Radiation-Free Percutaneous Coronary Intervention: Myth or Reality?
Abstract
1. Introduction
Methods
2. Discussion
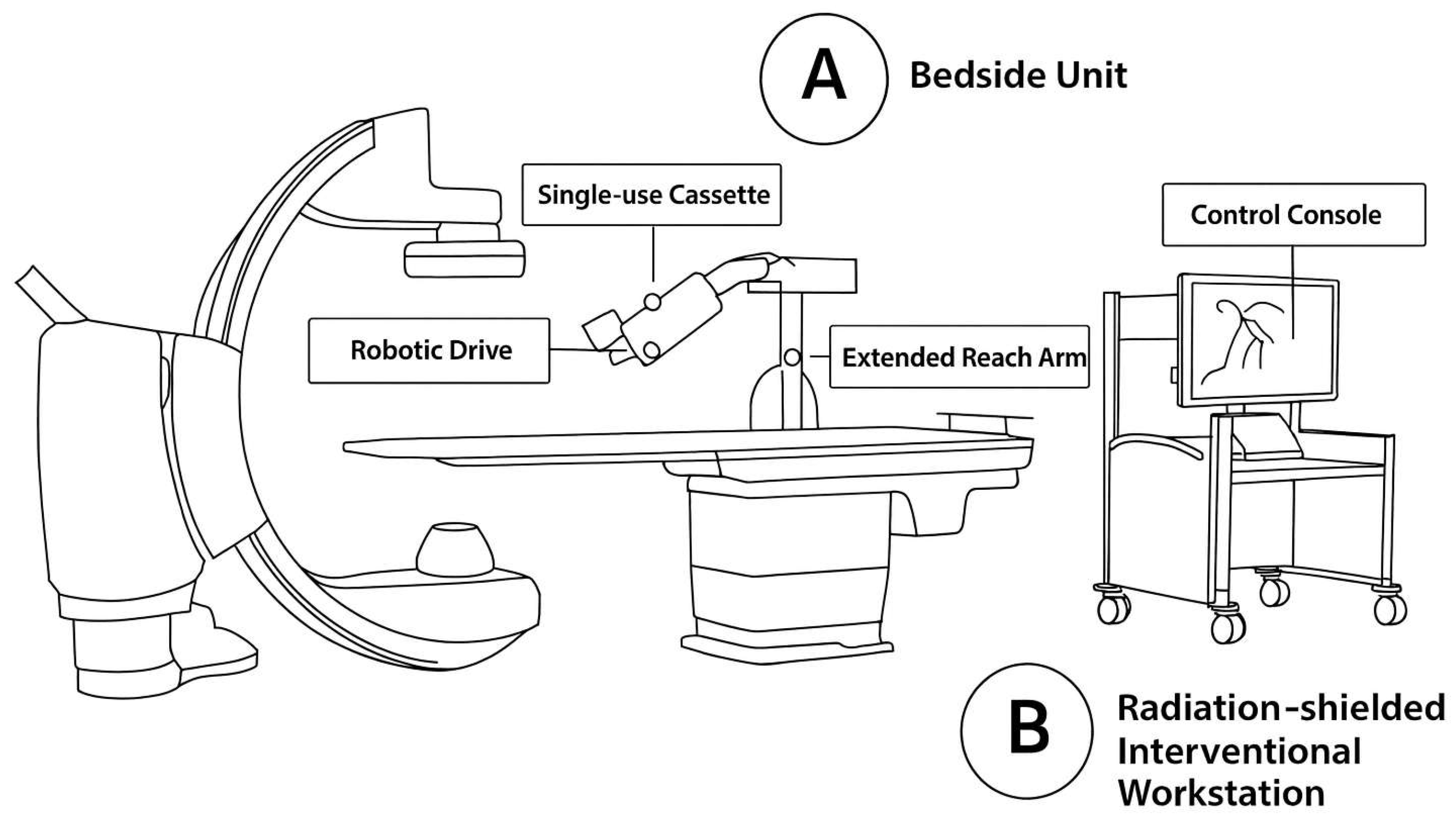
2.1. Robotic PCI
2.1.1. Safety and Feasibility
2.1.2. Radiation Exposure Reduction
2.2. Imaging Modalities’ Integration
2.3. Robotic PCI and Imaging Integration
2.4. Protective Equipment and Emerging Radiation Shielding Technologies
2.5. Radiation Exposure Reduction in Clinical Trials
2.6. Contrast Use and Procedural Efficiency
2.7. Limitations
2.8. The Philosophical Shift in Interventional Cardiology
2.9. Future Aspects
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| CCTA | Coronary computed tomography angiography |
| CTO | Chronic total occlusion |
| DAP | Dose area product |
| FFR | Fractional flow reserve |
| FFR-CT | CT-derived fractional flow reserve |
| fps | Frames per second |
| GRX | CorPath GRX robotic platform |
| IVUS | Intravascular ultrasound |
| iFR | Instantaneous wave-free ratio |
| M-PCI | Manual percutaneous coronary intervention |
| OCT | Optical coherence tomography |
| PCI | Percutaneous coronary intervention |
| R-PCI | Robotic percutaneous coronary intervention |
| STEMI | ST-elevation myocardial infarction |
References
- Townsend, N.; Nichols, M.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef]
- Grüntzig, A. Transluminal dilatation of coronary-artery stenosis. Lancet 1978, 1, 263. [Google Scholar] [CrossRef]
- Bangalore, S.; Toklu, B.; Feit, F. Response to letter regarding article, “Outcomes with coronary artery bypass graft surgery versus percutaneous coronary intervention for patients with diabetes mellitus: Can newer generation drug-eluting stents bridge the gap?”. Circ. Cardiovasc. Interv. 2014, 7, 729. [Google Scholar] [CrossRef][Green Version]
- Vano, E.; Kleiman, N.J.; Duran, A.; Rehani, M.M.; Echeverri, D.; Cabrera, M. Radiation-associated lens opacities in catheterization personnel. J. Vasc. Interv. Radiol. 2013, 24, 197–204. [Google Scholar] [CrossRef]
- Andreassi, M.G.; Piccaluga, E.; Guagliumi, G.; Greco, M.D.; Gaita, F.; Picano, E. Occupational Health Risks in Cardiac Catheterization Laboratory Workers. Circ. Cardiovasc. Interv. 2016, 9, e003273. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Balter, S.; Cowley, M.; Hodgson, J.; Klein, L.W. Occupational hazards of interventional cardiologists. Catheter. Cardiovasc. Interv. 2004, 63, 407–411. [Google Scholar] [CrossRef]
- Ciraj-Bjelac, O.; Rehani, M.M.; Sim, K.H.; Liew, H.B.; Vano, E.; Kleiman, N.J. Risk for radiation-induced cataract for staff in interventional cardiology. Catheter. Cardiovasc. Interv. 2010, 76, 826–834. [Google Scholar]
- Klein, L.W.; Miller, D.L.; Balter, S.; Laskey, W.; Naito, N.; Haines, D.; Ross, A.; Mauro, M.A.; Goldstein, J.A. Occupational health hazards in the interventional laboratory: Time for a Safer Environment. Radiology 2009, 250, 538–544. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Weisz, G. Robotic-assisted angioplasty: Current status and future possibilities. Curr. Cardiol. Rep. 2012, 14, 216–224. [Google Scholar] [CrossRef]
- Weisz, G.; Metzger, D.C.; Caputo, R.P.; Delgado, J.A.; Marshall, J.J.; Vetrovec, G.W.; Reisman, M.; Waksman, R.; Granada, J.F.; Novack, V.; et al. Safety and feasibility of robotic percutaneous coronary intervention: PRECISE (Percutaneous Robotically-Enhanced Coronary Intervention) Study. J. Am. Coll. Cardiol. 2013, 61, 1596–1600. [Google Scholar] [CrossRef]
- Mahmud, E.; Naghi, J.; Ang, L.; Harrison, J.; Behnamfar, O.; Pourdjabbar, A.; Reeves, R.; Patel, M. Complex robotically assisted PCI (CORA-PCI) study. JACC Cardiovasc. Interv. 2017, 10, 1320–1327. [Google Scholar] [CrossRef]
- Madder, R.D.; VanOosterhout, S.; Mulder, A.; Elmore, M.; Campbell, J.; Borgman, A.; Parker, J.; Wohns, D. Impact of robotics and a suspended lead suit on physician radiation exposure during percutaneous coronary intervention. Cardiovasc. Revascularization Med. 2017, 18, 190–196. [Google Scholar] [CrossRef]
- Wagener, M.; Onuma, Y.; Sharif, R.; Coen, E.; Wijns, W.; Sharif, F. Features and limitations of R-PCI: A systematic review. J. Clin. Med. 2024, 13, 5537. [Google Scholar] [CrossRef]
- Smitson, C.C.; Ang, L.; Pourdjabbar, A.; Reeves, R.; Patel, M.; Mahmud, E. Safety and feasibility of a novel, second-generation robotic-assisted system for percutaneous coronary intervention: First-in-human report. J. Invasive Cardiol. 2018, 30, 152–156. [Google Scholar] [CrossRef]
- Hirai, T.; Kearney, K.; Kataruka, A.; Gosch, K.L.; Brandt, H.; Nicholson, W.J.; Lombardi, W.L.; Grantham, J.A.; Salisbury, A.C. Initial report of safety and procedure duration of robotic-assisted chronic total occlusion coronary intervention. Catheter. Cardiovasc. 2020, 95, 165–169. [Google Scholar] [CrossRef]
- Durand, E.; Sabatier, R.; Smits, P.C.; Verheye, S.; Pereira, B.; Fajadet, J. Evaluation of the R-One robotic system for percutaneous coronary intervention: The R-EVOLUTION study. EuroIntervention 2023, 18, e1339–e1347. [Google Scholar] [CrossRef]
- Dou, K.F.; Song, C.X.; Mu, C.W.; Yang, W.X.; Zhu, C.G.; Feng, L.; Chen, J.; Song, L.; Ning, Y.; Xu, B. Feasibility and safety of robotic PCI in China: First in man experience in Asia. J. Geriatr. Cardiol. 2019, 16, 401–405. [Google Scholar]
- Koeda, Y.; Ishida, M.; Sasaki, K.; Kikuchi, S.; Yamaya, S.; Tsuji, K.; Ishisone, T.; Goto, I.; Kimura, T.; Shimoda, Y.; et al. Periprocedural and 30-day outcomes of robotic-assisted percutaneous coronary intervention using intravascular imaging guidance. Cardiovasc. Interv. Ther. 2023, 38, 39–48. [Google Scholar] [CrossRef]
- Gupta, R.; Malik, A.H.; Chan, J.S.K.; Lawrence, H.; Mehta, A.; Venkata, V.S.; Aedma, S.K.; Ranchal, P.; Dhaduk, K.; Aronow, W.S.; et al. Robotic assisted versus manual percutaneous coronary intervention: Systematic review and meta-analysis. Cardiol. Rev. 2024, 32, 24–29. [Google Scholar] [CrossRef]
- Viscusi, M.M.; Bermpeis, K.; Bertolone, D.T.; Mahendiran, T.; Belmonte, M.; Botti, G.; Gallinoro, E.; Paolisso, P.; Barbato, E.; Buytaert, D.; et al. Impact of Robotic Percutaneous Coronary Intervention (R-PCI) With and Without CCTA-Guidance on Clinical Outcomes and Hospital Economics: A Single Center Registry. Catheter. Cardiovasc. Interv. 2025, 105, 426–434. [Google Scholar] [CrossRef]
- Zyśk, A.; Wolny, R.; Kruk, M.; Kwieciński, J.; Dębski, A.; Barbero, U.; Kępka, C.; Demkow, M.; Witkowski, A.; Opolski, M.P.; et al. Computed tomography angiography-derived scores for prediction of chronic total occlusion percutaneous coronary intervention using the hybrid algorithm. J. Cardiovasc. Dev. Dis. 2023, 11, 3. [Google Scholar] [CrossRef]
- Patel, T.M.; Shah, S.C.; Pancholy, S.B.; Kini, A.S.; Mehran, R.; Kirtane, A.J. Comparison of robotic percutaneous coronary intervention with traditional percutaneous coronary intervention: A propensity score–matched analysis of a multicenter registry. Circ. Cardiovasc. Interv. 2020, 13, e008888. [Google Scholar] [CrossRef]
- Patel, T.M.; Shah, S.C.; Patel, A.T.; Patel, B.; Pancholy, S.B. Learning curve of robotic percutaneous coronary intervention: A single-center experience. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100508. [Google Scholar] [CrossRef]
- Tamargo, M.; Vázquez, M.E.; Gutiérrez, E.; Ramos, M.R.; Elízaga, J.; Fernández-Avilés, F. What have we learned from robotic-percutaneous coronary intervention so far? Early experience in a tertiary center. Rev. Esp. Cardiol. 2022, 75, 1075–1077. [Google Scholar] [CrossRef]
- Harrison, J.; Ang, L.; Naghi, J.; Behnamfar, O.; Pourdjabbar, A.; Patel, M.P.; Reeves, R.R.; Mahmud, E. Robotically-assisted percutaneous coronary intervention: Reasons for partial manual assistance or manual conversion. Cardiovasc. Revasc. Med. 2018, 19, 526–531. [Google Scholar] [CrossRef]
- Katsarou, M.; Zwiebel, B.; Vogler, J.; Shames, M.L.; Thayer, A.; Chowdhurry, R.P.; Money, S.R.; Bismuth, J. StemRad MD, an exoskeleton-based radiation protection system, reduces ergonomic posture risk based on a prospective observational study. J. Endovasc. Ther. 2024, 31, 668–674. [Google Scholar] [CrossRef]
- P4 Trial Investigators. Precise Procedural and PCI Plan (P4): A Multicentre, Randomised Trial of CCTA-Guided Versus IVUS-Guided PCI. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05253677 (accessed on 3 July 2025).
- Sonck, J.; Nagumo, S.; Norgaard, B.L.; Otake, H.; Ko, B.; Zhang, J.; Mizukami, T.; Maeng, M.; Andreini, D.; Takahashi, Y.; et al. Clinical Validation of a Virtual Planner for Coronary Interventions Based on Coronary CT Angiography. JACC Cardiovasc. Imaging 2022, 15, 1242–1255. [Google Scholar] [CrossRef]
- Räber, L.; Mintz, G.S.; Koskinas, K.C.; Johnson, T.W.; Holm, N.R.; Onuma, Y.; Radu, M.D.; Joner, M.; Yu, B.; Jia, H.; et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention 2018, 14, 656–677. [Google Scholar] [CrossRef]
- Carvalho, P.E.P.; Cavalcante, J.L.; Lesser, J.; Cheng, V.; Mutlu, D.; Strepkos, D.; Alexandrou, M.; Jalli, S.; Ser, O.S.; Rangan, B.; et al. Coronary Computed Tomography Angiography for Percutaneous Coronary Intervention: Initial United States Experience With FFRCT Based Virtual PCI. Catheter. Cardiovasc. Interv. 2025. [Google Scholar] [CrossRef] [PubMed]
- Häner, J.D.; Räber, L.; Moro, C.; Losdat, S.; Windecker, S. Robotic-assisted percutaneous coronary intervention: Experience in Switzerland. Front. Cardiovasc. Med. 2023, 10, 1294930. [Google Scholar] [CrossRef]
- Rizik, D.G.; Gosselin, K.P.; Burke, R.F.; Goldstein, J.A. Comprehensive radiation shield minimizes operator radiation exposure in coronary and structural heart procedures. Cardiovasc. Revasc. Med. 2024, 64, 70–75. [Google Scholar] [CrossRef]
- Domienik-Andrzejewska, J.; Mirowski, M.; Jastrzębski, M.; Górnik, T.; Masiarek, K.; Warchoł, I.; Grabowicz, W. Occupational exposure to physicians working with a Zero-Gravity™ protection system in haemodynamic and electrophysiology labs: Dosimetric assessment against ceiling suspended shields. Radiat. Environ. Biophys. 2022, 61, 293–300. [Google Scholar] [CrossRef]
- Crowhurst, J.A.; Tse, J.; Mirjalili, N.; Savage, M.L.; Raffel, O.C.; Gaikwad, N.; Walters, D.L.; Dautov, R. Trial of a Novel Radiation Shielding Device to Protect Staff in the Cardiac Catheter Laboratory. Am. J. Cardiol. 2023, 203, 429–435. [Google Scholar] [CrossRef]
- Katsarou, M.; Zwiebel, B.; Chowdhury, R.P.; Shames, M.; Berger, T.; Przybyla, B.; Bismuth, J. Experimental Analysis of Radiation Protection Offered by a Novel Exoskeleton-Based Radiation Protection System versus Conventional Lead Aprons. J. Vasc. Interv. Radiol. 2023, 34, 1345–1352. [Google Scholar] [CrossRef]
- Roguin, A.; Wu, P.; Cohoon, T.; Gul, F.; Nasr, G.; Premyodhin, N.; Kern, M.J. Update on Radiation Safety in the Cath Lab—Moving Toward a “Lead-Free” Environment. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101040. [Google Scholar] [CrossRef]
- Gupta, A.; Chhikara, S.; Vijayvergiya, R.; Barwad, P.; Prasad, K.; Datta, R.; Mahesh, N.K.; Maurya, P.; Singh, N. Radiation Exposure Reduction and Patient Outcome by Using Very Low Frame Rate Fluoroscopy Protocol (3.8 + 7.5 fps) During Percutaneous Coronary Intervention. Front. Cardiovasc. Med. 2021, 8, 625873. [Google Scholar] [CrossRef]
- Stocker, T.J.; Abdel-Wahab, M.; Möllmann, H.; Deseive, S.; Massberg, S.; Hausleiter, J. Trends and predictors of radiation exposure in percutaneous coronary intervention: The PROTECTION VIII study. EuroIntervention 2022, 18, e324–e332. [Google Scholar] [CrossRef]
- Beyar, R.; Gruberg, L.; Deleanu, D.; Roguin, A.; Almagor, Y.; Cohen, S.; Kumar, G.; Wenderow, T. Remote-control percutaneous coronary interventions: Concept, validation, and first-in-humans pilot clinical trial. J. Am. Coll. Cardiol. 2006, 47, 296–300. [Google Scholar] [CrossRef]
- Siemens Healthineers. Siemens Healthineers Discontinues CorPath GRX for Cardiovascular Interventions, Shifts Focus to Neurovascular Robotics. DOTmed HealthCare Business News. 12 May 2023. Available online: https://www.dotmed.com/news/story/60482 (accessed on 3 July 2025).
- Monlezun, D.J. Percutaneous coronary intervention mortality, cost, complications, and disparities after radiation therapy: Artificial intelligence-augmented, cost-effectiveness, and computational ethical analysis. J. Cardiovasc. Dev. Dis. 2023, 10, 445. [Google Scholar] [CrossRef]
- R-ONE® Robotically-Enhanced PCI Intervention Study [Internet]. ClinicalTrials.gov Identifier: NCT07135557. 2020. Available online: https://clinicaltrials.gov/study/NCT07135557 (accessed on 3 July 2025).
- Thirumurugan, E.; Gomathi, K.; Swathy, P.; Afrin, S.A.; Karthick, R. Robotic percutaneous coronary intervention (R-PCI): Time to focus on the pros and cons. Indian. Heart J. 2023, 75, 161–168. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhai, G.Y. Narrative review of latest research progress about robotic percutaneous coronary intervention. J. Geriatr. Cardiol. 2024, 21, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Samant, S.; Panagopoulos, A.N.; Wu, W.; Zhao, S.; Chatzizisis, Y.S. Artificial Intelligence in Coronary Artery Interventions: Preprocedural Planning and Procedural Assistance. J. Soc. Cardiovasc. Angiogr. Interv. 2025, 4, 102519. [Google Scholar] [CrossRef] [PubMed]
- Madder, R.D.; VanOosterhout, S.M.; Jacoby, M.E.; Collins, J.S.; Borgman, A.S.; Mulder, A.N.; Elmore, M.A.; Campbell, J.L.; McNamara, R.F.; Wohns, D.H. Percutaneous coronary intervention using a combination of robotics and telecommunications by an operator in a separate physical location from the patient: An early exploration into the feasibility of telestenting (the REMOTE-PCI study). EuroIntervention 2017, 12, 1569–1576. [Google Scholar] [CrossRef] [PubMed]


| Study (Year) | Design & Sample Size | Population/Lesion Type | Primary Endpoint | Radiation Reduction/Clinical Success | Key Limitations |
|---|---|---|---|---|---|
| PRECISE (2013) [10] | Prospective multicenter trial, n = 164 | Single-lesion PCI | Technical & clinical success | 95% ↓ operator dose | Limited to first-gen CorPath 200; no complex lesions |
| CORA-PCI (2017) [11] | Prospective registry, n = 315 | Complex B2/C lesions | Clinical & technical success | 93–100% clinical, 81–98.8% technical | Learning curve; lack of atherectomy and limited device compatibility |
| Smitson et al. (2018) [14] | Prospective single-center, n = 40 | 77.8% B2/C lesions | Technical success | 90% technical, 97.5% clinical | Device delivery failures in calcified lesions |
| Hirai et al. (2020) [15] | Retrospective single-center, n = 51 CTOs | CTO PCI (hybrid robotic-manual) | Procedural completion | 98% completed robotically | All CTOs crossed manually first |
| R-EVOLUTION (2023) [16] | Multicenter registry, n = 62 | De novo lesions, radial access | Technical & clinical success | 95.2% technical, 100% clinical | Limited sample size; early experience phase |
| Patel et al. (2020) [22] | Propensity-matched cohort, n = 560 | Mixed lesion types | Radiation exposure | ↓ air kerma & DAP in R-PCI (p < 0.01) | Observational; potential residual confounding |
| Study/Device | Type | Radiation Reduction | Key Features |
|---|---|---|---|
| REDUCE-PCI [36] | Clinical Trial | ↓ 45% operator dose | Real-time dosimetry, staff education, and shielding optimization |
| Gupta et al. 2021 [37] | Clinical Trial | ↓ 80% total dose | Low-frame-rate fluoroscopy (3.8 + 7.5 fps), reduced cine |
| PROTECTION VIII [38] | Multicenter Registry | ↓ 47% median DAP | Flat-panel detectors, optimized collimation, default low-dose settings |
| R-EVOLUTION Trial [16] | Clinical Trial (Robotic PCI) | ↓ 69% operator, ↓ 31% patient dose | Robotic PCI system (R-One platform), cockpit control, improved shielding |
| Rampart IC [34] | Shielding Device | ↓ 99.7% staff exposure | Lead-acrylic mobile full-body enclosure, apron-free configuration |
| Protego System [32] | Shielding Device | ↓ 95.9–99.8% (thyroid, waist) | Apron-free rigid and flexible barrier system for operator and assistant |
| StemRad MD [26] | Wearable Exoskeleton Shield | ↓ >90% (brain, eyes, thyroid) | Bismuth-antimony core, orthopedic support, full-body wearable protection |
| Study | Contrast Use (R-PCI vs. M-PCI) | Procedural Time (R-PCI vs. M-PCI) | Statistically Significant? |
|---|---|---|---|
| Smilowitz et al. [9] | 121 ± 47 mL vs. 137 ± 62 mL | 44 ± 32.7 vs. 61 ± 19 min | No |
| Madder et al. [12] | 167 ± 89 mL vs. 145 ± 92 mL | 55 ± 22 min vs. 45 ± 37 min | Yes (time only) |
| CORA-PCI [11] | 183.4 ± 78.7 vs. 202.5 ± 74 mL | 43 ± 26 min vs. 34 ± 17 min | Yes (time) |
| Hirai et al. [15] | 111 ± 39 mL vs. 118 ± 53 mL | 89.6 ± 27.1 vs. 93.4 ± 30.5 min (NS) | No (contrast, time) |
| Patel et al. [22] | 140 vs. 130 mL (NS) | 37 vs. 27 min (p < 0.0005) | Yes (time only) |
| R-EVOLUTION [16] | — | ↓ time with experience (>5 R-PCI cases) | Yes |
| Study | System | Population | Technical Success (%) | Clinical Success (%) | Conversion to M-PCI (%) |
|---|---|---|---|---|---|
| CORA-PCI [11] | CorPath GRX | Mixed lesion complexity | 97.6–98.8 | 100 | 5.5 |
| PRECISE [10] | CorPath 200 | Single-lesion PCI | 91.7–98.8 | 97.6–100 | ~4 |
| Smitson et al. [14] | CorPath GRX | 77.8% B2/C lesions | 90 | 97.5 | 10 |
| Hirai et al. [15] | CorPath GRX | CTO (hybrid R-PCI) | 98 | 100 | 2 |
| R-EVOLUTION [16] | R-One (Robocath) | de novo stenosis, radial | 95.2 | 100 | 3.2 |
| Patel et al. [22] | CorPath GRX | Matched cohort (n = 560) | — | — | Radiation outcomes only |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotoulas, S.C.; Triantafyllis, A.S.; Kontogiannis, N.; Tsinivizov, P.; Antoniades, K.; Aqeel, I.; Karapedi, E.; Kolyda, A.; Poulimenos, L.E. Radiation-Free Percutaneous Coronary Intervention: Myth or Reality? J. Cardiovasc. Dev. Dis. 2025, 12, 339. https://doi.org/10.3390/jcdd12090339
Kotoulas SC, Triantafyllis AS, Kontogiannis N, Tsinivizov P, Antoniades K, Aqeel I, Karapedi E, Kolyda A, Poulimenos LE. Radiation-Free Percutaneous Coronary Intervention: Myth or Reality? Journal of Cardiovascular Development and Disease. 2025; 12(9):339. https://doi.org/10.3390/jcdd12090339
Chicago/Turabian StyleKotoulas, Sotirios C., Andreas S. Triantafyllis, Nestoras Kontogiannis, Pavlos Tsinivizov, Konstantinos Antoniades, Ibraheem Aqeel, Eleni Karapedi, Angeliki Kolyda, and Leonidas E. Poulimenos. 2025. "Radiation-Free Percutaneous Coronary Intervention: Myth or Reality?" Journal of Cardiovascular Development and Disease 12, no. 9: 339. https://doi.org/10.3390/jcdd12090339
APA StyleKotoulas, S. C., Triantafyllis, A. S., Kontogiannis, N., Tsinivizov, P., Antoniades, K., Aqeel, I., Karapedi, E., Kolyda, A., & Poulimenos, L. E. (2025). Radiation-Free Percutaneous Coronary Intervention: Myth or Reality? Journal of Cardiovascular Development and Disease, 12(9), 339. https://doi.org/10.3390/jcdd12090339







