Abstract
Pentacuspid aortic valve is an exceptionally rare congenital anomaly that is often associated with functional deterioration and aortopathy. We report a case of a 39-year-old male presenting with severe aortic regurgitation and an ascending aortic aneurysm in the setting of a pentacuspid aortic valve. The patient underwent a successful Bentall and hemiarch replacement using a composite mechanical valved conduit. This case emphasizes the potential association between rare aortic valve morphologies and ascending aortic pathology and includes a brief review of the existing literature on the pentacuspid aortic valve.
1. Introduction
Congenital anomalies of the aortic valve (CAAVs) are rare, affecting approximately 1–2% of the general population [1,2,3,4]. Pentacuspid aortic valve (PAV), defined by the presence of five aortic cusps, is the rarest form, with an estimated prevalence of less than one in a million people [2]. Most of the very few reported PAV cases (Supplementary Materials: Table S1) are associated with valvular dysfunction, primarily aortic regurgitation (AR), and a subset are complicated by ascending aortic aneurysms [5,6,7,8,9,10,11,12,13,14,15,16,17]. The pathophysiological mechanisms linking abnormal cusp morphology and aortopathy remain incompletely understood, but they may share features with those seen in bicuspid aortic valve (BAV)-related aortopathy [3,18,19].
This case report describes a patient with severe AR and an ascending aortic aneurysm caused by a functionally pentacuspid valve and discusses its clinical and surgical implications in the context of the available literature.
2. Case Presentation
A 39-year-old male presented with symptoms of exertional dyspnea, orthopnea, and episodes of shortness of breath. Ten months prior to admission, he experienced pulmonary edema, which was managed medically at a regional hospital. The patient had no known comorbidities but was an active smoker.
Upon admission, he was normotensive (blood pressure 120/70 mmHg) and receiving the following medications: bisoprolol 2.5 mg once daily, ramipril 2.5 mg once daily, atorvastatin 20 mg once daily, furosemide once daily, and spironolactone 25 mg once daily. The electrocardiogram showed sinus rhythm with a heart rate of 76 beats per minute.
Transthoracic echocardiography (TTE) revealed a left ventricular end-diastolic diameter (LVEDD) of 65 mm and an end-systolic diameter (LVESD) of 48 mm, with a preserved ejection fraction (LVEF) of 56%. The aortic valve was severely calcified, with cusp fusion and decreased mobility (Figure 1A and Figure 2A). Severe aortic regurgitation (grade 4+) was present, with an effective regurgitant orifice area (EROA) of 0.41 cm2, a regurgitant volume (RV) of 65 mL/beat, and a regurgitant fraction (RF) of 52% [20]. The aortic annulus measured 27 mm, the sinotubular junction 40 mm, and the ascending aorta 54 mm. Suprasternal and high right parasternal views demonstrated normal diameters of the aortic arch and descending thoracic aorta, with no evidence of dilation, dissection, or coarctation. Mild mitral and tricuspid regurgitations (1+) were observed, with a systolic pulmonary artery pressure of 58 mmHg. The aortic valve was functionally described as bicuspid, though leaflet morphology was not clearly defined. Coronary angiography revealed a normal branching pattern of the coronary arteries and the absence of obstructive lesions.
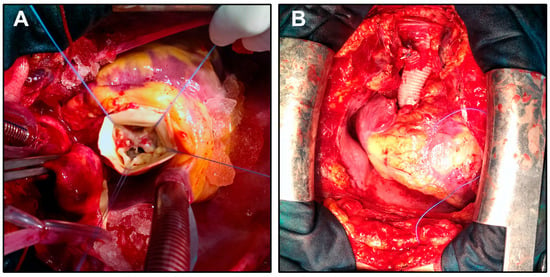
Figure 1.
Intraoperative views during surgical correction of a PAV and ascending aortic aneurysm. (A) Intraoperative exposure of a PAV during aortic root replacement. The valve shows five distinct cusps with asymmetric morphology. Stay sutures are placed to retract the aortic wall and expose the valve anatomy. (B) Completion of the procedure following valve and ascending aorta replacement with a composite graft. The graft anastomosis and surrounding structures are shown in situ prior to chest closure.
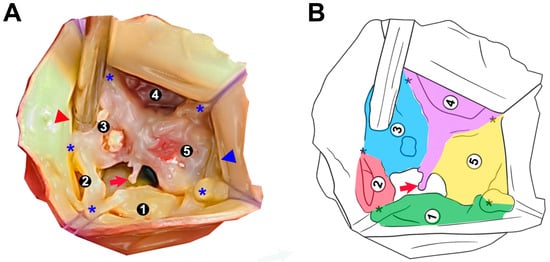
Figure 2.
Morphological anatomy of a pentacuspid aortic valve (PAV): surgical and schematic views. (A) Intraoperative photograph demonstrating a severely dysmorphic PAV with five identifiable cusp regions labeled 1–5 (the same as Figure 1A but zoomed in and cropped). Note the irregular cusp sizes, variable commissural spacing, and presence of fused and rudimentary cusp tissue. Blue asterisks (*) denote commissures and pseudocommissures. The red arrow indicates the presence of an anomalous cord extending from the raphe of the conjoined cusp. The red triangle depicts the position of the LMCA ostium. The blue triangle indicates the position of the RCA ostium. (B) Corresponding schematic illustration of the valve morphology shown in (A), highlighting the asymmetry and heterogeneous cusp arrangement. Each cusp is distinctly color-coded (green, red, blue, purple, and yellow) and labeled for correlation with panel (A). Note the fusion between cusps 3, 4, and 5, as well as the underdeveloped morphology of cusp 2.
The patient underwent surgery via anterograde partial cardiopulmonary bypass (CPB). A Bentall procedure with hemiarch replacement was performed using a composite On-X Ascending Aortic Prosthesis (25 mm) with Vascutek Gelweave Valsalva Graft (26 mm) (Terumo Cardiovascular Systems, Ann Arbor, MI). The cardiopulmonary bypass time was 185 min, the aortic cross-clamp (ACC) time was 105 min, and the circulatory arrest (CA) time was 10 min (open distal anastomosis) at a core temperature of 19 °C and jugular venous oxygen saturation of 100% (Figure 1). Intraoperative transesophageal echocardiography (TEE) showed normal prosthetic valve function and satisfactory hemodynamic performance.
Intraoperative aortic root analysis (Figure 2) revealed a severely dysmorphic PAV with five identifiable cusp regions of irregular sizes and partial fusion, predisposing central malcoaptation and eccentric aortic regurgitation. Cusp 1 (green) appears well-developed and free-standing, corresponding with the non-coronary cusp of the normal tricuspid valve, with true commissures towards neighboring cusps. Cusp 2 (red) is the smallest accessory cusp. Between cusps 3 (blue), 4 (purple), and 5 (yellow), there appears to be a fusion zone forming raphe-like structures. The commissures between these cusps are asymmetric and seem shallower or less developed, which is consistent with pseudocommissures [4]. Partial cusp fusion is visible between cusps 2 and 3. An anomalous cord, extending from the raphe of the conjoined cusp, protrudes into an eccentric orifice [21]. The present morphology with cusp asymmetry and fusion is likely to exhibit bicuspid physiology, as seen on TEE.
Both coronary ostia were of normal size and free of atherosclerotic disease. In relation to the asymmetric PAV anatomy, the left main coronary artery (LMCA) ostium had a slightly lower take-off and corresponded to cusp 3. The right coronary artery (RCA) ostium had a normal take-off, corresponding to cusp 5, and was positioned slightly closer to the adjacent commissure (between cusp 1 and cusp 5) than in a typical tricuspid configuration. Coronary flow was unaffected, as demonstrated by preoperative angiography and confirmed intraoperatively by unimpeded cardioplegia delivery (Figure 2A).
The postoperative period was marked by prolonged leukocytosis and neutrophilia (until postoperative day 6), with leukocyte counts up to 19 × 109/L and neutrophil counts up to 85%. A de-escalation antibiotic regimen including vancomycin and meropenem was administered. The patient remained afebrile throughout hospitalization. Urine and blood cultures, as well as surgical wound swabs, were negative for infection. Upon normalization of white blood cell counts (Leu 9.1 × 109/L; Neu 65.7%), the patient was discharged on postoperative day 13 in a stable hemodynamic condition and without signs of systemic or local infection.
At the 11-year follow-up, the patient remains in good general condition with normal function of the aortic prosthesis and preserved left ventricular systolic function.
3. Discussion
Structural CAAVs affect approximately 1–2% of the general population, with BAV being the most common, occurring in 0.5–1.4% of individuals [22,23]. Unicuspid aortic valve (UAV) is considerably rarer, with an estimated prevalence of 0.02% [24], while a quadricuspid aortic valve (QAV) is observed in 0.003–0.013% of the population [25]. In contrast, PAV is extraordinarily rare, with only isolated case reports in the literature and no robust epidemiological data, suggesting a prevalence likely well below 0.0001%, or fewer than 1 in 1 million [4]. This extreme rarity has precluded formal population-based prevalence estimates and highlights the need for systematic reporting and surveillance.
A review of 14 reported PAV cases (Supplementary Materials: Table S1) reveals a consistent association between the young age (24–39 years), asymmetric cusp morphology, severe eccentric AR, and aortic dilatation, particularly of the root or ascending aorta, in four (28.6%) cases, including the current report [6,9,10]. Of those, three cases underwent root and ascending aorta reconstructions (i.e., Robicsek and Bentall), in addition to AV replacement [9,10]. In our case, the patient underwent a Bentall procedure with hemiarch replacement, which remains the treatment of choice in patients with combined valvular insufficiency and ascending aortic aneurysm [26]. In this case, the decision to use a mechanical composite graft was appropriate given the patient’s young age, preserved ventricular function, and the need for durable correction. The uneventful recovery and excellent 11-year follow-up outcome reinforce the long-term efficacy of this approach when tailored appropriately.
The observed asymmetry, including a rudimentary cusp (cusp 2) and raphe formation between cusps 3, 4, and 5 (Figure 2), supports the hypothesis that cusp malformation and fusion contribute to poor coaptation and progressive AR [4]. In addition, the anomalous cord arising from the raphe’s free margin and floating within the regurgitant orifice (Figure 2) adds a unique structural abnormality not previously described with PAV. A similar structure, with its free portion attached to the aorta, has been documented in some BAV cases, contributing to chronic and acute AR [21].
The co-occurrence of root and ascending aortic dilatation in current and previously reported cases contributes to the hemodynamic burden on the aortic wall, either directly (via eccentric regurgitant jets) or indirectly (through altered flow patterns and wall stress), promoting aneurysmal formation and remodeling [4]. There is also accumulating evidence that CAAVs share some genetics and embryological defects involving both valvulogenesis and aortic wall maturation, predisposing individuals to aortopathy. A rare histological examination of the resected PAV demonstrated myxomatous degeneration, which may support a possible underlying connective tissue abnormality [27].
Genetic studies in BAV and associated aortopathy have implicated a limited number of genes, most notably NOTCH1, SMAD6, and ACTA2. In a large BAV/TAA cohort, SMAD6 variants were specifically enriched among patients with concurrent thoracic aortic aneurysms [28,29], while NOTCH1 and ACTA2 mutations were also documented in familial or heritable forms of aortic valve disease and aortic wall pathology [30]. However, no analogous genetic associations have been reported for PAV, which remains exceedingly rare and lacks familial or syndromic clustering. Nevertheless, in patients with PAV and concomitant ascending aortic dilation, it is plausible that similar developmental pathways affecting valve morphogenesis and aortic wall structure may be involved, and an echocardiographic screening of first-degree relatives may be reasonable in selected cases.
The decision to perform a Bentall procedure in this patient was multifactorial. First, although preoperative sinus of Valsalva measurements were unavailable, we did not expect a normal root configuration, and this was confirmed by intraoperative assessment. Second, while no definitive association between PAV and aortopathy has been demonstrated, the possibility of intrinsic root wall pathology and future dilation could not be excluded. Third, technical challenges were anticipated in adapting commercially available tubular grafts to a markedly dilated STJ proximally and a normal-sized arch distally. Finally, the use of a composite Valsalva graft offered the advantage of addressing all pathologies in a single reconstruction while restoring near-physiological root geometry, a procedure well within the extensive experience of our surgical team.
Associated congenital anomalies were reported in two cases: patent foramen ovale [8] and renal artery dysplasia [13].
Despite the rarity of PAV, this case underscores the importance of considering it in the differential diagnosis of unexplained AR, especially in young people and when associated with ascending aortic dilation. Preoperative recognition remains difficult but may improve with advances in imaging techniques such as 3D echocardiography, MDCT, and cardiac MRI, which can better delineate the commissural architecture and leaflet morphology [31].
4. Conclusions
Pentacuspid aortic valve represents an exceptionally rare congenital anomaly, most often presenting with asymmetric cusp morphology, severe aortic regurgitation, and, in a subset of patients, ascending aortic dilatation. A review of the literature reveals a reproducible pattern of functional insufficiency and structural aortopathy, frequently necessitating combined valvular and aortic surgical intervention. These findings suggest a pathophysiological overlap with BAV-associated aortopathy and support the notion that PAV should be approached as a valvulo-aortic disease entity.
However, current understanding is severely limited by the rarity of PAV, lack of centralized registries, heterogeneous reporting, and scarce histological or genetic characterization. As a result, long-term outcomes, optimal timing of intervention, and risk stratification remain largely undefined.
Future efforts should prioritize the development of multicenter registries and standardized morphological classification schemes. Advanced imaging techniques may enhance preoperative recognition, while systematic histological and molecular investigations could uncover developmental or genetic substrates underlying PAV and its associated aortopathy. Such coordinated strategies are essential to changing PAV from an anecdotal curiosity to a better-understood clinical and surgical entity.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcdd12090330/s1, Table S1: A literature review of the reported PAV cases.
Author Contributions
Conceptualization, M.J.K. and N.K.; methodology, M.G. and M.K.; investigation, M.K. and I.Đ.; writing—original draft preparation, N.K., M.G. and D.L.; writing—review and editing, M.J.K. and N.K.; visualization, I.Đ. and M.K.; supervision, M.J.K. and D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent was obtained from the patient to publish this paper.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CAAVs | Congenital Anomalies of the Aortic Valve |
| PAV | Pentacuspid Aortic Valve |
| AR | Aortic Regurgitation |
| BAV | Bicuspid Aortic Valve |
| TEE | Transthoracic Echocardiography |
| LVEDD | Left Ventricular End-Diastolic Diameter |
| LVESD | Left Ventricular End-Systolic Diameter |
| LVEF | Left Ventricular Ejection Fraction |
| EROA | Effective Regurgitant Orifice Area |
| RV | Regurgitant Volume |
| RF | Regurgitant Fraction |
| CPB | Cardiopulmonary Bypass |
| ACC | Aortic Cross-Clamp |
| CA | Circulatory Arrest |
| TEE | Transesophageal Echocardiography |
| LMCA | Left Main Coronary Artery |
References
- Hinton, R.B.; Yutzey, K.E. Heart valve structure and function in development and disease. Annu. Rev. Physiol. 2011, 73, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Perloff, J.K. The Howard Gilman Foundation Lecture. Where have we come from and where are we going? Valve management past, present and future. In Pathophysiology, Evaluation and Management of Valvular Heart Diseases Vol. 2; Advances in Cardiology; Borer, J.S., Isom, O.W., Eds.; Karger: New York, NY, USA, 2004; Volume 41, pp. 1–8. [Google Scholar] [CrossRef]
- Michelena, H.I.; Corte, A.D.; Evangelista, A.; Maleszewski, J.J.; Edwards, W.D.; Roman, M.J.; Devereux, R.B.; Fernández, B.; Asch, F.M.; Barker, A.J.; et al. International Consensus Statement on Nomenclature and Classification of the Congenital Bicuspid Aortic Valve and Its Aortopathy, for Clinical, Surgical, Interventional and Research Purposes. Radiol. Cardiothorac. Imaging 2021, 3, e200496. [Google Scholar] [CrossRef]
- Dallard, J.; Boodhwani, M.; Labrosse, M. Aortic Valve Mechanics. In Cardiovascular Mechanics; Labrosse, M., Ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 279–318. [Google Scholar]
- Simonds, J. Congenital malformations of the aortic and pulmonary valves. Am. J. Med. Sci. (1827–1924) 1923, 166, 584. [Google Scholar] [CrossRef]
- Bogers, J.J.; Aytug, Z.; Hendriks, F.F.; Huysmans, H.A. Quinticuspid aortic valve causing aortic valve incompetence and stenosis. Thorax 1982, 37, 542–543. [Google Scholar] [CrossRef]
- Yates, A. Editorial comment. J. Cardiovasc. Surg. 1984, 25, 254. [Google Scholar] [CrossRef]
- Cemri, M.; Cengel, A.; Timurkaynak, T. Pentacuspid aortic valve diagnosed by transoesophageal echocardiography. Heart 2000, 84, E9. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, L.; Zhang, Z.; Wang, Y.; Yang, X. Cardiovascular magnetic resonance of quinticuspid aortic valve with aortic regurgitation and dilated ascending aorta. J. Cardiovasc. Magn. Reson. 2009, 11, 28. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.; Zhang, H.; Wang, J.; Han, J. Pentacuspid Aortic Valve with Severe Aortic Regurgitation. Ann. Thorac. Surg. 2010, 89, 2034–2036. [Google Scholar] [CrossRef]
- Kuroki, H.; Hirooka, K.; Ohnuki, M. Pentacuspid aortic valve causing severe aortic regurgitation. J. Thorac. Cardiovasc. Surg. 2012, 143, e11–e12. [Google Scholar] [CrossRef][Green Version]
- Ozyilmaz, S.; Akgul, O.; Guzeltas, A.; Ozyilmaz, I. Diagnosis of pentacuspid aortic valve with severe regurgitation using three-dimensional transesophageal echocardiography. Echocardiography 2015, 32, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Patanè, F.; Ceresa, F.; Ferrazzo, G.; de Gregorio, C. Pentacuspid aortic valve associated with bilateral renal artery dysplasia. J. Cardiovasc. Med. 2020, 21, 717–719. [Google Scholar] [CrossRef]
- Al Ansari, A.E.; Abdulrahman, A.; Shaikho, N.M.G.; Saif, S.A. A very rare cause of aortic regurgitation: Pentacuspid aortic valve. Eur. Heart J. Case Rep. 2021, 5, ytab038. [Google Scholar] [CrossRef]
- Beddingfield, R.H.; Rashid, Z.A.; Pagel, P.S. A Five-Leaf Clover or an Exceptionally Rare Cause of Severe Aortic Insufficiency? J. Cardiothorac. Vasc. Anesth. 2022, 36, 4534–4537. [Google Scholar] [CrossRef]
- Motoki, T.; Ikeno, Y.; Suehiro, Y.; Kurushima, A.; Okita, Y.; Fukumura, Y. A successful repair of pentacuspid aortic valve. JTCVS Tech. 2022, 14, 69–72. [Google Scholar] [CrossRef]
- Albogmi, D.M.; Alharthi, D.A.; Alharbi, D.M.; Alareef, D.M.; Aldohan, D.M. Case Report of a Pentacuspid Aortic Valve with Aortic Insufficiency. Int. J. Appl. Sci. Res. 2023, 6, 27–30. [Google Scholar] [CrossRef]
- Ahmed, A.; Wang, T.K.M. Non-Trileaflet Aortic Valve Aortopathies. Life 2025, 15, 713. [Google Scholar] [CrossRef]
- Henderson, D.J.; Eley, L.; Chaudhry, B. New Concepts in the Development and Malformation of the Arterial Valves. J. Cardiovasc. Dev. Dis. 2020, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef] [PubMed]
- Vowels, T.J.; Gonzalez-Stawinski, G.V.; Ko, J.M.; Trachiotis, G.D.; Roberts, B.J.; Roberts, C.S.; Roberts, W.C. Anomalous cord from the raphe of a congenitally bicuspid aortic valve to the aortic wall producing either acute or chronic aortic regurgitation. J. Am. Coll. Cardiol. 2014, 63, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Sillesen, A.S.; Vøgg, O.; Pihl, C.; Raja, A.A.; Sundberg, K.; Vedel, C.; Zingenberg, H.; Jørgensen, F.S.; Vejlstrup, N.; Iversen, K.; et al. Prevalence of Bicuspid Aortic Valve and Associated Aortopathy in Newborns in Copenhagen, Denmark. JAMA 2021, 325, 561–567. [Google Scholar] [CrossRef]
- Rizza, V.; Ancona, F.; Ingallina, G.; Stella, S.; Margonato, D.; Tavernese, A.; Belli, M.; Biondi, F.; Fiore, G.; Barki, M.; et al. Prevalence, clinical characterization, management and evolution of bicuspid aortic valve classified according to the 2021 International Consensus Statement in a tertiary care hospital. Eur. J. Cardio-Thorac. Surg. 2025, 67, ezaf109. [Google Scholar] [CrossRef] [PubMed]
- Kumanayaka, D.D.; Otto, A.; Chauhan, M.; Belinschi, V.; Mirza, N.; Rayad, M.N.; Suleiman, A. Unicuspid Aortic Valve, an Extremely Rare Congenital Anomaly in Adults: A Systemic Review. World J. Cardiovasc. Dis. 2023, 13, 283–288. [Google Scholar] [CrossRef]
- Janssens, U.; Klues, H.G.; Hanrath, P. Congenital quadricuspid aortic valve anomaly associated with hypertrophic non-obstructive cardiomyopathy: A case report and review of the literature. Heart 1997, 78, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Vendramin, I.; Bortolotti, U.; De Manna, D.N.; Lechiancole, A.; Sponga, S.; Livi, U. Combined Replacement of Aortic Valve and Ascending Aorta—A 70-Year Evolution of Surgical Techniques. Aorta 2021, 9, 118–123. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Bicuspid aortic valve-associated aortopathy: Where do we stand? J. Mol. Cell. Cardiol. 2019, 133, 76–85. [Google Scholar] [CrossRef]
- Gillis, E.; Kumar, A.A.; Luyckx, I.; Preuss, C.; Cannaerts, E.; Van De Beek, G.; Wieschendorf, B.; Alaerts, M.; Bolar, N.; Vandeweyer, G.; et al. Candidate Gene Resequencing in a Large Bicuspid Aortic Valve-Associated Thoracic Aortic Aneurysm Cohort: SMAD6 as an Important Contributor. Front. Physiol. 2017, 8, 400. [Google Scholar] [CrossRef]
- Giusti, B.; Sticchi, E.; De Cario, R.; Magi, A.; Nistri, S.; Pepe, G. Genetic Bases of Bicuspid Aortic Valve: The Contribution of Traditional and High-Throughput Sequencing Approaches on Research and Diagnosis. Front. Physiol. 2017, 8, 612. [Google Scholar] [CrossRef]
- Bravo-Jaimes, K.; Prakash, S.K. Genetics in bicuspid aortic valve disease: Where are we? Prog. Cardiovasc. Dis. 2020, 63, 398–406. [Google Scholar] [CrossRef]
- D’Alonzo, M.; Grande, A.M.; Casale, I.; Fiore, A. Multimodal imaging in the assessment of quadricuspid aortic valve. J. Cardiothorac. Surg. 2025, 20, 148. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).