holm, N.R.; Christiansen, E.H.; et al. Clinical Likelihood Prediction of Hemodynamically Obstructive Coronary Artery Disease in Patients with Stable Chest Pain. JACC Cardiovasc. Imaging 2024, 17, 1199–1210. [Google Scholar] [CrossRef]
- Greco, A.; Capodanno, D. Differences in coronary artery disease and outcomes of percutaneous coronary intervention with drug-eluting stents in women and men. Expert. Rev. Cardiovasc. Ther. 2021, 19, 301–312. [Google Scholar] [CrossRef]
- Occhipinti, G.; Greco, A.; Angiolillo, D.J.; Capodanno, D. Gender differences in efficacy and safety of antiplatelet strategies for acute coronary syndromes. Expert. Opin. Drug Saf. 2023, 22, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Capodanno, D. Therapeutic uncertainties: First finding of atrial fibrillation in acute coronary syndrome. Eur. Heart J. Suppl. 2022, 24, I43–I46. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Laudani, C.; Rochira, C.; Capodanno, D. Antithrombotic Management in AF Patients Following Percutaneous Coronary Intervention: A European Perspective. Interv. Cardiol. 2023, 18, e05. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Costa, F.; Lokhnygina, Y.; Clare, R.M.; Wallentin, L.; Moliterno, D.J.; Armstrong, P.W.; White, H.D.; Held, C.; Aylward, P.E.; et al. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: Lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur. Heart J. 2016, 38, 804–810. [Google Scholar] [CrossRef]
- Nathan, A.S.; Sen, S.; Yeh, R.W. The risk of bleeding with the use of antiplatelet agents for the treatment of cardiovascular disease. Expert. Opin. Drug Saf. 2017, 16, 561–572. [Google Scholar] [CrossRef]
- Capodanno, D.; Greco, A. Dual antiplatelet therapy in patients at high bleeding risk: Less is more-more or less. Eur. Heart J. 2023, 44, 969–971. [Google Scholar] [CrossRef]
- Greco, A.; Capodanno, D. Shortening Dual Antiplatelet Therapy Duration in High-Risk Patients Undergoing Percutaneous Coronary Intervention. JACC Asia 2023, 3, 47–50. [Google Scholar] [CrossRef]
- Capodanno, D.; Greco, A. Short Dual Antiplatelet Therapy in High Bleeding Risk Patients: 1 Month or 3 Months? JACC Cardiovasc. Interv. 2023, 16, 2511–2513. [Google Scholar] [CrossRef]
- Greco, A.; Capodanno, D.; Angiolillo, D. The Conundrum Surrounding Racial Differences on Ischaemic and Bleeding Risk with Dual Anti-Platelet Therapy. Thromb. Haemost. 2019, 119, 9–13. [Google Scholar] [CrossRef]
- Capodanno, D.; Mehran, R.; Krucoff, M.W.; Baber, U.; Bhatt, D.L.; Capranzano, P.; Collet, J.-P.; Cuisset, T.; Luca, G.D.; Luca, L.D.; et al. Defining Strategies of Modulation of Antiplatelet Therapy in Patients with Coronary Artery Disease: A Consensus Document from the Academic Research Consortium. Circulation 2023, 147, 1933–1944. [Google Scholar] [CrossRef]
- Jneid, H.; Bhatt, D.L. Advances in antiplatelet therapy. Expert. Opin. Emerg. Drugs 2003, 8, 349–363. [Google Scholar] [CrossRef]
- Oliva, A.; Cao, D.; Spirito, A.; Nicolas, J.; Pileggi, B.; Kamaleldin, K.; Vogel, B.; Mehran, R. Personalized Approaches to Antiplatelet Treatment for Cardiovascular Diseases: An Umbrella Review. Pharmacogenomics Pers. Med. 2023, 16, 973–990. [Google Scholar] [CrossRef]
- Sibbing, D. Modulation of Antiplatelet Therapy in PCI-Treated Patients: A Rocky Road Toward More Individualized Treatment. J. Am. Coll. Cardiol. 2024, 83, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Gao, X.; Lee, S.H.; Kan, J.; Zhang, J.J.; Lee, S.J.; Hong, S.J.; Ahn, C.M.; Kim, J.S.; Kim, B.K.; et al. De-escalating Dual Antiplatelet Therapy to Ticagrelor Monotherapy in Acute Coronary Syndrome: A Systematic Review and Individual Patient Data Meta-analysis of Randomized Clinical Trials. Ann. Intern. Med. 2025, 178, 533–542. [Google Scholar] [CrossRef]
- Greco, A.; Scilletta, S.; Faro, D.C.; Agnello, F.; Mauro, M.S.; Laudani, C.; Occhipinti, G.; Spagnolo, M.; Rochira, C.; Finocchiaro, S.; et al. Eligibility to Intensified Antithrombotic Regimens for Secondary Prevention in Patients Who Underwent Percutaneous Coronary Intervention. Am. J. Cardiol. 2023, 199, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Baber, U.; Leisman, D.E.; Cohen, D.J.; Gibson, C.M.; Henry, T.D.; Dangas, G.; Moliterno, D.; Kini, A.; Krucoff, M.; Colombo, A.; et al. Tailoring Antiplatelet Therapy Intensity to Ischemic and Bleeding Risk. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e004945. [Google Scholar] [CrossRef]
- Farag, M.; Jeyalan, V.; Ferreiro, J.L.; Jeong, Y.H.; Geisler, T.; Gorog, D.A. Reduction or de-escalation of dual antiplatelet therapy intensity or duration in patients with acute coronary syndromes undergoing percutaneous coronary intervention: A mini-review. Front. Cardiovasc. Med. 2022, 9, 1018649. [Google Scholar] [CrossRef]
- Landi, A.; Caglioni, S.; Valgimigli, M. De-escalation in intensity or duration of dual antiplatelet therapy in patients with coronary artery disease: More than alternative treatment options. Eur. J. Intern. Med. 2023, 110, 16–18. [Google Scholar] [CrossRef]
- Gorog, D.A.; Ferreiro, J.L.; Ahrens, I.; Ako, J.; Geisler, T.; Halvorsen, S.; Huber, K.; Jeong, Y.H.; Navarese, E.P.; Rubboli, A.; et al. De-escalation or abbreviation of dual antiplatelet therapy in acute coronary syndromes and percutaneous coronary intervention: A Consensus Statement from an international expert panel on coronary thrombosis. Nat. Rev. Cardiol. 2023, 20, 830–844. [Google Scholar] [CrossRef]
- Greco, A.; Mauro, M.S.; Capodanno, D.; Angiolillo, D.J. P2Y12 Inhibitor Monotherapy: Considerations for Acute and Long-term Secondary Prevention post-PCI. Rev. Cardiovasc. Med. 2022, 23, 348. [Google Scholar] [CrossRef]
- Greco, A.; Spagnolo, M.; Capodanno, D. Stent type selection in high bleeding risk patients. Expert. Rev. Med. Devices 2024, 21, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Scalia, L.; Laudani, C.; Spagnolo, M.; Mauro, M.S.; Sammartino, S.; Capranzano, P.; Capodanno, D. Downstream cangrelor versus upstream ticagrelor in patients with ST-segment elevation myocardial infarction: A propensity score-matched analysis. Int. J. Cardiol. 2025, 418, 132660. [Google Scholar] [CrossRef] [PubMed]
- Greco, A. Antiplatelet Monotherapy After Short DAPT in ACS. JACC Cardiovasc. Interv. 2025, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Subherwal, S.; Bach, R.G.; Chen, A.Y.; Gage, B.F.; Rao, S.V.; Newby, L.K.; Wang, T.Y.; Gibler, W.B.; Ohman, E.M.; Roe, M.T.; et al. Baseline Risk of Major Bleeding in Non-ST-Segment-Elevation Myocardial Infarction: The CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) Bleeding Score. Circulation 2009, 119, 1873–1882. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, R.; Zhang, Y.; Zhao, X.; Nie, S. Development and validation of a modified CRUSADE score for predicting in-hospital major bleeding risk in patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention. J. Am. Coll. Cardiol. 2018, 72, C103. [Google Scholar] [CrossRef]
- Mehran, R.; Pocock, S.J.; Nikolsky, E.; Clayton, T.; Dangas, G.D.; Kirtane, A.J.; Parise, H.; Fahy, M.; Manoukian, S.V.; Feit, F.; et al. A Risk Score to Predict Bleeding in Patients with Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2010, 55, 2556–2566. [Google Scholar] [CrossRef]
- Mathews, R.; Peterson, E.D.; Chen, A.Y.; Wang, T.Y.; Chin, C.T.; Fonarow, G.C.; Cannon, C.P.; Rumsfeld, J.S.; Roe, M.T.; Alexander, K.P. In-Hospital Major Bleeding During ST-Elevation and Non–ST-Elevation Myocardial Infarction Care: Derivation and Validation of a Model from the ACTION Registry®-GWTG™. Am. J. Cardiol. 2011, 107, 1136–1143. [Google Scholar] [CrossRef]
- Baber, U.; Mehran, R.; Giustino, G.; Cohen, D.J.; Henry, T.D.; Sartori, S.; Ariti, C.; Litherland, C.; Dangas, G.; Gibson, C.M.; et al. Coronary Thrombosis and Major Bleeding after PCI with Drug-Eluting Stents Risk Scores from Paris. J. Am. Coll. Cardiol. 2016, 67, 2224–2234. [Google Scholar] [CrossRef]
- Costa, F.; van Klaveren, D.; James, S.; Heg, D.; Räber, L.; Feres, F.; Pilgrim, T.; Hong, M.-K.; Kim, H.-S.; Colombo, A.; et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet 2017, 389, 1025–1034. [Google Scholar] [CrossRef]
- Raposeiras-Roubín, S.; Faxén, J.; Íñiguez-Romo, A.; Henriques, J.P.S.; D’Ascenzo, F.; Saucedo, J.; Szummer, K.; Jernberg, T.; James, S.K.; Juanatey, J.R.G.; et al. Development and external validation of a post-discharge bleeding risk score in patients with acute coronary syndrome: The BleeMACS score. Int. J. Cardiol. 2018, 254, 10–15. [Google Scholar] [CrossRef]
- Capodanno, D.; Greco, A. Risk Stratification for Bleeding in the Elderly with Acute Coronary Syndrome: Not so Simple. Thromb. Haemost. 2018, 118, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Simonsson, M.; Winell, H.; Olsson, H.; Szummer, K.; Alfredsson, J.; Hall, M.; Dondo, T.B.; Gale, C.P.; Jernberg, T. Development and Validation of a Novel Risk Score for In-Hospital Major Bleeding in Acute Myocardial Infarction:-The SWEDEHEART Score. J. Am. Heart Assoc. 2019, 8, e012157. [Google Scholar] [CrossRef] [PubMed]
- Mauri, L.; Kereiakes, D.J.; Yeh, R.W.; Driscoll-Shempp, P.; Cutlip, D.E.; Steg, P.G.; Normand, S.-L.T.; Braunwald, E.; Wiviott, S.D.; Cohen, D.J.; et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N. Engl. J. Med. 2014, 371, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation 2019, 140, 240–261. [Google Scholar] [CrossRef]
- Ueki, Y.; Bär, S.; Losdat, S.; Otsuka, T.; Zanchin, C.; Zanchin, T.; Gragnano, F.; Gargiulo, G.; Siontis, G.C.M.; Praz, F.; et al. Validation of the Academic Research Consortium for High Bleeding Risk (ARC-HBR) criteria in patients undergoing percutaneous coronary intervention and comparison with contemporary bleeding risk scores. EuroIntervention 2020, 16, 371–379. [Google Scholar] [CrossRef]
- Urban, P.; Gregson, J.; Owen, R.; Mehran, R.; Windecker, S.; Valgimigli, M.; Varenne, O.; Krucoff, M.; Saito, S.; Baber, U.; et al. Assessing the Risks of Bleeding vs Thrombotic Events in Patients at High Bleeding Risk After Coronary Stent Implantation. JAMA Cardiol. 2021, 6, 410. [Google Scholar] [CrossRef]
- Marquis-Gravel, G.; Neely, M.L.; Valgimigli, M.; Costa, F.; Van Klaveren, D.; Altner, R.; Bhatt, D.L.; Armstrong, P.W.; Fox, K.A.A.; White, H.D.; et al. Long-Term Bleeding Risk Prediction with Dual Antiplatelet Therapy After Acute Coronary Syndromes Treated Without Revascularization. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006582. [Google Scholar] [CrossRef]
- Lim, C.-E.; Simonsson, M.; Pasternak, B.; Jernberg, T.; Edgren, G.; Ueda, P. Discordance and Performance of the ARC-HBR and PRECISE-DAPT High Bleeding Risk Definitions After Coronary Stenting. JACC Cardiovasc. Interv. 2025, 18, 637–650. [Google Scholar] [CrossRef]
- Gragnano, F.; van Klaveren, D.; Heg, D.; Räber, L.; Krucoff, M.W.; Raposeiras-Roubän, S.; ten Berg, J.M.; Leonardi, S.; Kimura, T.; Corpataux, N.; et al. Derivation and Validation of the PRECISE-HBR Score to Predict Bleeding After Percutaneous Coronary Intervention. Circulation 2025, 151, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; De Filippo, O.; Gallone, G.; Mittone, G.; Deriu, M.A.; Iannaccone, M.; Ariza-Solé, A.; Liebetrau, C.; Manzano-Fernández, S.; Quadri, G.; et al. Machine learning-based prediction of adverse events following an acute coronary syndrome (PRAISE): A modelling study of pooled datasets. Lancet 2021, 397, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Sau, A.; Pastika, L.; Sieliwonczyk, E.; Patlatzoglou, K.; Ribeiro, A.H.; McGurk, K.A.; Zeidaabadi, B.; Zhang, H.; Macierzanka, K.; Mandic, D.; et al. Artificial intelligence-enabled electrocardiogram for mortality and cardiovascular risk estimation: A model development and validation study. Lancet Digit. Health 2024, 6, e791–e802. [Google Scholar] [CrossRef]
- Pezel, T.; Toupin, S.; Bousson, V.; Hamzi, K.; Hovasse, T.; Lefevre, T.; Chevalier, B.; Unterseeh, T.; Sanguineti, F.; Champagne, S.; et al. A Machine Learning Model Using Cardiac CT and MRI Data Predicts Cardiovascular Events in Obstructive Coronary Artery Disease. Radiology 2025, 314, e233030. [Google Scholar] [CrossRef]
- Batra, P.; Khera, A.V. Machine learning to assess coronary artery disease status-is it helpful? Lancet 2023, 401, 173–175. [Google Scholar] [CrossRef]
- Overmars, L.M.; van Es, B.; Groepenhoff, F.; De Groot, M.C.H.; Pasterkamp, G.; den Ruijter, H.M.; van Solinge, W.W.; Hoefer, I.E.; Haitjema, S. Preventing unnecessary imaging in patients suspect of coronary artery disease through machine learning of electronic health records. Eur. Heart J. Digit. Health 2022, 3, 11–19. [Google Scholar] [CrossRef]
- Rao, G.M.; Ramesh, D.; Sharma, V.; Sinha, A.; Hassan, M.M.; Gandomi, A.H. AttGRU-HMSI: Enhancing heart disease diagnosis using hybrid deep learning approach. Sci. Rep. 2024, 14, 7833. [Google Scholar] [CrossRef]
- Vu, T.; Kokubo, Y.; Inoue, M.; Yamamoto, M.; Mohsen, A.; Martin-Morales, A.; Dawadi, R.; Inoue, T.; Tay, J.T.; Yoshizaki, M.; et al. Machine Learning Model for Predicting Coronary Heart Disease Risk: Development and Validation Using Insights From a Japanese Population–Based Study. JMIR Cardio 2025, 9, e68066. [Google Scholar] [CrossRef]
- Li, Y.; Guan, L.; Ning, C.; Zhang, P.; Zhao, Y.; Liu, Q.; Ping, P.; Fu, S. Machine learning-based models to predict one-year mortality among Chinese older patients with coronary artery disease combined with impaired glucose tolerance or diabetes mellitus. Cardiovasc. Diabetol. 2023, 22, 139. [Google Scholar] [CrossRef]
- Hofweber, T.; Walker, R.L. Machine Learning in Health Care: Ethical Considerations Tied to Privacy, Interpretability, and Bias. N. Carol. Med. J. 2024, 85, 240–245. [Google Scholar] [CrossRef]
- Dong, T.; Sinha, S.; Zhai, B.; Fudulu, D.; Chan, J.; Narayan, P.; Judge, A.; Caputo, M.; Dimagli, A.; Benedetto, U.; et al. Performance Drift in Machine Learning Models for Cardiac Surgery Risk Prediction: Retrospective Analysis. JMIRx Med. 2024, 5, e45973. [Google Scholar] [CrossRef]
- Pencina, M.J.; McCall, J.; Economou-Zavlanos, N.J. A Federated Registration System for Artificial Intelligence in Health. JAMA 2024, 332, 789–790. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Z.; Cui, Q.; Liu, F.; Li, J.; Niu, X.; Shen, C.; Hu, D.; Huang, K.; Chen, J.; et al. A polygenic risk score improves risk stratification of coronary artery disease: A large-scale prospective Chinese cohort study. Eur. Heart J. 2022, 43, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Garot, J. Improving Risk Stratification of Patients with Known CAD. JACC Cardiovasc. Imaging 2022, 15, 72–74. [Google Scholar] [CrossRef]
- Roberts, R.; Campillo, A.; Schmitt, M. Prediction and management of CAD risk based on genetic stratification. Trends Cardiovasc. Med. 2020, 30, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Vernick, W.; Fleisher, L.A. Risk stratification. Best Pract. Res. Clin. Anaesthesiol. 2008, 22, 1–21. [Google Scholar] [CrossRef]
- Sauerbrei, W.; Royston, P.; Binder, H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat. Med. 2007, 26, 5512–5528. [Google Scholar] [CrossRef]
- Forina, M.; Lanteri, S.; Oliveros, M.C.; Millan, C.P. Selection of useful predictors in multivariate calibration. Anal. Bioanal. Chem. 2004, 380, 397–418. [Google Scholar] [CrossRef]
- Poldrack, R.A.; Huckins, G.; Varoquaux, G. Establishment of Best Practices for Evidence for Prediction: A Review. JAMA Psychiatry 2020, 77, 534–540. [Google Scholar] [CrossRef]
- Toll, D.B.; Janssen, K.J.; Vergouwe, Y.; Moons, K.G. Validation, updating and impact of clinical prediction rules: A review. J. Clin. Epidemiol. 2008, 61, 1085–1094. [Google Scholar] [CrossRef]
- Kuijpers, T.; van der Heijden, G.J.; Vergouwe, Y.; Twisk, J.W.; Boeke, A.J.; Bouter, L.M.; van der Windt, D.A. Good generalizability of a prediction rule for prediction of persistent shoulder pain in the short term. J. Clin. Epidemiol. 2007, 60, 947–953. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Bleeker, S.E.; Moll, H.A.; Grobbee, D.E.; Moons, K.G. Internal and external validation of predictive models: A simulation study of bias and precision in small samples. J. Clin. Epidemiol. 2003, 56, 441–447. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Persaud, N.; Mamdani, M.M. External validity: The neglected dimension in evidence ranking. J. Eval. Clin. Pract. 2006, 12, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ariza, C.D.; Cabrera-Villamizar, A.; Rodríguez-Pulido, A.L.; Callegari, S.; Ossa Rodríguez, N.A.; Pinilla-Roncancio, M.; Moreno López, S.M.; Sánchez-Vallejo, C.A. External validation of the ACC/AHA ASCVD risk score in a Colombian population cohort. Sci. Rep. 2023, 13, 6139. [Google Scholar] [CrossRef] [PubMed]
- Rodondi, N.; Locatelli, I.; Aujesky, D.; Butler, J.; Vittinghoff, E.; Simonsick, E.; Satterfield, S.; Newman, A.B.; Wilson, P.W.F.; Pletcher, M.J.; et al. Framingham Risk Score and Alternatives for Prediction of Coronary Heart Disease in Older Adults. PLoS ONE 2012, 7, e34287. [Google Scholar] [CrossRef]
- Blythe, R.; Naicker, S.; White, N.; Donovan, R.; Scott, I.A.; McKelliget, A.; McPhail, S.M. Clinician perspectives and recommendations regarding design of clinical prediction models for deteriorating patients in acute care. BMC Med. Inf. Inform. Decis. Mak. 2024, 24, 241. [Google Scholar] [CrossRef]
- Hulot, J.S.; Clopton, P. When Natural Peptides Meet Artificial Intelligence to Improve Risk Prediction. J. Am. Coll. Cardiol. 2021, 78, 1632–1634. [Google Scholar] [CrossRef]
- Gennari, A.G.; Rossi, A.; De Cecco, C.N.; van Assen, M.; Sartoretti, T.; Giannopoulos, A.A.; Schwyzer, M.; Huellner, M.W.; Messerli, M. Artificial intelligence in coronary artery calcium score: Rationale, different approaches, and outcomes. Int. J. Cardiovasc. Imaging 2024, 40, 951–966. [Google Scholar] [CrossRef]
- Correia, L.; Lopes, D.; Porto, J.V.; Lacerda, Y.F.; Correia, V.C.A.; Bagano, G.O.; Pontes, B.S.B.; Melo, M.H.V.; Silva, T.E.A.; Meireles, A.C. Validation of an Artificial Intelligence Algorithm for Diagnostic Prediction of Coronary Disease: Comparison with a Traditional Statistical Model. Arq. Bras. Cardiol. 2021, 117, 1061–1070. [Google Scholar] [CrossRef]
- Unterhuber, M.; Kresoja, K.P.; Rommel, K.P.; Besler, C.; Baragetti, A.; Klöting, N.; Ceglarek, U.; Blüher, M.; Scholz, M.; Catapano, A.L.; et al. Proteomics-Enabled Deep Learning Machine Algorithms Can Enhance Prediction of Mortality. J. Am. Coll. Cardiol. 2021, 78, 1621–1631. [Google Scholar] [CrossRef]
- Pieszko, K.; Slomka, P.J. Assessing Performance of Machine Learning. JAMA Cardiol. 2021, 6, 1465. [Google Scholar] [CrossRef]
- Khera, R.; Haimovich, J.; Hurley, N.C.; McNamara, R.; Spertus, J.A.; Desai, N.; Rumsfeld, J.S.; Masoudi, F.A.; Huang, C.; Normand, S.L.; et al. Use of Machine Learning Models to Predict Death After Acute Myocardial Infarction. JAMA Cardiol. 2021, 6, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Engelhard, M.M.; Navar, A.M.; Pencina, M.J. Incremental Benefits of Machine Learning-When Do We Need a Better Mousetrap? JAMA Cardiol. 2021, 6, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Mustafiz, C.; Mutahar, D.; Zaka, A.; Parvez, R.; Mridha, N.; Stretton, B.; Kovoor, J.G.; Bacchi, S.; Ramponi, F.; et al. Machine Learning vs Traditional Approaches to Predict All-Cause Mortality for Acute Coronary Syndrome: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2025, 41, 1564–1583. [Google Scholar] [CrossRef] [PubMed]
- Petch, J.; Di, S.; Nelson, W. Opening the Black Box: The Promise and Limitations of Explainable Machine Learning in Cardiology. Can. J. Cardiol. 2022, 38, 204–213. [Google Scholar] [CrossRef]
- Staffa, S.J.; Zurakowski, D. Statistical Development and Validation of Clinical Prediction Models. Anesthesiology 2021, 135, 396–405. [Google Scholar] [CrossRef]
- Krittanawong, C.; Johnson, K.W.; Rosenson, R.S.; Wang, Z.; Aydar, M.; Baber, U.; Min, J.K.; Tang, W.H.W.; Halperin, J.L.; Narayan, S.M. Deep learning for cardiovascular medicine: A practical primer. Eur. Heart J. 2019, 40, 2058–2073. [Google Scholar] [CrossRef]
- Karako, K.; Tang, W. Applications of and issues with machine learning in medicine: Bridging the gap with explainable AI. Biosci. Trends 2025, 18, 497–504. [Google Scholar] [CrossRef]
- Price, W.N. Big data and black-box medical algorithms. Sci. Transl. Med. 2018, 10, eaao5333. [Google Scholar] [CrossRef]
- Krittanawong, C.; Zhang, H.; Wang, Z.; Aydar, M.; Kitai, T. Artificial Intelligence in Precision Cardiovascular Medicine. J. Am. Coll. Cardiol. 2017, 69, 2657–2664. [Google Scholar] [CrossRef]
- Wang, Y.; Aivalioti, E.; Stamatelopoulos, K.; Zervas, G.; Mortensen, M.B.; Zeller, M.; Liberale, L.; Di Vece, D.; Schweiger, V.; Camici, G.G.; et al. Machine learning in cardiovascular risk assessment: Towards a precision medicine approach. Eur. J. Clin. Investig. 2025, 55 (Suppl. S1), e70017. [Google Scholar] [CrossRef]
- Corral-Acero, J.; Margara, F.; Marciniak, M.; Rodero, C.; Loncaric, F.; Feng, Y.; Gilbert, A.; Fernandes, J.F.; Bukhari, H.A.; Wajdan, A.; et al. The ‘Digital Twin’ to enable the vision of precision cardiology. Eur. Heart J. 2020, 41, 4556–4564. [Google Scholar] [CrossRef]

| Score | Timing of Assessment | Clinical Setting | Predicted Event and Timeframe | Input Variables | C-Statistics | External Validation |
|---|---|---|---|---|---|---|
| Framingham Risk Score | Before disease onset | General population | 10-year risk of cardiovascular disease | Clinical: age, sex, systolic blood pressure, treatment for hypertension, smoking status, diabetes, family history of premature cardiovascular disease Laboratory: HDL-C, total cholesterol | 0.69 (men), 0.72 (women) | 0.61–0.86 in different populations |
| SCORE | Before disease onset | General population aged 40–65 years | 10-year risk of fatal cardiovascular events | Clinical: age, sex, systolic blood pressure, smoking status Laboratory: total cholesterol or cholesterol/HDL-C ratio | 0.71–0.84 in different cohorts | 0.75 in Spanish without medical history |
| SCORE2 | Before disease onset | General population aged 40–69 years | 10-year risk of fatal or nonfatal cardiovascular events | Clinical: age, sex, systolic blood pressure, smoking status Laboratory: total cholesterol, HDL-C | 0.70–0.81 across age and regional cohorts | 0.64–0.81 in different populations |
| SCORE2-OP | Before disease onset | General population aged 70–89 years | 10-year risk of fatal or nonfatal cardiovascular events | Clinical: age, sex, systolic blood pressure, smoking status Laboratory: total cholesterol, HDL-C | 0.73–0.77 across age and regional cohorts | 0.59–0.67 in different populations |
| Pooled Cohort Equations | Before disease onset | General population aged 40–79 years | 10-year risk of a first cardiovascular event | Clinical: age, sex, race, systolic blood pressure, use of anti-hypertensive therapy, diabetes, smoking status Laboratory: total cholesterol, HDL-C | 0.71–0.82 across sex and race cohorts | 0.58–0.71 in different populations |
| Score | Timing of Assessment | Clinical Setting | Predicted Event and Timeframe | Input Variables | C-Statistics | External Validation |
|---|---|---|---|---|---|---|
| GRACE | Before treatment | ACS | In-hospital and six-month mortality | Clinical: age, heart rate, systolic blood pressure, cardiac arrest at admission, Killip class Laboratory: eGFR, abnormal cardiac enzymes Electrocardiographic: ST-segment deviation | 0.83 (in-hospital), 0.81 (6 months) | 0.80–0.86 in different populations |
| GRACE 2.0 | At admission or at hospital discharge | ACS | One-year mortality and death or MI, and three-year death | Clinical: age, heart rate, PAD, systolic blood pressure, Killip class, cardiac arrest at admission Laboratory: serum creatinine, elevated cardiac biomarkers Electrocardiographic: ST-segment deviation | 0.83 (1-year death), 0.75 (1-year death or MI), 0.78 (3-year death) | 0.74–0.81 in different populations |
| TIMI | Before treatment | STEMI | Thirty-day mortality | Clinical: age, systolic blood pressure, heart rate, Killip class, diabetes or history of hypertension or angina, weight Electrocardiographic: ST-segment deviation or LBBB Procedural: time to treatment | 0.78 | 0.64–0.67 in different populations |
| SIMPLE risk index | Before treatment | STEMI | Thirty-day mortality | Clinical: age, heart rate, systolic blood pressure | 0.78 | 0.77 in STEMI and NSTEMI |
| ACTION–GWTG | Before treatment | MI | In-hospital mortality | Clinical: age, heart rate, systolic blood pressure, cardiac arrest at presentation, cardiogenic shock, heart failure Laboratory: eGFR, troponin ratio Electrocardiographic: ST-segment elevation | 0.88 | NA |
| ZWOLLE | After treatment | STEMI | Thirty-day mortality | Clinical: age, anterior MI, Killip class Procedural: ischemic time, postprocedural TIMI flow, multivessel disease | 0.91 | 0.72–0.98 in different populations |
| Dynamic TIMI | Hospital discharge | STEMI | One-year mortality | Clinical: age, systolic blood pressure, heart rate, Killip class, diabetes or history of hypertension or angina, weight Electrocardiographic: ST-segment deviation or LBBB Procedural: time to treatment In-hospital complications: AF, ventricular fibrillation, ventricular tachycardia, renal failure, heart failure, cardiogenic shock | 0.76 | NA |
| RISK-PCI | After treatment | STEMI | Thirty-day MACE and mortality | Clinical: age, prior MI, LVEF <40% Laboratory: eGFR, WBC, blood glucose Electrocardiographic: anterior MI, LBBB, third-degree atrioventricular block Procedural: reference vessel diameter ≤2.5 mm, preprocedural TIMI flow 0, postprocedural TIMI flow <3 | 0.83 (MACE) and 0.87 (death) | 0.75–0.87 in STEMI |
| EPICOR | After treatment | ACS | Two-year mortality | Clinical: age, male sex, education level, BMI, LVEF, quality of life, previous cardiac disease, COPD, no revascularization or thrombolysis, Killip class, diagnosis of STEMI, in-hospital cardiac complications Laboratory: serum creatinine, blood glucose, hemoglobin Therapy: diuretics at discharge, aldosterone inhibitor at discharge, | 0.80 | 0.78 in Asian patients |
| Simplified EPICOR | After treatment | ACS | Two-year mortality | Clinical: age, male sex, LVEF, quality of life, previous cardiac disease, COPD, no revascularization or thrombolysis, diagnosis of STEMI Laboratory: serum creatinine, blood glucose, hemoglobin | 0.79 | NA |
| APEX-AMI | After treatment | STEMI | Ninety-day mortality | Clinical: age, systolic blood pressure, Killip class, heart rate Laboratory: serum creatinine Electrocardiographic: sum of ST-segment deviations, anterior MI | 0.81 | 0.71 in patients with MI |
| Score | Timing of Assessment | Clinical Setting | Predicted Event and Timeframe | Input Variables | C-Statistics | External Validation |
|---|---|---|---|---|---|---|
| SYNTAX II | Before treatment, after ICA | PCI or CABG | Four-year mortality | Clinical: age, female sex, LVEF, PAD, COPD Laboratory: serum creatinine Anatomical: SYNTAX score, unprotected left main disease | 0.73 | 0.72–0.73 in different populations |
| SYNTAX II 2020 | Before treatment, after ICA | PCI or CABG | Ten-year mortality and five-year MACE | Clinical: age, diabetes, current smoker, LVEF, PAD, COPD Laboratory: creatinine clearance Anatomical: SYNTAX score, 3-vessel disease or unprotected left main disease | 0.72 (10-year death), 0.67/0.62 (5-year MACE for PCI or CABG patients) | 0.62–0.72 in different populations |
| CONFIRM | Before ICA | Suspected CAD | All-cause mortality up to thirty months | Clinical: NCEP ATP III risk Computed tomography: proximal mixed or calcified plaque, proximal stenosis >50% | 0.75 | NA |
| ACEF | Before ICA | Patients undergoing elective cardiac operation | Thirty-day and two-year all-cause mortality | Clinical: age, LVEF Laboratory: creatinine | 0.75 (30 days), 0.77 (2 years) | 0.63–0.79 in different populations |
| ACEF II | Before ICA | Patients undergoing elective cardiac operation | Thirty-day and two-year all-cause mortality | Clinical: age, LVEF, emergency surgery Laboratory: creatinine, anemia | 0.77 (30 days), 0.69 (2 years) | 0.70–0.83 in different populations |
| RF-CL | Before ICA | Suspected CAD | Obstructive CAD | Clinical: age, sex, type of symptoms, family history of CAD, smoking, dyslipidemia, hypertension, diabetes, BMI Laboratory: reduced glomerular filtration rate | 0.75 | 0.78–0.79 in different populations |
| CACS-CL | Before ICA | Suspected CAD | Obstructive CAD | Clinical: age, sex, type of symptoms, family history of CAD, smoking, dyslipidemia, hypertension, diabetes, BMI Laboratory: reduced glomerular filtration rate Computed tomography: coronary artery calcium score | 0.88 | 0.82–0.86 in different populations |
| Score | Timing of Assessment | Clinical Setting | Predicted Event and Timeframe | Input Variables | C-Statistics | External Validation |
|---|---|---|---|---|---|---|
| CRUSADE | After PCI | High-risk NSTEMI | In-hospital major bleeding | Clinical: systolic blood pressure, heart rate, sex, signs of heart failure, vascular disease, diabetes Laboratory: creatinine clearance, hematocrit | 0.72 | 0.71–0.81 in different populations |
| Modified CRUSADE | After PCI | High-risk NSTEMI | In-hospital major bleeding | Clinical and laboratory: CRUSADE score Procedural: puncture pathway Therapy: P2Y12 inhibitor therapy, use of GPI during PCI, use of GPI after PCI | 0.83 | NA |
| ACUITY-HORIZONS-AMI | After PCI | ACS | 30-day major bleeding | Clinical: age, female sex, STEMI or NSTEMI Laboratory: creatinine, WBC, anemia Therapy: heparin plus a GPI or bivalirudin alone | 0.74 | 0.70–0.84 in different populations |
| ACTION | At the time of PCI | ACS | In-hospital major bleeding | Clinical: age, female sex, heart rate, systolic blood pressure, body weight, heart failure or shock presentation, diabetes, PAD Laboratory: serum creatinine, hemoglobin Electrocardiographic: ECG changes Therapy: warfarin use at home | 0.73 | 0.78 in STEMI |
| PARIS | After treatment | Patients on DAPT after PCI | Coronary thrombotic events and major bleeding at 2 years | PARIS thrombosis Clinical: diabetes, ACS presentation, current smoking, prior PCI, prior CABG Laboratory: eGFR PARIS bleeding Clinical: age, BMI, smoking status Laboratory: anemia, eGFR <60 mL/min Therapy: triple therapy at discharge | 0.70 (thrombotic events), 0.72 (bleeding) | 0.59–0.64 in different populations |
| PRECISE-DAPT | At the time of PCI | ACS and CCS | 2-year bleeding | Clinical: age, previous bleed Laboratory: hemoglobin, WBC, eGFR | 0.73 | 0.62–0.70 in different populations |
| BleeMACS | At the time of PCI | ACS | 1-year serious spontaneous bleeding | Clinical: age, hypertension, PAD, previous bleeding, malignancy Laboratory: serum creatinine, hemoglobin | 0.71 | 0.63–0.65 in non-PCI and PCI patients |
| SWEDEHEART | Before PCI | ACS | In-hospital major bleeding | Clinical: age, sex Laboratory: serum creatinine, hemoglobin, C reactive protein | 0.81 | 0.60 in East-Asian patients |
| DAPT | After 12 months of uneventful DAPT | Patients on DAPT after PCI | Coronary thrombotic events and bleeding at 12–30 months | Clinical: age, smoking, diabetes, MI at presentation, prior PCI or prior MI, heart failure or LVEF <30% Procedural: paclitaxel-eluting stent, stent diameter <3 mm, vein graft stenting | 0.70 (thrombotic events), 0.68 (bleeding) | 0.49–0.64 in different populations |
| ARC-HBR | After PCI | Patients undergoing PCI | BARC type 3 or 5 bleeding at 1 year | Clinical: age, use of long-term oral anticoagulation, spontaneous bleeding requiring hospitalization or transfusion, chronic bleeding diathesis, liver cirrhosis with portal hypertension, long-term use of oral NSAIDs or steroids, active malignancy, previous stroke, intracranial hemorrhage or known brain arteriovenous malformation, nondeferrable major surgery on DAPT, recent major surgery or major trauma Laboratory: eGFR, hemoglobin, platelet count | NR | 0.64–0.69 in different populations |
| PRECISE-HBR | After PCI | Patients undergoing PCI | BARC type 3 or 5 bleeding at 1 year | Clinical: age, previous bleeding, oral anticoagulation, ARC-HBR criteria Laboratory: estimated glomerular filtration rate, hemoglobin, WBC | 0.73 | 0.73–0.74 in different populations |
| Score | Timing of Assessment | Clinical Setting | Predicted Event and Timeframe | Input Variables | C-Statistics | External Validation |
|---|---|---|---|---|---|---|
| PRAISE | At discharge | ACS | All-cause death, MI and major bleeding at 1-year | Clinical: age, LVEF, sex, hypertension, hyperlipidemia, PAD, prior MI, prior CABG, prior stroke, prior bleeding, malignancy, STEMI or NSTEMI, diabetes Laboratory: hemoglobin, eGFR Angiographic or procedural: multivessel disease, complete revascularization, vascular access, drug-eluting stent Therapy: beta-blockers, ACE-inhibitors or ARBs, statins, PPI, OAC | 0.82 (death), 0.74 (MI), 0.70 (bleeding) | 0.61–0.75 in Asian patients |
| AIRE | Any | Volunteers, primary and secondary care patients | Mortality risk and time-to-death | Electrocardiogram: AI-based prediction model | 0.78 | NA |
| Pezel et al. | Before diagnosis | Symptomatic patients without known CAD referred for CCTA | MACE at up to 7 years | Computed tomography: number of proximal stenoses >50%, number of segments with noncalcified plaques, number of vessels with obstructive CAD Magnetic resonance: number of ischemic segments, number of LGE segments, LVEF | 0.86 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, A.; Capodanno, D. Personalized Treatment of Patients with Coronary Artery Disease: The Value and Limitations of Predictive Models. J. Cardiovasc. Dev. Dis. 2025, 12, 344. https://doi.org/10.3390/jcdd12090344
Greco A, Capodanno D. Personalized Treatment of Patients with Coronary Artery Disease: The Value and Limitations of Predictive Models. Journal of Cardiovascular Development and Disease. 2025; 12(9):344. https://doi.org/10.3390/jcdd12090344
Chicago/Turabian StyleGreco, Antonio, and Davide Capodanno. 2025. "Personalized Treatment of Patients with Coronary Artery Disease: The Value and Limitations of Predictive Models" Journal of Cardiovascular Development and Disease 12, no. 9: 344. https://doi.org/10.3390/jcdd12090344
APA StyleGreco, A., & Capodanno, D. (2025). Personalized Treatment of Patients with Coronary Artery Disease: The Value and Limitations of Predictive Models. Journal of Cardiovascular Development and Disease, 12(9), 344. https://doi.org/10.3390/jcdd12090344







