AI Applied to Cardiac Magnetic Resonance for Precision Medicine in Coronary Artery Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
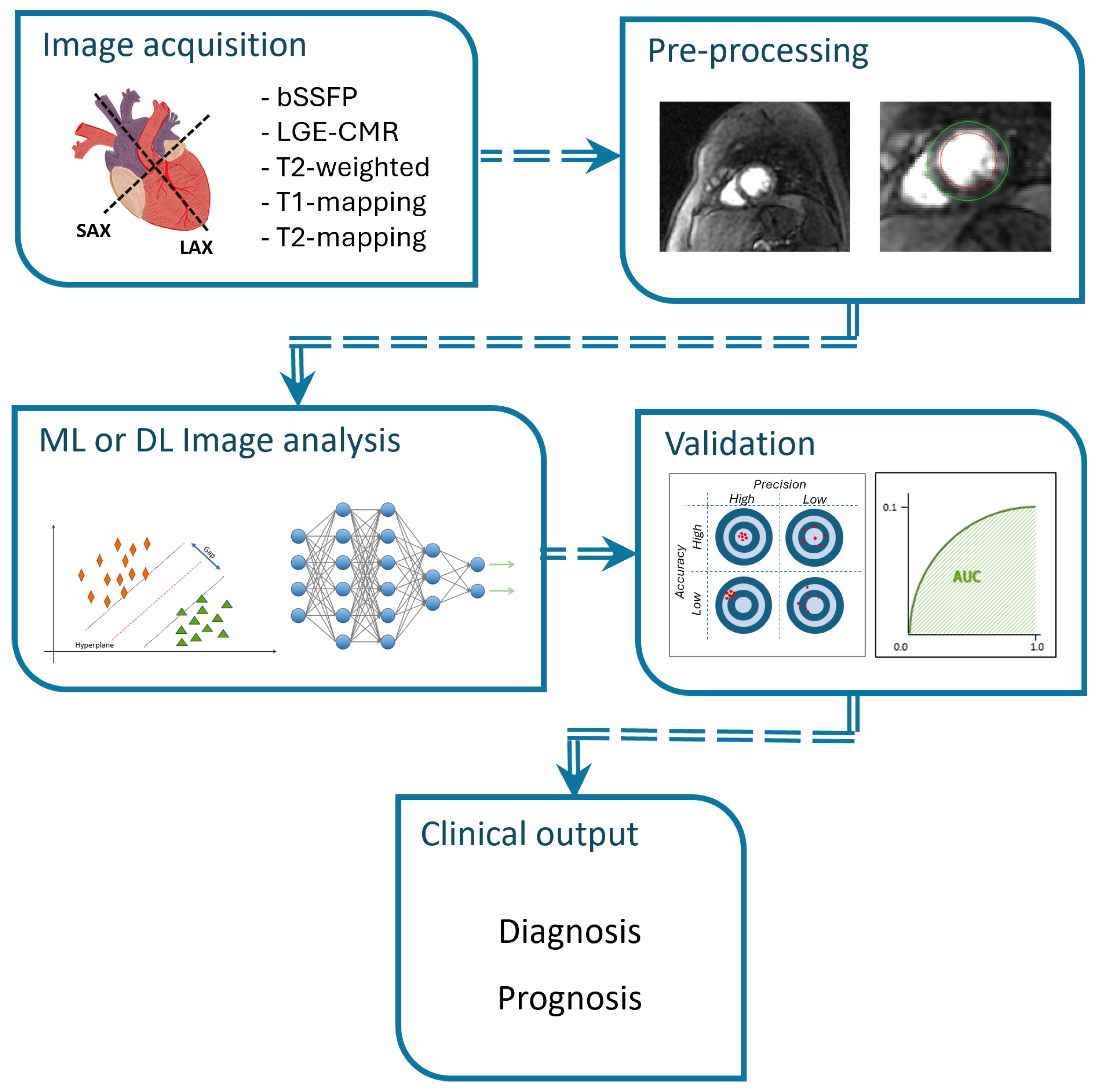
3. Results
3.1. Classification
3.1.1. Classification Between Healthy Volunteers and Patients with Cardiovascular Diseases
3.1.2. Risk Stratification for Major Adverse Cardiovascular Events (MACE)
3.1.3. Risk Stratification for Arrhythmia-Induced Mortality in CAD Patients
3.1.4. Early Identification of Left Ventricular Remodelling (LVR)
3.1.5. Identification of Normal and Infarcted Myocardial Segments
3.1.6. Other Classifications Findings
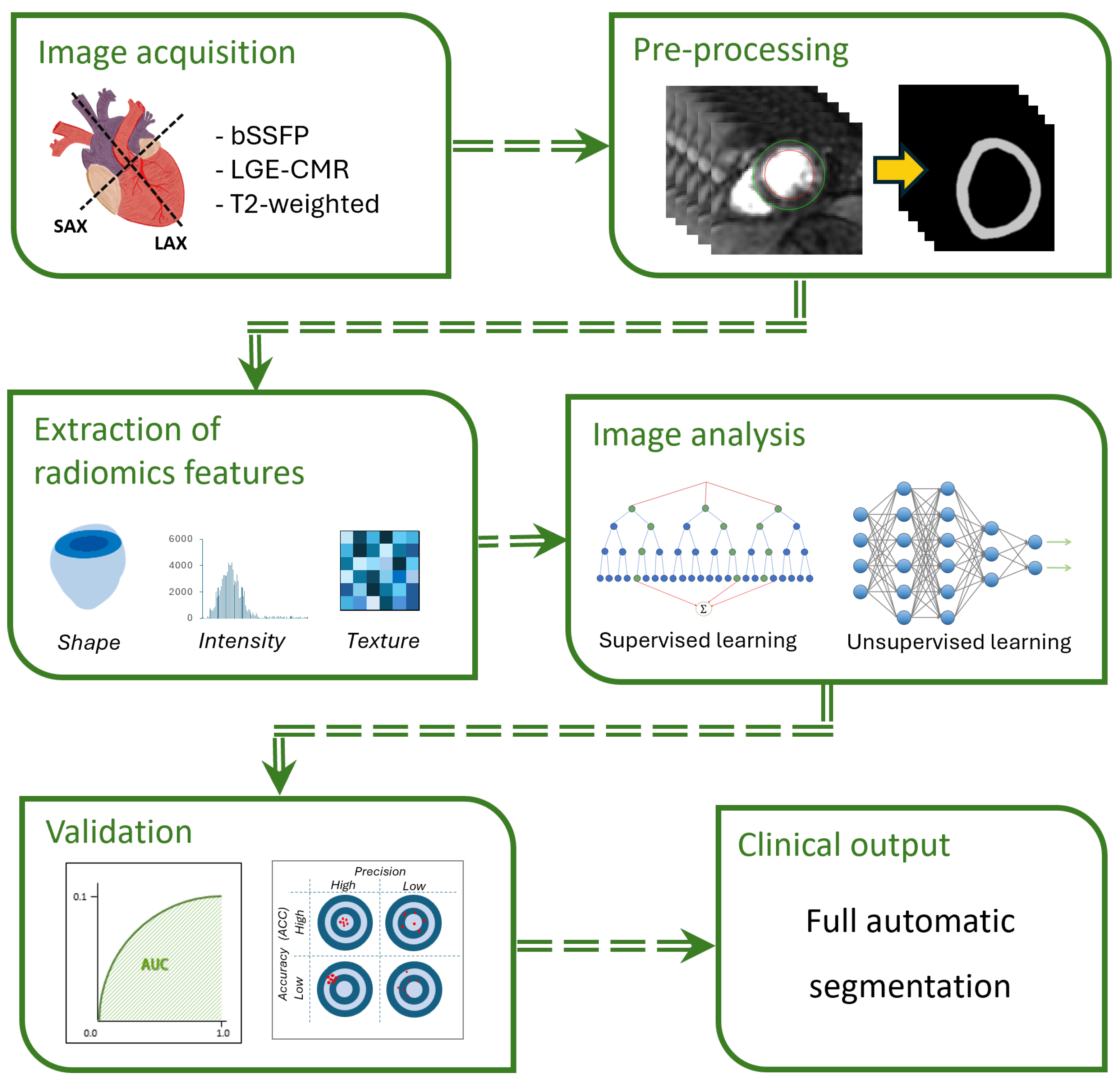
3.2. Classification Using Radiomics
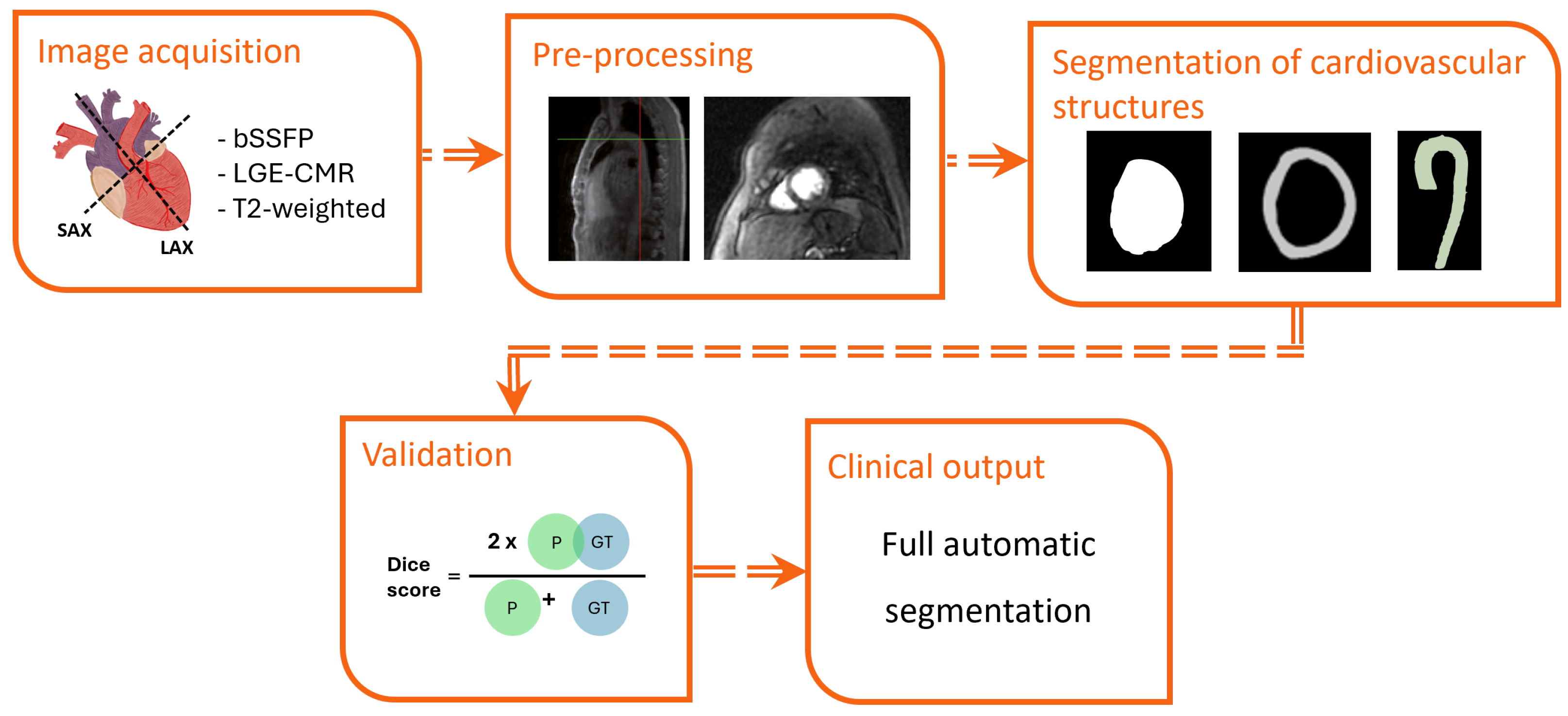
3.3. Segmentation
| Reference | # Subject (M/F) | Age(y) Mean ± Std | CMR Seq. | AI Model | Target | Performance |
|---|---|---|---|---|---|---|
| Gröschel, et al. [95] | 136 (91/45) | HS 44 ± 16 CAD 68 ± 11 | LGE | Deep CNN | LV-Myo | Healthy: DSC = 0.85 Patients: DSC = 0.80 |
| Mosquera- Rojas, et al. [106] | 274 (NR) | NR | LGE | DualUNet | LV-Myo | DSC = 0.84 |
| Barbaroux, et al. [92] | 271 (197/73) | 48 ± 14 | LGE | DynU-Net | LV-Myo | SAx: DSC = 0.83 LAx: DSC = 0.82 |
| Yan, et al. [121] | 1354 (NR) | NR | LGE | SegNet model | LV-Myo | Train: DSC = 0.94 Validation: DSC = 0.87 Test: DSC = 0.94 |
| Scannell, et al. [99] | 175 (136/39) | 64 ± 10 | T1-w | U-Net | LV-Myo | DSC = 0.80 |
| Ahmad, et al. [113] | 56 (NR) | 58 | LGE | DL | LV-Myo | DSC = 0.85 |
| Kim, et al. [115] | 35 (NR) | NR | bSSFP | DL (CNN- U-Net) | LV-Myo | DSC = 0.80 |
| Liu, et al. [123] | 32 (NR) | NR | T2-w LGE | CLS | LV-Myo | DSC = 0.84 DSC = 0.78 |
| Tan, et al. [124] | 1340 (NR) | NR | bSSFP | CNN (three networks (LM, CTR, MB)) | LV-Myo | DSC = 0.86 |
| Chen, et al. [119] | 150 (NR) | NR | T1-w LGE | Res-UNet | LV-ED LV-ES RV-ED RV-ES Myo ED Myo ES | DSC = 0.89 DSC = 0.81 DSC = 0.81 DSC = 0.70 DSC = 0.72 DSC = 0.76 |
| Papetti, et al. [107] | 144 (NR) | NR | LGE | CNN | LV-Myo MIS | DSC = 0.79 DSC = 0.78 |
| Lecesne, et al. [125] | 150 (NR) | NR | LGE | U-Net | LV-Myo MI | DSC = 0.92 DSC = 0.92 |
| Lin, et al. [97] | 34 (29/5) | NR | LGE | CTAEM-Net | MIS | DSC = 0.90 |
| Mamalakis, et al. [120] | 20 (NR) | NR | LGE | BZ- SOCRATIS | Myo Core Scar Border Scar Myo Core Scar Border Scar | Internal: DSC = 0.81 DSC = 0.60 DSC = 0.43 External: DSC = 0.70 DSC = 0.44 DSC = 0.54 |
| Xu, et al. [110] | 165 (NR) | NR | LGE | BMAnet | MI | Labeled 33: DSC = 0.59 Labeled 66: DSC = 0.65 |
| Chen, et al. [70] | 195 (NR) | NR | LGE | U-Net | MI | DSC = 0.84 |
| Heidenreich, et al. [96] | 78 (64/14) | 64 | LGE | nnU-nets | Myo MIS | DSC = 0.83 DSC = 0.72 |
| Xu, et al. [109] | 165 (NR) | NR | bSSFP | DSTGAN | MIS | DSC = 0.92 |
| Zabihollahy, et al. [100] | 34 (29/5) | 51 ± 12 | LGE | CNN-based | MIS | DSC = 0.93 |
| Moccia, et al. [98] | 30 (26/4) | NR | LGE | FCNN | MIS | DSC = 0.71 |
| Li, et al. [134] | NR (NR) | NR | bSSFP LGE T2-w | NVTrans- UNet | MI MI+ME | DSC = 0.64 DSC = 0.57 |
| Qiu, et al. [116] | NR (NR) | NR | Multi- sequence: bSSFP LGE T2-w T1-mapping T2-mapping | MyoPS-Net | MIS ME | DSC = 0.65 DSC = 0.74 |
| Cui, et al. [128] | 45 (NR) | NR | LGE T2-w bSSFP | U-Net++ (Deep supervision) + EfficientSeg- B1 (Ours) | MIS MIS + ME Average | DSC = 0.71 DSC = 0.74 DSC = 0.72 |
| Li, et al. [129] | 45 (NR) | NR | LGE T2-w bSSFP | TAUNet | LV RV Myo MIS ME | DSC = 0.94 DSC = 0.91 DSC = 0.91 DSC = 0.62 DSC = 0.78 |
| Cui, et al. [127] | 45 (NR) | NR | LGE T2-w bSSFP | Deep U-net Deep U-net+ DFM | MIS MIS+ME MIS MIS+ME | DSC = 0.68 DSC = 0.70 DSC = 0.69 DSC = 0.70 |
| Brahim, et al. [93] | 150 (89/61) | NR | LGE | ICPIU-Net | Myo MI MVO Classification | DSC = 0.95 DSC = 0.78 DSC = 0.77 ACC = 98,00 |
| de la Rosa, et al. [130] | 100 (NR) | NR | LGE | CNN | MI+MVO | DSC = 0.77 |
| Brahim, et al. [131] | 150 (NR) | NR | LGE | 3D pretrained Autoencoder network and the 3D U-Net | Myo MI MVO | DSC = 0.95 DSC = 0.76 DSC = 0.73 |
| Chen, et al. [94] | 150 (92/58) | HS 32 ± 12 CAD 61 ± 12 | LGE | 3SUnet | Cardiac adipose tissue | HF = 15.62 |
| Arega, et al. [114] | 295 (NR) | NR | T1-mapping (Native) T1-mapping (Post- Contrast) T1-mapping (Native) T1-mapping (Post- Contrast) | Swin-based U-Net CNN-based U-Net | LV MYO RV LV MYO RV LV MYO RV LV MYO RV | DSC = 0.97 DSC = 0.91 DSC = 0.92 DSC = 0.95 DSC = 0.88 DSC = 0.89 DSC = 0.96 DSC = 0.90 DSC = 0.90 DSC = 0.94 DSC = 0.85 DSC = 0.86 |
| Popescu, et al. [135] | 401 (NR) | NR | LGE bSSFP | DNN | LV Myo MIS | DSC = 0.93 DSC = 0.57 |
| Mamalakis, et al [136] | 60 (NR) | NR | LGE | MA- SOCRATIS | LV Myo MIS | intra-observer: DSC = 0.81 inter-observer: DSC = 0.70 intra-observer: DSC = 0.70 inter-observer: DSC = 0.70 |
| Al-antari, et al. [101] | 150 (89/61) | NR | LGE | ResU-Net | MI+MVO | ACC = 88.50 |
| Jani, et al. [112] | 501 (431/70) | 59 ± 12 | LGE | Cascaded U-Net | LV-Myo MIS | DSC = 0.66 DSC = 0.75 |
| Qi, et al. [108] | 415 (370/45) | 59 ± 10 | CGE | DGL | MIS | ACC = 0.92 |
| Yalcinkaya, et al. [111] | 150 (NR) | 60 ± 14 | LGE bSSFP | DNN | LV-Myo | Internal: DSC = 0.89 External: DSC = 0.88 |
| Lin, et al. [105] | 174 (119/55) | 51 ± 12 | Cine | U-Net | Coronary artery | Training: DSC = 0.95 Validation: DSC = 0.94 |
| Ben Khalifa, et al. [103] | 163 (40/123) | HS 42 ± 14 CAD 58 ± 11 | LGE bSSFP | U-Net | LV-Myo Classification MI | DSC = 0.92 ACC = 0.96 |
| Li, et al. [104] | 45 (NR) | NR | LGE T2-w bSSFP | DNN (MPS- Mamba) | MIS ME | DSC = 0.71 DSC = 0.73 |
| Bernardo, et al. [118] | 171 (NR) | NR | NR | U-Net | LV Myo | ED: DSC = 0.94 ES: DSC = 0.79 ED: DSC = 0.81 ES: DSC = 0.69 |
| Jafari, et al. [102] | 55 (37/18) | 50 ± 17 | DCE | U-Net | LV-Myo | DSC = 0.78 |
3.4. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| CAD | Coronary artery disease |
| MI | Myocardial infarction |
| CMR | Cardiac magnetic resonance |
| CTA | Computed tomography angiogram |
| LGE | Late gadolinium enhancement |
| LVEF | Left ventricular ejection fraction |
| ML | Machine learning |
| DL | Deep learning |
| SVM | Support vector machine |
| MACE | Major adverse cardiovascular events |
| ACC | Accuracy |
| AUC | Area under curve |
| LVR | Left ventricular remodelling |
| AHA | American Heart Association |
| SDAE | Stack denoising autoencode |
| CNN | Convolutional neural network |
References
- Halle, M.; Papadakis, M. Correction to: 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2024, 45, 1145. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Habib, B.; Calmon, R.; Khraiche, D.; Boddaert, N.; Bonnet, D.; Raimondi, F. Radiation dose reduction in paediatric coronary computed tomography: Assessment of effective dose and image quality. Eur. Radiol. 2016, 26, 2030–2038. [Google Scholar] [CrossRef]
- Habib, B.; Calmon, R.; Donciu, V.; Khraiche, D.; Warin-Fresse, K.; Bonnet, D.; Boddaert, N.; Raimondi, F. Low-dose paediatric cardiac and thoracic computed tomography with prospective triggering: Is it possible at any heart rate? Phys. Medica 2018, 49, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhao, S.; Xu, H.; He, W.; Yin, L.; Yao, Z.; Xu, Z.; Jin, H.; Wu, D.; Li, C.; et al. Comparison of machine learning-based CT fractional flow reserve with cardiac MR perfusion mapping for ischemia diagnosis in stable coronary artery disease. Eur. Radiol. 2024, 34, 5654–5665. [Google Scholar] [CrossRef]
- Ali, L.A.; Marrone, C.; Martins, D.S.; Khraiche, D.; Festa, P.; Martini, N.; Santoro, G.; Todiere, G.; Panaioli, E.; Bonnet, D.; et al. Prognostic factors in hypertrophic cardiomyopathy in children: An MRI based study. Int. J. Cardiol. 2022, 364, 141–147. [Google Scholar] [CrossRef]
- Martins, D.; Khraiche, D.; Legendre, A.; Boddaert, N.; Raisky, O.; Bonnet, D.; Raimondi, F. Aortic angle is associated with neo-aortic root dilatation and regurgitation following arterial switch operation. Int. J. Cardiol. 2019, 280, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.S.; Ait-Ali, L.; Khraiche, D.; Festa, P.; Barison, A.; Martini, N.; Benadjaoud, Y.; Anjos, R.; Boddaert, N.; Bonnet, D.; et al. Evolution of acute myocarditis in a pediatric population: An MRI based study. Int. J. Cardiol. 2021, 329, 226–233. [Google Scholar] [CrossRef]
- Raimondi, F.; Martins, D.; Coenen, R.; Panaioli, E.; Khraiche, D.; Boddaert, N.; Bonnet, D.; Atkins, M.; El-Said, H.; Alshawabkeh, L.; et al. Prevalence of Venovenous Shunting and High-Output State Quantified with 4D Flow MRI in Patients with Fontan Circulation. Radiol. Cardiothorac. Imaging 2021, 3, e210161. [Google Scholar] [CrossRef]
- von Knobelsdorff-Brenkenhoff, F.; Schulz-Menger, J. Cardiovascular magnetic resonance in the guidelines of the European Society of Cardiology: A comprehensive summary and update. J. Cardiovasc. Magn. Reson. 2023, 25, 42. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 1–18. [Google Scholar] [CrossRef]
- Hajhosseiny, R.; Bustin, A.; Munoz, C.; Rashid, I.; Cruz, G.; Manning, W.J.; Prieto, C.; Botnar, R.M. Coronary Magnetic Resonance Angiography: Technical Innovations Leading Us to the Promised Land? JACC Cardiovasc. Imaging 2020, 13, 2653–2672. [Google Scholar] [CrossRef]
- Aidi, H.E.; Adams, A.; Moons, K.G.; Ruijter, H.M.D.; Mali, W.P.M.; Doevendans, P.A.; Nagel, E.; Schalla, S.; Bots, M.L.; Leiner, T. Cardiac Magnetic Resonance Imaging Findings and the Risk of Cardiovascular Events in Patients With Recent Myocardial Infarction or Suspected or Known Coronary Artery Disease: A Systematic Review of Prognostic Studies. J. Am. Coll. Cardiol. 2014, 63, 1031–1045. [Google Scholar] [CrossRef]
- Gronda, E.; Vanoli, E.; Sacchi, S.; Grassi, G.; Ambrosio, G.; Napoli, C. Risk of heart failure progression in patients with reduced ejection fraction: Mechanisms and therapeutic options. Heart Fail. Rev. 2020, 25, 295–303. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488. [Google Scholar] [CrossRef]
- Polidori, T.; Santis, D.D.; Rucci, C.; Tremamunno, G.; Piccinni, G.; Pugliese, L.; Zerunian, M.; Guido, G.; Pucciarelli, F.; Bracci, B.; et al. Radiomics applications in cardiac imaging: A comprehensive review. Radiol. Medica 2023, 128, 922–933. [Google Scholar] [CrossRef]
- Hani, S.H.B.; Ahmad, M.M. Machine-learning Algorithms for Ischemic Heart Disease Prediction: A Systematic Review. Curr. Cardiol. Rev. 2022, 19, 87–99. [Google Scholar] [CrossRef]
- Zhang, Q.; Fotaki, A.; Ghadimi, S.; Wang, Y.; Doneva, M.; Wetzl, J.; Delfino, J.G.; O’Regan, D.P.; Prieto, C.; Epstein, F.H. Improving the efficiency and accuracy of cardiovascular magnetic resonance with artificial intelligence—review of evidence and proposition of a roadmap to clinical translation. J. Cardiovasc. Magn. Reson. 2024, 26, 101051. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Olanisa, O.O.; Nzeako, T.; Shahrokhi, M.; Esfahani, E.; Fakher, N.; Tabari, M.A.K. Revolutionizing Cardiac Imaging: A Scoping Review of Artificial Intelligence in Echocardiography, CTA, and Cardiac MRI. J. Imaging 2024, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Infante, T.; Cavaliere, C.; Punzo, B.; Grimaldi, V.; Salvatore, M.; Napoli, C. Radiogenomics and Artificial Intelligence Approaches Applied to Cardiac Computed Tomography Angiography and Cardiac Magnetic Resonance for Precision Medicine in Coronary Heart Disease: A Systematic Review. Circ. Cardiovasc. Imaging 2021, 14, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Tejani, A.S.; Klontzas, M.E.; Gatti, A.A.; Mongan, J.T.; Moy, L.; Park, S.H.; Kahn, C.E. Checklist for Artificial Intelligence in Medical Imaging (CLAIM): 2024 Update. Radiol. Artif. Intell. 2024, 6, e240300. [Google Scholar] [CrossRef] [PubMed]
- Maleckar, M.M.; Myklebust, L.; Uv, J.; Florvaag, P.M.; Strøm, V.; Glinge, C.; Jabbari, R.; Vejlstrup, N.; Engstrøm, T.; Ahtarovski, K.; et al. Combined In-silico and Machine Learning Approaches Toward Predicting Arrhythmic Risk in Post-infarction Patients. Front. Physiol. 2021, 12, 745349. [Google Scholar] [CrossRef]
- Alskaf, E.; Crawley, R.; Scannell, C.M.; Suinesiaputra, A.; Young, A.; Masci, P.G.; Perera, D.; Chiribiri, A. Hybrid artificial intelligence outcome prediction using features extraction from stress perfusion cardiac magnetic resonance images and electronic health records. J. Med. Artif. Intell. 2024, 7, 3. [Google Scholar] [CrossRef]
- Alskaf, E.; Scannell, C.M.; Suinesiaputra, A.; Crawley, R.; Masci, P.; Young, A.; Perera, D.; Chiribiri, A. Qualitative American Heart Association plot of late gadolinium enhancement with mortality and ventricular arrhythmia prediction using artificial intelligence. J. Med. Artif. Intell. 2025, 8, 2. [Google Scholar] [CrossRef]
- Backhaus, S.J.; Aldehayat, H.; Kowallick, J.T.; Evertz, R.; Lange, T.; Kutty, S.; Bigalke, B.; Gutberlet, M.; Hasenfuß, G.; Thiele, H.; et al. Artificial intelligence fully automated myocardial strain quantification for risk stratification following acute myocardial infarction. Sci. Rep. 2022, 12, 12220. [Google Scholar] [CrossRef]
- Böttcher, B.; Beller, E.; Busse, A.; Cantré, D.; Yücel, S.; Öner, A.; Ince, H.; Weber, M.A.; Meinel, F.G. Fully automated quantification of left ventricular volumes and function in cardiac MRI: Clinical evaluation of a deep learning-based algorithm. Int. J. Cardiovasc. Imaging 2020, 36, 2239–2247. [Google Scholar] [CrossRef]
- Cau, R.; Pisu, F.; Pintus, A.; Palmisano, V.; Montisci, R.; Suri, J.S.; Salgado, R.; Saba, L. Cine-cardiac magnetic resonance to distinguish between ischemic and non-ischemic cardiomyopathies: A machine learning approach. Eur. Radiol. 2024, 34, 5691–5704. [Google Scholar] [CrossRef]
- Chen, B.H.; Wu, C.W.; An, D.A.; Zhang, J.L.; Zhang, Y.H.; Yu, L.Z.; Watson, K.; Wesemann, L.; Hu, J.; Chen, W.B.; et al. A deep learning method for the automated assessment of paradoxical pulsation after myocardial infarction using multicenter cardiac MRI data. Eur. Radiol. 2023, 33, 8477–8487. [Google Scholar] [CrossRef]
- Chen, M.; Fang, L.; Zhuang, Q.; Liu, H. Deep Learning Assessment of Myocardial Infarction from MR Image Sequences. IEEE Access 2019, 7, 5438–5446. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, J.; Pommier, T.; Cottin, Y.; Salomon, M.; Decourselle, T.; Lalande, A.; Couturier, R. Prediction of Myocardial Infarction From Patient Features With Machine Learning. Front. Cardiovasc. Med. 2022, 9, 754609. [Google Scholar] [CrossRef]
- Acero, J.C.; Lamata, P.; Eitel, I.; Zacur, E.; Evertz, R.; Lange, T.; Backhaus, S.J.; Stiermaier, T.; Thiele, H.; Bueno-Orovio, A.; et al. Comprehensive characterization of cardiac contraction for improved post-infarction risk assessment. Sci. Rep. 2024, 14, 8951. [Google Scholar] [CrossRef] [PubMed]
- Frøysa, V.; Berg, G.J.; Singsaas, E.; Eftestøl, T.; Woie, L.; Ørn, S. Texture-based probability mapping for automatic assessment of myocardial injury in late gadolinium enhancement images after revascularized STEMI. Int. J. Cardiol. 2025, 427, 133107. [Google Scholar] [CrossRef] [PubMed]
- GhaffariJolfayi, A.; Salmanipour, A.; Heshmat-Ghahdarijani, K.; MozafaryBazargany, M.H.; Azimi, A.; Pirouzi, P.; Mohammadzadeh, A. Machine learning-based interpretation of non-contrast feature tracking strain analysis and T1/T2 mapping for assessing myocardial viability. Sci. Rep. 2025, 15, 753. [Google Scholar] [CrossRef]
- Ghanbari, F.; Joyce, T.; Lorenzoni, V.; Guaricci, A.I.; Pavon, A.G.; Fusini, L.; Andreini, D.; Rabbat, M.G.; Aquaro, G.D.; Abete, R.; et al. AI Cardiac MRI Scar Analysis Aids Prediction of Major Arrhythmic Events in the Multicenter DERIVATE Registry. Radiology 2023, 307, e222239. [Google Scholar] [CrossRef]
- Guglielmo, M.; Penso, M.; Carerj, M.L.; Giacari, C.M.; Volpe, A.; Fusini, L.; Baggiano, A.; Mushtaq, S.; Annoni, A.; Cannata, F.; et al. DEep LearnIng-based QuaNtification of epicardial adipose tissue predicts MACE in patients undergoing stress CMR. Atherosclerosis 2024, 397, 117549. [Google Scholar] [CrossRef]
- Jacob, A.J.; Chitiboi, T.; Schoepf, U.J.; Sharma, P.; Aldinger, J.; Baker, C.; Lautenschlager, C.; Emrich, T.; Varga-Szemes, A. Deep-Learning-Based Disease Classification in Patients Undergoing Cine Cardiac MRI. J. Magn. Reson. Imaging JMRI 2025, 61, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Knott, K.D.; Seraphim, A.; Augusto, J.B.; Xue, H.; Chacko, L.; Aung, N.; Petersen, S.E.; Cooper, J.A.; Manisty, C.; Bhuva, A.N.; et al. The Prognostic Significance of Quantitative Myocardial Perfusion: An Artificial Intelligence-Based Approach Using Perfusion Mapping. Circulation 2020, 141, 1282–1291. [Google Scholar] [CrossRef]
- Lalande, A.; Chen, Z.; Pommier, T.; Decourselle, T.; Qayyum, A.; Salomon, M.; Ginhac, D.; Skandarani, Y.; Boucher, A.; Brahim, K.; et al. Deep learning methods for automatic evaluation of delayed enhancement-MRI. The results of the EMIDEC challenge. Med. Image Anal. 2022, 79, 102428. [Google Scholar] [CrossRef]
- Li, G.; Zheng, C.; Cui, Y.; Si, J.; Yang, Y.; Li, J.; Lu, J. Diagnostic efficacy of complexity metrics from cardiac MRI myocardial segmental motion curves in detecting late gadolinium enhancement in myocardial infarction patients. Heliyon 2024, 10, e31889. [Google Scholar] [CrossRef]
- Mauger, C.A.; Gilbert, K.; Suinesiaputra, A.; Bluemke, D.A.; Wu, C.O.; Lima, J.A.; Young, A.A.; Ambale-Venkatesh, B. Multi-Ethnic Study of Atherosclerosis: Relationship between Left Ventricular Shape at Cardiac MRI and 10-year Outcomes. Radiology 2023, 306, e220122. [Google Scholar] [CrossRef] [PubMed]
- Okada, D.R.; Miller, J.; Chrispin, J.; Prakosa, A.; Trayanova, N.; Jones, S.; Maggioni, M.; Wu, K.C. Substrate Spatial Complexity Analysis for the Prediction of Ventricular Arrhythmias in Patients With Ischemic Cardiomyopathy. Circ. Arrhythmia Electrophysiol. 2020, 13, E007975. [Google Scholar] [CrossRef] [PubMed]
- Paciorek, A.M.; von Schacky, C.E.; Foreman, S.C.; Gassert, F.G.; Gassert, F.T.; Kirschke, J.S.; Laugwitz, K.L.; Geith, T.; Hadamitzky, M.; Nadjiri, J. Automated assessment of cardiac pathologies on cardiac MRI using T1-mapping and late gadolinium phase sensitive inversion recovery sequences with deep learning. BMC Med. Imaging 2024, 24, 43. [Google Scholar] [CrossRef]
- Pezel, T.; Sanguineti, F.; Garot, P.; Unterseeh, T.; Champagne, S.; Toupin, S.; Morisset, S.; Hovasse, T.; Faradji, A.; Ah-Sing, T.; et al. Machine-Learning Score Using Stress CMR for Death Prediction in Patients With Suspected or Known CAD. JACC Cardiovasc. Imaging 2022, 15, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Pezel, T.; Garot, P.; Toupin, S.; Hovasse, T.; Sanguineti, F.; Champagne, S.; Morisset, S.; Chitiboi, T.; Jacob, A.J.; Sharma, P.; et al. Prognostic impact of artificial intelligence-based fully automated global circumferential strain in patients undergoing stress CMR. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1269–1279. [Google Scholar] [CrossRef]
- Pezel, T.; Toupin, S.; Bousson, V.; Hamzi, K.; Hovasse, T.; Lefevre, T.; Chevalier, B.; Unterseeh, T.; Sanguineti, F.; Champagne, S.; et al. A Machine Learning Model Using Cardiac CT and MRI Data Predicts Cardiovascular Events in Obstructive Coronary Artery Disease. Radiology 2025, 314, e233030. [Google Scholar] [CrossRef]
- Popescu, D.M.; Shade, J.K.; Lai, C.; Aronis, K.N.; Ouyang, D.; Moorthy, M.V.; Cook, N.R.; Lee, D.C.; Kadish, A.; Albert, C.M.; et al. Arrhythmic sudden death survival prediction using deep learning analysis of scarring in the heart. Nat. Cardiovasc. Res. 2022, 1, 334–343. [Google Scholar] [CrossRef]
- Righetti, F.; Rubiu, G.; Penso, M.; Moccia, S.; Carerj, M.L.; Pepi, M.; Pontone, G.; Caiani, E.G. Deep learning approaches for the detection of scar presence from cine cardiac magnetic resonance adding derived parametric images. Med. Biol. Eng. Comput. 2024, 63, 59–73. [Google Scholar] [CrossRef]
- Schuster, A.; Lange, T.; Backhaus, S.J.; Strohmeyer, C.; Boom, P.C.; Matz, J.; Kowallick, J.T.; Lotz, J.; Steinmetz, M.; Kutty, S.; et al. Fully Automated Cardiac Assessment for Diagnostic and Prognostic Stratification Following Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, 18. [Google Scholar] [CrossRef]
- Udin, M.H.; Armstrong, S.; Kai, A.; Doyle, S.; Ionita, C.N.; Pokharel, S.; Sharma, U.C. Lightweight preprocessing and template matching facilitate streamlined ischemic myocardial scar classification. J. Med. Imaging 2024, 11, 024503. [Google Scholar] [CrossRef]
- Wang, S.; Abdelaty, A.M.; Parke, K.; Arnold, J.R.; McCann, G.P.; Tyukin, I.Y. MyI-Net: Fully Automatic Detection and Quantification of Myocardial Infarction from Cardiovascular MRI Images. Entropy 2023, 25, 431. [Google Scholar] [CrossRef]
- Wu, X.; Tang, L.; Li, W.; He, S.; Yue, X.; Peng, P.; Wu, T.; Zhang, X.; Wu, Z.; He, Y.; et al. Feasibility of accelerated non-contrast-enhanced whole-heart bSSFP coronary MR angiography by deep learning–constrained compressed sensing. Eur. Radiol. 2023, 33, 8180–8190. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Deng, L.; Li, W.; Peng, P.; Yue, X.; Tang, L.; Pu, Q.; Ming, Y.; Zhang, X.; Huang, X.; et al. Deep Learning-Based Acceleration of Compressed Sensing for Noncontrast-Enhanced Coronary Magnetic Resonance Angiography in Patients With Suspected Coronary Artery Disease. J. Magn. Reson. Imaging 2023, 58, 1521–1530. [Google Scholar] [CrossRef]
- Wu, X.; Yue, X.; Peng, P.; Tan, X.; Huang, F.; Cai, L.; Li, L.; He, S.; Zhang, X.; Liu, P.; et al. Accelerated 3D whole-heart non-contrast-enhanced mDIXON coronary MR angiography using deep learning-constrained compressed sensing reconstruction. Insights Imaging 2024, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, L.; Zhang, H.; Zhang, Y.; Du, X.; Li, S. A novel machine-learning algorithm to estimate the position and size of myocardial infarction for MRI sequence. Computing 2018, 101, 653–665. [Google Scholar] [CrossRef]
- Zaidi, H.A.; Jones, R.E.; Hammersley, D.J.; Hatipoglu, S.; Balaban, G.; Mach, L.; Halliday, B.P.; Lamata, P.; Prasad, S.K.; Bishop, M.J. Machine learning analysis of complex late gadolinium enhancement patterns to improve risk prediction of major arrhythmic events. Front. Cardiovasc. Med. 2023, 10, 1082778. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, G.; Gao, Z.; Xu, C.; Zhang, Y.; Shi, R.; Keegan, J.; Xu, L.; Zhang, H.; Fan, Z.; et al. Deep learning for diagnosis of chronic myocardial infarction on nonenhanced cardiac cine MRI. Radiology 2019, 291, 606–607. [Google Scholar] [CrossRef]
- Attallah, O.; Ragab, D.A. Auto-MyIn: Automatic diagnosis of myocardial infarction via multiple GLCMs, CNNs, and SVMs. Biomed. Signal Process. Control 2023, 80, 104273. [Google Scholar] [CrossRef]
- Bekheet, M.; Sallah, M.; Alghamdi, N.S.; Rusu-Both, R.; Elgarayhi, A.; Elmogy, M. Cardiac Fibrosis Automated Diagnosis Based on FibrosisNet Network Using CMR Ischemic Cardiomyopathy. Diagnostics 2024, 14, 255. [Google Scholar] [CrossRef]
- Dieu, X.; Chabrun, F.; Prunier, F.; Angoulvant, D.; Mewton, N.; Roubille, F.; Reynier, P.; Ferre, M.; Moal, V.; Cottin, L.; et al. Post-infarct cardiac remodeling predictions with machine learning. Int. J. Cardiol. 2022, 355, 1–4. [Google Scholar] [CrossRef]
- Goldfarb, J.W.; Craft, J.; Cao, J.J. Water–fat separation and parameter mapping in cardiac MRI via deep learning with a convolutional neural network. J. Magn. Reson. Imaging 2019, 50, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Joloudari, J.H.; Saadatfar, H.; Gol, M.G.; Alizadehsani, R.; Sani, Z.A.; Hasanzadeh, F.; Hassannataj, E.; Sharifrazi, D.; Mansor, Z. FCM-DNN: Diagnosing coronary artery disease by deep accuracy fuzzy C-means clustering model. Math. Biosci. Eng. MBE 2022, 19, 3609–3635. [Google Scholar] [CrossRef]
- Kim, Y.C.; Chung, Y.; Choe, Y.H. Deep learning for classification of late gadolinium enhancement lesions based on the 16-segment left ventricular model. Phys. Medica 2024, 117, 103193. [Google Scholar] [CrossRef]
- Chen, C. Protection of Ivabradine Combined with Trimetazidine on Myocardial Injury after Percutaneous Coronary Intervention in Patients with Coronary Artery Disease Evaluated by Magnetic Resonance Image under Convolutional Neural Network. Contrast Media Mol. Imaging 2021, 2021, 3150938. [Google Scholar] [CrossRef]
- Feng, Y.; Xulei, Y.; Kng, T.S.; Lee, G.; Liang, Z.; Tan, R.S.; Yi, S. Multi-dimensional proprio-proximus machine learning for assessment of myocardial infarction. Comput. Med. Imaging Graph. 2018, 70, 63–72. [Google Scholar] [CrossRef]
- Hernandez-Casillas, A.; Del-Canto, I.; Ruiz-Espana, S.; Lopez-Lereu, M.P.; Monmeneu, J.V.; Moratal, D. Detection and Classification of Myocardial Infarction Transmurality Using Cardiac MR Image Analysis and Machine Learning Algorithms. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference, Scotland, UK, 11–15 July 2022; Volume 2022, pp. 1686–1689. [Google Scholar] [CrossRef]
- Iqbal, T.; Khalid, A.; Ullah, I. Explaining decisions of a light-weight deep neural network for real-time coronary artery disease classification in magnetic resonance imaging. J. Real-Time Image Process. 2024, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, M.; Kavitha, G. Deep CNN with LM learning based myocardial ischemia detection in cardiac magnetic resonance images. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference, Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 824–827. [Google Scholar] [CrossRef]
- Chen, Z.; Lalande, A.; Salomon, M.; Decourselle, T.; Pommier, T.; Qayyum, A.; Shi, J.; Perrot, G.; Couturier, R. Automatic deep learning-based myocardial infarction segmentation from delayed enhancement MRI. Comput. Med. Imaging Graph. 2022, 95, 102014. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart. Circulation 2002, 105, 539–542. [Google Scholar] [CrossRef]
- Raisi-Estabragh, Z.; Izquierdo, C.; Campello, V.M.; Martin-Isla, C.; Jaggi, A.; Harvey, N.C.; Lekadir, K.; Petersen, S.E. Cardiac magnetic resonance radiomics: Basic principles and clinical perspectives. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 349–356. [Google Scholar] [CrossRef]
- Abdulkareem, M.; Kenawy, A.A.; Rauseo, E.; Lee, A.M.; Sojoudi, A.; Amir-Khalili, A.; Lekadir, K.; Young, A.A.; Barnes, M.R.; Barckow, P.; et al. Predicting post-contrast information from contrast agent free cardiac MRI using machine learning: Challenges and methods. Front. Cardiovasc. Med. 2022, 9, 894503. [Google Scholar] [CrossRef] [PubMed]
- Arian, F.; Amini, M.; Mostafaei, S.; Kalantari, K.R.; Avval, A.H.; Shahbazi, Z.; Kasani, K.; Rajabi, A.B.; Chatterjee, S.; Oveisi, M.; et al. Myocardial Function Prediction After Coronary Artery Bypass Grafting Using MRI Radiomic Features and Machine Learning Algorithms. J. Digit. Imaging 2022, 35, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Avard, E.; Shiri, I.; Hajianfar, G.; Abdollahi, H.; Kalantari, K.R.; Houshmand, G.; Kasani, K.; Bitarafan-rajabi, A.; Deevband, M.R.; Oveisi, M.; et al. Non-contrast Cine Cardiac Magnetic Resonance image radiomics features and machine learning algorithms for myocardial infarction detection. Comput. Biol. Med. 2022, 141, 105145. [Google Scholar] [CrossRef]
- Baessler, B.; Mannil, M.; Oebel, S.; Maintz, D.; Alkadhi, H.; Manka, R. Subacute and Chronic Left Ventricular Myocardial Scar: Accuracy of Texture Analysis on Nonenhanced Cine MR Images. Radiology 2018, 286, 103–112. [Google Scholar] [CrossRef]
- Berg, B.V.; Keyzer, F.D.; Cernicanu, A.; Claus, P.; Masci, P.G.; Bogaert, J.; Dresselaers, T. Radiomics-based detection of acute myocardial infarction on noncontrast enhanced midventricular short-axis cine CMR images. Int. J. Cardiovasc. Imaging 2024, 40, 1211–1220. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, L.; Li, Y.; Yu, Y.; Zhang, J.; Liao, B.; Luo, G.; Tian, J.; Zhou, H.; Tang, H. Integration of Cine-cardiac Magnetic Resonance Radiomics and Machine Learning for Differentiating Ischemic and Dilated Cardiomyopathy. Acad. Radiol. 2024, 31, 2704–2714. [Google Scholar] [CrossRef]
- Durmaz, E.S.; Karabacak, M.; Ozkara, B.B.; Kargın, O.A.; Raimoglu, U.; Tokdil, H.; Durmaz, E.; Adaletli, I. Radiomics-based machine learning models in STEMI: A promising tool for the prediction of major adverse cardiac events. Eur. Radiol. 2023, 33, 4611–4620. [Google Scholar] [CrossRef]
- Frøysa, V.; Berg, G.J.; Eftestøl, T.; Woie, L.; Ørn, S. Texture-based probability mapping for automatic scar assessment in late gadolinium-enhanced cardiovascular magnetic resonance images. Eur. J. Radiol. Open 2021, 8, 100387. [Google Scholar] [CrossRef]
- Khozeimeh, F.; Sharifrazi, D.; Izadi, N.H.; Joloudari, J.H.; Shoeibi, A.; Alizadehsani, R.; Tartibi, M.; Hussain, S.; Sani, Z.A.; Khodatars, M.; et al. RF-CNN-F: Random forest with convolutional neural network features for coronary artery disease diagnosis based on cardiac magnetic resonance. Sci. Rep. 2022, 12, 11178. [Google Scholar] [CrossRef] [PubMed]
- Kotu, L.P.; Engan, K.; Borhani, R.; Katsaggelos, A.K.; Ørn, S.; Woie, L.; Eftestøl, T. Cardiac magnetic resonance image-based classification of the risk of arrhythmias in post-myocardial infarction patients. Artif. Intell. Med. 2015, 64, 205–215. [Google Scholar] [CrossRef]
- Larroza, A.; Materka, A.; López-Lereu, M.P.; Monmeneu, J.V.; Bodí, V.; Moratal, D. Differentiation between acute and chronic myocardial infarction by means of texture analysis of late gadolinium enhancement and cine cardiac magnetic resonance imaging. Eur. J. Radiol. 2017, 92, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Larroza, A.; López-Lereu, M.P.; Monmeneu, J.V.; Gavara, J.; Chorro, F.J.; Bodí, V.; Moratal, D. Texture analysis of cardiac cine magnetic resonance imaging to detect nonviable segments in patients with chronic myocardial infarction. Med. Phys. 2018, 45, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, T.; Peng, Y.; Yuan, J.; Wang, S.; Xu, W.; Gong, J. Non-contrast cine cardiovascular magnetic resonance-based radiomics nomogram for predicting microvascular obstruction after reperfusion in ST-segment elevation myocardial infarction. Front. Cardiovasc. Med. 2023, 10, 1274267. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, Y.; Yu, T.; Sun, Z.; Hou, Y. Radiomics of Non-Contrast-Enhanced T1 Mapping: Diagnostic and Predictive Performance for Myocardial Injury in Acute ST-Segment-Elevation Myocardial Infarction. Korean J. Radiol. 2021, 22, 535. [Google Scholar] [CrossRef] [PubMed]
- Noto, T.D.; von Spiczak, J.; Mannil, M.; Gantert, E.; Soda, P.; Manka, R.; Alkadhi, H. Radiomics for distinguishing myocardial infarction from myocarditis at late gadolinium enhancement at mri: Comparison with subjective visual analysis. Radiol. Cardiothorac. Imaging 2019, 1, e180026. [Google Scholar] [CrossRef]
- Pujadas, E.R.; Raisi-Estabragh, Z.; Szabo, L.; McCracken, C.; Morcillo, C.I.; Campello, V.M.; Martín-Isla, C.; Atehortua, A.M.; Vago, H.; Merkely, B.; et al. Prediction of incident cardiovascular events using machine learning and CMR radiomics. Eur. Radiol. 2023, 33, 3488–3500. [Google Scholar] [CrossRef]
- Raisi-Estabragh, Z.; Martin-Isla, C.; Nissen, L.; Szabo, L.; Campello, V.M.; Escalera, S.; Winther, S.; Bøttcher, M.; Lekadir, K.; Petersen, S.E. Radiomics analysis enhances the diagnostic performance of CMR stress perfusion: A proof-of-concept study using the Dan-NICAD dataset. Front. Cardiovasc. Med. 2023, 10, 1141026. [Google Scholar] [CrossRef]
- Rauseo, E.; Morcillo, C.I.; Raisi-Estabragh, Z.; Gkontra, P.; Aung, N.; Lekadir, K.; Petersen, S.E. New Imaging Signatures of Cardiac Alterations in Ischaemic Heart Disease and Cerebrovascular Disease Using CMR Radiomics. Front. Cardiovasc. Med. 2021, 8, 716577. [Google Scholar] [CrossRef]
- Wang, D.; Taher, H.J.; Al-Fatlawi, M.; Abdullah, B.A.; Ismailova, M.K.; Abedi-Firouzjah, R. Multi-parametric assessment of cardiac magnetic resonance images to distinguish myocardial infarctions: A tensor-based radiomics feature. J. X-Ray Sci. Technol. 2024, 32, 735–749. [Google Scholar] [CrossRef]
- Barbaroux, H.; Kunze, K.P.; Neji, R.; Nazir, M.S.; Pennell, D.J.; Nielles-Vallespin, S.; Scott, A.D.; Young, A.A. Automated segmentation of long and short axis DENSE cardiovascular magnetic resonance for myocardial strain analysis using spatio-temporal convolutional neural networks. J. Cardiovasc. Magn. Reson. 2023, 25, 16. [Google Scholar] [CrossRef] [PubMed]
- Brahim, K.; Arega, T.W.; Boucher, A.; Bricq, S.; Sakly, A.; Meriaudeau, F. An Improved 3D Deep Learning-Based Segmentation of Left Ventricular Myocardial Diseases from Delayed-Enhancement MRI with Inclusion and Classification Prior Information U-Net (ICPIU-Net). Sensors 2022, 22, 2084. [Google Scholar] [CrossRef]
- Chen, S.; An, D.; Feng, C.; Bian, Z.; Wu, L.M. Segmentation of Pericardial Adipose Tissue in CMR Images: A Benchmark Dataset MRPEAT and a Triple-Stage Network 3SUnet. IEEE Trans. Med. Imaging 2023, 42, 2386–2399. [Google Scholar] [CrossRef] [PubMed]
- Gröschel, J.; Kuhnt, J.; Viezzer, D.; Hadler, T.; Hormes, S.; Barckow, P.; Schulz-Menger, J.; Blaszczyk, E. Comparison of manual and artificial intelligence based quantification of myocardial strain by feature tracking—A cardiovascular MR study in health and disease. Eur. Radiol. 2024, 34, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, J.F.; Gassenmaier, T.; Ankenbrand, M.J.; Bley, T.A.; Wech, T. Self-configuring nnU-net pipeline enables fully automatic infarct segmentation in late enhancement MRI after myocardial infarction. Eur. J. Radiol. 2021, 141, 109817. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Jiang, M.; Zhao, M.; Ukwatta, E.; White, J.A.; Chiu, B. Cascaded Triplanar Autoencoder M-Net for Fully Automatic Segmentation of Left Ventricle Myocardial Scar From Three-Dimensional Late Gadolinium-Enhanced MR Images. IEEE J. Biomed. Health Inform. 2022, 26, 2582–2593. [Google Scholar] [CrossRef]
- Moccia, S.; Banali, R.; Martini, C.; Muscogiuri, G.; Pontone, G.; Pepi, M.; Caiani, E.G. Development and testing of a deep learning-based strategy for scar segmentation on CMR-LGE images. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 187–195. [Google Scholar] [CrossRef]
- Scannell, C.M.; Veta, M.; Villa, A.D.; Sammut, E.C.; Lee, J.; Breeuwer, M.; Chiribiri, A. Deep-Learning-Based Preprocessing for Quantitative Myocardial Perfusion MRI. J. Magn. Reson. Imaging JMRI 2020, 51, 1689–1696. [Google Scholar] [CrossRef]
- Zabihollahy, F.; White, J.A.; Ukwatta, E. Convolutional neural network-based approach for segmentation of left ventricle myocardial scar from 3D late gadolinium enhancement MR images. Med. Phys. 2019, 46, 1740–1751. [Google Scholar] [CrossRef]
- Al-antari, M.A.; Shaaf, Z.F.; Jamil, M.M.A.; Samee, N.A.; Alkanhel, R.; Talo, M.; Al-Huda, Z. Deep learning myocardial infarction segmentation framework from cardiac magnetic resonance images. Biomed. Signal Process. Control 2024, 89, 105710. [Google Scholar] [CrossRef]
- Jafari, R.; Verma, R.; Aggarwal, V.; Gupta, R.K.; Singh, A. Deep learning-based segmentation of left ventricular myocardium on dynamic contrast-enhanced MRI: A comprehensive evaluation across temporal frames. Int. J. Comput. Assist. Radiol. Surg. 2024, 19, 2055–2062. [Google Scholar] [CrossRef]
- Khalifa, A.B.; Mili, M.; Maatouk, M.; Abdallah, A.B.; Abdellali, M.; Gaied, S.; Ali, A.B.; Lahouel, Y.; Bedoui, M.H.; Zrig, A. Deep Transfer Learning for Classification of Late Gadolinium Enhancement Cardiac MRI Images into Myocardial Infarction, Myocarditis, and Healthy Classes: Comparison with Subjective Visual Evaluation. Diagnostics 2025, 15, 207. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wang, P.; Liu, K.; Wei, B.; Cong, J. An enhanced visual state space model for myocardial pathology segmentation in multi-sequence cardiac MRI. Med. Phys. 2025, 52, 4355–4370. [Google Scholar] [CrossRef]
- Lin, L.; Zheng, Y.; Li, Y.; Jiang, D.; Cao, J.; Wang, J.; Xiao, Y.; Mao, X.; Zheng, C.; Wang, Y. Automatic vessel segmentation and reformation of non-contrast coronary magnetic resonance angiography using transfer learning-based three-dimensional U-net with attention mechanism. J. Cardiovasc. Magn. Reson. 2025, 27, 101126. [Google Scholar] [CrossRef]
- Mosquera-Rojas, G.; Ouadah, C.; Hadadi, A.; Lalande, A.; Leclerc, S. Automatic Myocardium Segmentation in Delayed-Enhancement MRI with Pathology-Specific Data Augmentation and Deep Learning Architectures. Algorithms 2023, 16, 488. [Google Scholar] [CrossRef]
- Papetti, D.M.; Abeelen, K.V.; Davies, R.; Menè, R.; Heilbron, F.; Perelli, F.P.; Artico, J.; Seraphim, A.; Moon, J.C.; Parati, G.; et al. An accurate and time-efficient deep learning-based system for automated segmentation and reporting of cardiac magnetic resonance-detected ischemic scar. Comput. Methods Programs Biomed. 2023, 229, 107321. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Qian, P.; Tang, L.; Chen, B.; An, D.; Wu, L.M. Predicting Late Gadolinium Enhancement of Acute Myocardial Infarction in Contrast-Free Cardiac Cine MRI Using Deep Generative Learning. Circ. Cardiovasc. Imaging 2024, 17, 16786. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Howey, J.; Ohorodnyk, P.; Roth, M.; Zhang, H.; Li, S. Segmentation and quantification of infarction without contrast agents via spatiotemporal generative adversarial learning. Med. Image Anal. 2020, 59, 101568. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Zhang, D.; Han, L.; Zhang, Y.; Chen, J.; Li, S. BMAnet: Boundary Mining With Adversarial Learning for Semi-Supervised 2D Myocardial Infarction Segmentation. IEEE J. Biomed. Health Inform. 2023, 27, 87–96. [Google Scholar] [CrossRef]
- Yalcinkaya, D.M.; Youssef, K.; Heydari, B.; Wei, J.; Merz, N.B.; Judd, R.; Dharmakumar, R.; Simonetti, O.P.; Weinsaft, J.W.; Raman, S.V.; et al. Improved robustness for deep learning-based segmentation of multi-center myocardial perfusion cardiovascular MRI datasets using data-adaptive uncertainty–guided space-time analysis. J. Cardiovasc. Magn. Reson. 2024, 26, 101082. [Google Scholar] [CrossRef]
- Jani, V.P.; Ostovaneh, M.; Chamera, E.; Kato, Y.; Lima, J.A.; Ambale-Venkatesh, B. Deep learning for automatic volumetric segmentation of left ventricular myocardium and ischaemic scar from multi-slice late gadolinium enhancement cardiovascular magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 829–838. [Google Scholar] [CrossRef]
- Ahmad, I.; Qayyum, A.; Gupta, B.B.; Alassafi, M.O.; Alghamdi, R.A. Ensemble of 2D Residual Neural Networks Integrated with Atrous Spatial Pyramid Pooling Module for Myocardium Segmentation of Left Ventricle Cardiac MRI. Mathematics 2022, 10, 627. [Google Scholar] [CrossRef]
- Arega, T.W.; Bricq, S.; Legrand, F.; Jacquier, A.; Lalande, A.; Meriaudeau, F. Automatic uncertainty-based quality controlled T1 mapping and ECV analysis from native and post-contrast cardiac T1 mapping images using Bayesian vision transformer. Med. Image Anal. 2023, 86, 102773. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, K.R.; Choe, Y.H. Automatic myocardial segmentation in dynamic contrast enhanced perfusion MRI using Monte Carlo dropout in an encoder-decoder convolutional neural network. Comput. Methods Programs Biomed. 2020, 185, 105150. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, L.; Wang, S.; Zhang, K.; Chen, Y.; Yang, S.; Zhuang, X. MyoPS-Net: Myocardial pathology segmentation with flexible combination of multi-sequence CMR images. Med. Image Anal. 2023, 84, 102694. [Google Scholar] [CrossRef]
- Tan, X.W.; Zheng, Q.; Shi, L.; Gao, F.; Allen, J.C.J.; Coenen, A.; Baumann, S.; Schoepf, U.J.; Kassab, G.S.; Lim, S.T.; et al. Combined diagnostic performance of coronary computed tomography angiography and computed tomography derived fractional flow reserve for the evaluation of myocardial ischemia: A meta-analysis. Int. J. Cardiol. 2017, 236, 100–106. [Google Scholar] [CrossRef]
- Bernardo, A.; Mato, G.; Calandrelli, M.; Medus, J.; Curiale, A. A novel deep learning based method for myocardial strain quantification. Biomed. Phys. Eng. Express 2024, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, W.; Zhang, J.; Qiu, H.; Zeng, D.; Shi, Y.; Yuan, H.; Zhuang, J.; Jia, Q.; Zhang, Y.; et al. Myocardial Segmentation of Cardiac MRI Sequences With Temporal Consistency for Coronary Artery Disease Diagnosis. Front. Cardiovasc. Med. 2022, 9, 804442. [Google Scholar] [CrossRef]
- Mamalakis, M.; Garg, P.; Nelson, T.; Lee, J.; Swift, A.J.; Wild, J.M.; Clayton, R.H. Automatic development of 3D anatomical models of border zone and core scar regions in the left ventricle. Comput. Med. Imaging Graph. 2023, 103, 102152. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Su, Y.; Sun, H.; Yu, H.; Ma, W.; Chi, H.; Cao, H.; Chang, Q. SegNet-based left ventricular MRI segmentation for the diagnosis of cardiac hypertrophy and myocardial infarction. Comput. Methods Programs Biomed. 2022, 227, 107197. [Google Scholar] [CrossRef]
- Yeung, M.; Rundo, L.; Nan, Y.; Sala, E.; Schönlieb, C.B.; Yang, G. Calibrating the Dice Loss to Handle Neural Network Overconfidence for Biomedical Image Segmentation. J. Digit. Imaging 2023, 36, 739–752. [Google Scholar] [CrossRef]
- Liu, J.; Xie, H.; Zhang, S.; Gu, L. Multi-sequence myocardium segmentation with cross-constrained shape and neural network-based initialization. Comput. Med. Imaging Graph. 2019, 71, 49–57. [Google Scholar] [CrossRef]
- Tan, L.K.; McLaughlin, R.A.; Lim, E.; Aziz, Y.F.A.; Liew, Y.M. Fully automated segmentation of the left ventricle in cine cardiac MRI using neural network regression. J. Magn. Reson. Imaging JMRI 2018, 48, 140–152. [Google Scholar] [CrossRef]
- Lecesne, E.; Simon, A.; Garreau, M.; Barone-Rochette, G.; Fouard, C. Segmentation of cardiac infarction in delayed-enhancement MRI using probability map and transformers-based neural networks. Comput. Methods Programs Biomed. 2023, 242, 107841. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Lin, L.; Wang, Y.; Liu, P.; Wang, Q.; Chen, W.; Wang, W.; Xia, Q.; Huang, N.; et al. Identification of Myocardial Scarring Using Contrast-Free Cardiac MRI in Patients With Autoimmune Rheumatic Diseases. J. Magn. Reson. Imaging JMRI 2023, 60, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Jiang, L.; Yuwen, C.; Xia, Y.; Zhang, Y. Deep U-Net architecture with curriculum learning for myocardial pathology segmentation in multi-sequence cardiac magnetic resonance images. Knowl.-Based Syst. 2022, 249, 108942. [Google Scholar] [CrossRef]
- Cui, H.; Li, Y.; Jiang, L.; Wang, Y.; Xia, Y.; Zhang, Y. Improving myocardial pathology segmentation with U-Net++ and EfficientSeg from multi-sequence cardiac magnetic resonance images. Comput. Biol. Med. 2022, 151, 106218. [Google Scholar] [CrossRef]
- Li, D.; Peng, Y.; Guo, Y.; Sun, J. TAUNet: A triple-attention-based multi-modality MRI fusion U-Net for cardiac pathology segmentation. Complex Intell. Syst. 2022, 8, 2489–2505. [Google Scholar] [CrossRef]
- de la Rosa, E.; Sidibé, D.; Decourselle, T.; Leclercq, T.; Cochet, A.; Lalande, A. Myocardial Infarction Quantification from Late Gadolinium Enhancement MRI Using Top-Hat Transforms and Neural Networks. Algorithms 2021, 14, 249. [Google Scholar] [CrossRef]
- Brahim, K.; Qayyum, A.; Lalande, A.; Boucher, A.; Sakly, A.; Meriaudeau, F. A 3D Network Based Shape Prior for Automatic Myocardial Disease Segmentation in Delayed-Enhancement MRI. IRBM 2021, 42, 424–434. [Google Scholar] [CrossRef]
- Karimi, D.; Salcudean, S.E. Reducing the Hausdorff Distance in Medical Image Segmentation with Convolutional Neural Networks. IEEE Trans. Med. Imaging 2020, 39, 499–513. [Google Scholar] [CrossRef]
- Nawaz, M.; Uvaliyev, A.; Bibi, K.; Wei, H.; Abaxi, S.M.D.; Masood, A.; Shi, P.; Ho, H.P.; Yuan, W. Unraveling the complexity of Optical Coherence Tomography image segmentation using machine and deep learning techniques: A review. Comput. Med. Imaging Graph. 2023, 108, 102269. [Google Scholar] [CrossRef]
- Li, B.; Yang, T.; Zhao, X. NVTrans-UNet: Neighborhood vision transformer based U-Net for multi-modal cardiac MR image segmentation. J. Appl. Clin. Med. Phys. 2023, 24. [Google Scholar] [CrossRef]
- Popescu, D.M.; Abramson, H.G.; Yu, R.; Lai, C.; Shade, J.K.; Wu, K.C.; Maggioni, M.; Trayanova, N.A. Anatomically informed deep learning on contrast-enhanced cardiac magnetic resonance imaging for scar segmentation and clinical feature extraction. Cardiovasc. Digit. Health J. 2022, 3, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Mamalakis, M.; Garg, P.; Nelson, T.; Lee, J.; Wild, J.M.; Clayton, R.H. MA-SOCRATIS: An automatic pipeline for robust segmentation of the left ventricle and scar. Comput. Med. Imaging Graph. 2021, 93, 101982. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, J.; Fan, G.; Zhao, H.; Wang, X.; Zhang, J.; Zhang, P.; Wang, W. Imaging transparent intact cardiac tissue with single-cell resolution. Biomed. Opt. Express 2018, 9, 423–436. [Google Scholar] [CrossRef] [PubMed]

| Database | Search Parameters |
|---|---|
| Pubmed | ((((coronary artery disease) OR (ischemic heart disease)) OR (myocardial infarction)) AND ((((MRI) OR (Magnetic resonance imaging)) OR (CMR)) OR (Cardiac Magnetic resonance))) AND (((artificial intelligence) OR (machine learning)) OR (deep learning)) |
| WOS | (((ALL = (coronary artery disease)) OR ALL = (ischemic heart disease)) OR ALL = (myocardial infarc-tion)) AND (((((ALL = (MRI)) OR ALL = (Magnetic resonance imaging))) OR ALL = (CMR)) OR ALL = (Cardiac Magnetic resonance)) AND (((ALL = (artificial intelligence)) OR ALL = (machine learn-ing)) OR ALL = (deep learning)) |
| Scopus | (coronary AND artery AND disease OR ischemic AND heart AND disease OR myocardial AND infarction) AND (mri OR magnetic AND resonance AND imaging OR cmr OR cardiac AND mag-netic AND resonance) AND (artificial AND intelligence OR machine AND learning OR deep AND learning) |
| Reference | # Subject (M/F) | Age(y) Mean ± Std | CMR Seq. | Target | (Category) AI Model | Performance |
|---|---|---|---|---|---|---|
| Bekheet, et al. [60] | 1140 (NR) | NR | LGE | Myo Fibrosis +/− | (ML) MobileNetV2 GoogleNet ResNet50 FibrosisNet | ACC = 87.13 ACC = 88.60 ACC = 88.45 ACC = 96.05 |
| Lalande, et al. [40] | 150 (89/61) | MI 66 ± 14 HS 59 ± 12 | LGE | MI +/− | (DL) Multi-input classification: CNN, RF. | ACC = 92.00 AUC = 0.96 |
| Chen, et al. [32] | 150 (89/61) | MI 66 ± 14 HS 59 ± 12 | LGE | MI +/− | (ML) RF Regressor | Infarction: ACC = 88.67 PMVO: ACC = 77.33 |
| Muthulakshmi, et al. [69] | 21 (NR) | NR | bSSFP | MI +/− | (DL) Levenberg- Marquardt learning CNN | ACC = 86.39 |
| Xu, et al. [56] | 58 (36/22) | 51 ± 16 | LGE | MI +/− | (ML) SVM | ACC = 93.30 |
| Attallah, et al [59] | 100 (NR) | NR | LGE | MI +/− | (DL) Auto-MyIn | ACC = 98.40 |
| Zhang, et al. [58] | 299 (213/86) | HS 40 ± 13 CAD 56 ± 11 | Non- contrast bSSFP | Chronic MI +/− | (DL) NR | AUC = 0.94 |
| Joloudari, et al. [63] | 30 (NR) | NR | NR | CAD +/− | (DL) FCM-DNN | ACC = 99.91 AUC = 1.00 |
| Iqbal, et al. [68] | 63151 (NR) | NR | LGE Perfusion T2w bSSFP | CAD +/− | (ML) LWNN (adapted version of LeNET5 model) NN | ACC = 99.35 AUC = 0.99 |
| Wu, et al. [54] | 64 (33/31) | 59 ± 10 | Non- contrast bSSFP | CAD +/− | (DL) CSAI | Patient: ACC = 87.50 Vessel: ACC = 91.10 Segment: ACC = 96.60 |
| Chen [65] | 120 (63/57) | Group A 64 ± 9 Group B 62 ± 8 Group C 62 ± 9 | LGE | Myo injury | (DL) CNN | ACC = 91.04 AUC = 0.96 |
| Paciorek, et al. [44] | 200 (132/68) | 53 ± 19 | LGE T1-mapping | Normal/ Abnormal | (DL) DenseNet-161 (LGE PSIR) DenseNet-161 (T1 mapping) | ACC = 88.00 AUC = 0.75 ACC = 70.00 AUC = 0.69 |
| Backhaus, et al. [27] | 1095 (820/275) | 64 | bSSFP | MACE +/− | (DL) Commercial software | Auto-GLS: AUC = 0.69 Auto-GCS: AUC = 0.66 |
| Schuster, et al. [50] | 1017 (763/254) | 64 | LGE | MACE +/− | (DL) Commercial software | Auto-mated: AUC = 0.67 Auto corrected: AUC = 0.68 |
| Pezel, et al. [46] | 2152 (1653/499) | 66 ± 12 | LGE | MACE +/− | (ML) U-net Dijkstra’s algorithm | ICC = 0.83 (95% CI) |
| Knott, et al. [39] | 1049 (702/347) | 60 ± 13 | Perfusion | Stress MBF and MPR associated with death or MACE | (NR) Commercial software | MBF: ICC = 0.68 (95%CI) MPR: ICC = 0.68 (95%CI) |
| Popescu, et al. [48] | 269 (233/36) | 61 ± 11 | LGE | SCDA risk +/− | (DL) NN Architecture SSCAR | Internal: ACC = 77.00 External: ACC = 73.00 |
| Pezel, et al. [45] | 31762 (20,879/10,883) | 63 ± 12 | LGE | SCDA risk +/− | (ML) RSF | AUC = 0.75 |
| Maleckar, et al. [24] | 30 (NR) | NR | LGE | Arrhythmia risk +/− | (ML) NR | ACC = 86.00 |
| Ghanbari, et al. [36] | 761 (671/90) | 65 ± 11 | LGE | Arrhythmia risk +/− | (ML) Ternaus network (Multivariable Cox models– total scar) CNN | AUC = 0.67 |
| Okada, et al. [43] | 122 (106/16) | 60 ± 11 | LGE | Arrhythmia risk +/− | (ML) SVM+poly | ACC = 81.00 |
| Zaidi, et al. [57] | 397 (346/51) | 64 ± 9 | LGE | Major arrhythmic event +/− | (ML) Multivariate cox regression analysis | AUC = 0.81 |
| Chen, et al. [30] | 311 (294/17) | NR | T2w-STIR bSSFP T2-mapping LGE | Paradoxical pulsation +/− | (DL) CNN | Internal: ACC = 85.00 AUC = 0.91 External: ACC = 84.00 AUC = 0.83 |
| Paciorek, et al. [44] | 200 (132/68) | 53 ± 19 | LGE T1-mapping | Normal/ Abnormal | (DL) DenseNet-161 (LGE PSIR) DenseNet-161 (T1 mapping) | ACC = 88.00 AUC = 0.75 ACC = 70.00 AUC = 0.69 |
| Chen, et al. [31] | 73 (51/22) | NR | LGE | Segment Infarct +/− | (DL) SDAE+SVM | ACC = 87.60 |
| Feng, et al. [66] | 30 (NR) | NR | bSSFP LGE | Segment Infarct +/− | (ML) SVM-RFE | Basal: ACC = 80.50 Middle: ACC = 87.90 Apical: ACC = 81.00 |
| Kim, et al. [64] | 170 (NR) | NR | LGE | Segment Infarct +/− | (DL) ResNet50 | ACC = 81.10 AUC = 0.87 |
| Wang, et al. [52] | 301 (172/129) | 57 | LGE | Segment Infarct +/− | (DL) MI-ResNet50- AC CNN | AUC = 0.86 |
| Hernández- Casillas, et al. [67] | 35 (NR) | NR | LGE | Segment Infarct +/− | (ML) Naïve Bayes | AUC = 0.69 |
| Mauger, et al. [42] | 5098 (2451/2565) | HS 60 ± 9 CAD 66 ± 9 | GRE | Relationship between LV 3D shape CMR and incident cardio- vascular events | (ML) Model 3 (model 1+30 event-specific remodeling signatures derived from the PLS analysis) | AUC = 0.77 |
| Dieu, et al. [61] | 443 (NR) | NR | NR | LV remodeling +/− | (ML) LR | AUC = 0.78 |
| Böttcher, et al. [28] | 50 (37/13) | 57 | bSSFP | Myo function | (DL) Commercially available software | LV EDV: ICC = 0.99 LV ESV: ICC = 0.99 LV SV: ICC = 0.89 LV EF: ICC = 0.97 LV mass: ICC = 0.99 |
| Goldfarb, et al. [62] | 64 (NR) | NR | bSSFP | Water–Fat | (DL) U-Net | R2 ≥ 0.97 (p < 0.001) |
| Wu, et al. [53] | 50 (15/35) |
HS 24 ± 8 CAD 60 ± 12 | Non- contrast bSSFP | Angiography | (DL) CSAI | Patient: ACC = 90.00 Vessel: ACC = 91.70 Segment: ACC = 97.30 |
| Paciorek, et al. [44] | 200 (132/68) | 53 ± 19 | LGE T1-mapping | Normal/ Abnormal | (DL) DenseNet-161 (LGE PSIR) DenseNet-161 (T1 mapping) | ACC = 88.00 AUC = 0.75 ACC = 70.00 AUC = 0.69 |
| Cau, et al. [29] | 107 (72/35) | 61 | bSSFP | CAD +/− | (ML) GB-GAM | AUC = 0.82 |
| Alskaf, et al. [25] | 1286 (845/441) | <65 65–75 >75 | Perfusion | Mortality risk +/− | (ML) HNN | AUC = 0.82 |
| Alskaf, et al. [26] | 2740 (1726/1014) | <65 65–75 >75 | LGE | Mortality risk +/− Arrhythmia risk +/− | (ML) HNN | Mortality: AUC = 0.77 Arrhythmia: AUC = 0.75 |
| Corral-Acero, et al. [33] | 1021 (NR) | 63 | LGE T1-w | MACE +/− | (DL) UNet | AUC = 0.77 |
| Li, et al. [41] | 42 (33/9) | 60 71 ± 11 | LGE bSSFP | Remote Viable Unviable | (ML) SVM XGBoost NN | Remote vs. Viable: AUC = 0.65 Viable vs. Unviable: AUC = 0.77 Remote vs. Unviable: AUC = 0.89 |
| Udin, et al. [51] | 279 (168/111) | HS 58 CAD 63 | LGE | MI +/− | (ML) ResNet50 ResNet152V2 | Without LWP: AUC = 0.76 With LWP: AUC = 0.88 Without LWP: AUC = 0.76 With LWP: AUC = 0.90 |
| Frøysa, et al. [34] | 41 (33/8) | 58 ± 12 | LGE | MI +/− | (ML) Texture-based probability mapping | R2 (p < 0.001) |
| Ghaffari- Jolfayi, et al. [35] | 79 (52/27) | 47 ± 12 | LGE, T1 mapping T2 mapping | Segment Infarct +/− | (ML) RF | LAD territory: AUC = 0.89 RCA territory: AUC = 0.90 LCX territory: AUC = 0.92 |
| Jacob, et al. [38] | 1337 (602/735) | HS 50 ± 16 CAD 63 ± 12 | bSSFP | CAD +/− | (DL) RF XGBoost | ACC = 0.81 AUC = 0.85 |
| Righetti, et al. [49] | 206 (164/42) | 67 | bSSFP | CAD +/− | (DL) U-Net | ACC = 79.00 |
| Paciorek, et al. [44] | 200 (132/68) | 53 ± 19 | LGE T1-mapping | Normal/ Abnormal | (DL) DenseNet-161 (LGE PSIR) DenseNet-161 (T1 mapping) | ACC = 88.00 AUC = 0.75 ACC = 70.00 AUC = 0.69 |
| Wu, et al. [55] | 99 (49/50) | HS 28 ± 11 CAD 59 ± 10 | bSSFP | CAD +/− | (DL) DL-CS mDIXON | ACC = 84.10 |
| Guglielmo, et al. [37] | 730 (616/114) | 63 ± 10 | LGE | MACE +/− | (DL) FCN U-Net | HR = 1.08 (95% CI) |
| Pezel, et al. [47] | 2038 (947/1091) | 70 ± 12 | LGE Perfusion | MACE +/− | (ML) XGBoost | Internal: AUC = 0.86 External: AUC = 0.84 AUC = 0.92 |
| Reference | # Subject (M/F) | Age(y) Mean ± Std | CMR Seq. | Target | AI Model | Performance |
|---|---|---|---|---|---|---|
| Arian, et al. [74] | 43 (34/39) | 58 ± 11 | LGE | Myo function | SCAD- penalized SVM RP algorithm | AUC = 0.78 AUC = 0.65 |
| Avard, et al. [75] | 72 (NR) | NR | non- contrast bSSFP | MI Viable Normal | LR SVM | AUC = 0.93 ACC = 86.00 AUC = 0.92 ACC = 85.00 |
| Ma, et al. [86] | 68 (57/11) | 55 ± 10 | non- contrast T1-maps | MVO SLS | T1values+ RS | MVO: AUC = 0.86 SLS: AUC = 0.77 |
| Abdulkareem, et al. [73] | 272 (NR) | NR | bSSFP LGE | Segment Myo+MIS | SVM DT | AUC = 0.58 AUC = 0.57 |
| Larroza, et al. [84] | 50 (45/5) | 61 ± 12 | bSSFP LGE (2D+t) | Nonviable Viable Remote segments | RBF-SVM classifier | AUC = 0.84 |
| Liu, et al. [85] | 167 (149/18) | 52 ± 11 | LGE | MVO +/− | LASSO | AUC = 0.78 |
| Frøysa, et al. [80] | 52 (40/12) | 64 | LGE | MIS size | The texture- based probability mapping method | DSC = 0.69 |
| Durmaz, et al. [79] | 60 (55/5) | MACE: 57 ± 9 No MACE: 55 ± 9 | LGE | MACE +/− | NN | AUC = 0.96 ACC = 89.40 |
| Raisi- Estabragh, et al. [89] | 92 (56/36) | NR | Perfusion | Rest and stress radiomics features | Model 4- Per territory (delta to histogram) | Sen = 53.00 Spec = 86.00 |
| Khozeimeh, et al. [81] | 63648 (NR) | NR | LGE Perfusion T2-w bSSFP | CAD +/− | Ensemble of CNNs and RF+Adam (optimizer) | AUC = 0.99 ACC = 99.18 |
| Di Noto, et al. [87] | 173 (153/20) | 66 ± 9 | LGE | MI Myo- carditis | SVM: (2D Features + RFE) LDA: (3D Features + PCA) | ACC = 88.00 ACC = 85.00 |
| Kotu, et al. [82] | 54 (NR) | NR | LGE | Arrhythmic risk | Several built-in classification schemes from matrix laboratory (matlab) | AUC = 0.96 ACC = 94.44 |
| Rauseo, et al. [90] | 2457 (NR) | HS 59 ± 7 CAD 67 ± 6 | bSSFP | CAD CVD | SVM | IHD: AUC = 0.82 CVD: AUC = 0.79 MI: AUC = 0.87 IS: AUC = 0.81 |
| Larroza, et al. [83] | 44 (40/4) | 61 ± 9 | LGE bSSFP | Acute MI Chronic MI | SVM + poly | AUC = 0.86 AUC = 0.82 |
| Baessler, et al. [76] | 180 (138/42) | HS 48 ± 17 CAD 64 ± 10 | Non- contrast bSSFP | Subacute MI Chronic MI | LR | Teta 1: AUC = 0.93 Perc.01: AUC = 0.92 |
| Pujadas, et al. [88] | 819 (NR) | 66 ± 7 | bSSFP | MI Other vascular pathologies | SVM | AUC = 0.76 ACC = 71.00 |
| Wang, et al. [91] | 115 (NR) | NR | LGE T1-w- transverse sBTFE T1+sBTFE | CAD +/− | Lasso RF/LR | AUC = 0.93 ACC = 0.93 |
| Vande Berg, et al. [77] | 148 (NR) | HS 48 ± 12 CAD 58 ± 12 | Cine bSSFP T2w | CAD +/− | Lasso | ES: ACC = 0.84 ED: ACC = 0.76 |
| Deng, et al. [78] | 115 (89/26) | 58 ± 11 | Cine | CAD +/− | GNB | AUC = 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Jara, C.; Salas, R.; Díaz-Navarro, R.; Chabert, S.; Andia, M.E.; Vega, J.; Urbina, J.; Uribe, S.; Sekine, T.; Raimondi, F.; et al. AI Applied to Cardiac Magnetic Resonance for Precision Medicine in Coronary Artery Disease: A Systematic Review. J. Cardiovasc. Dev. Dis. 2025, 12, 345. https://doi.org/10.3390/jcdd12090345
Jiménez-Jara C, Salas R, Díaz-Navarro R, Chabert S, Andia ME, Vega J, Urbina J, Uribe S, Sekine T, Raimondi F, et al. AI Applied to Cardiac Magnetic Resonance for Precision Medicine in Coronary Artery Disease: A Systematic Review. Journal of Cardiovascular Development and Disease. 2025; 12(9):345. https://doi.org/10.3390/jcdd12090345
Chicago/Turabian StyleJiménez-Jara, Cristina, Rodrigo Salas, Rienzi Díaz-Navarro, Steren Chabert, Marcelo E. Andia, Julián Vega, Jesús Urbina, Sergio Uribe, Tetsuro Sekine, Francesca Raimondi, and et al. 2025. "AI Applied to Cardiac Magnetic Resonance for Precision Medicine in Coronary Artery Disease: A Systematic Review" Journal of Cardiovascular Development and Disease 12, no. 9: 345. https://doi.org/10.3390/jcdd12090345
APA StyleJiménez-Jara, C., Salas, R., Díaz-Navarro, R., Chabert, S., Andia, M. E., Vega, J., Urbina, J., Uribe, S., Sekine, T., Raimondi, F., & Sotelo, J. (2025). AI Applied to Cardiac Magnetic Resonance for Precision Medicine in Coronary Artery Disease: A Systematic Review. Journal of Cardiovascular Development and Disease, 12(9), 345. https://doi.org/10.3390/jcdd12090345







