Real-World Use of ARNI Within GDMT in HFrEF Patients with and Without Atrial Fibrillation: A Retrospective Analysis of Cardiac and Renal Functions and Clinical Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Definitions
2.2. Follow-Up and Adverse Events
2.3. Statistical Analysis
3. Results
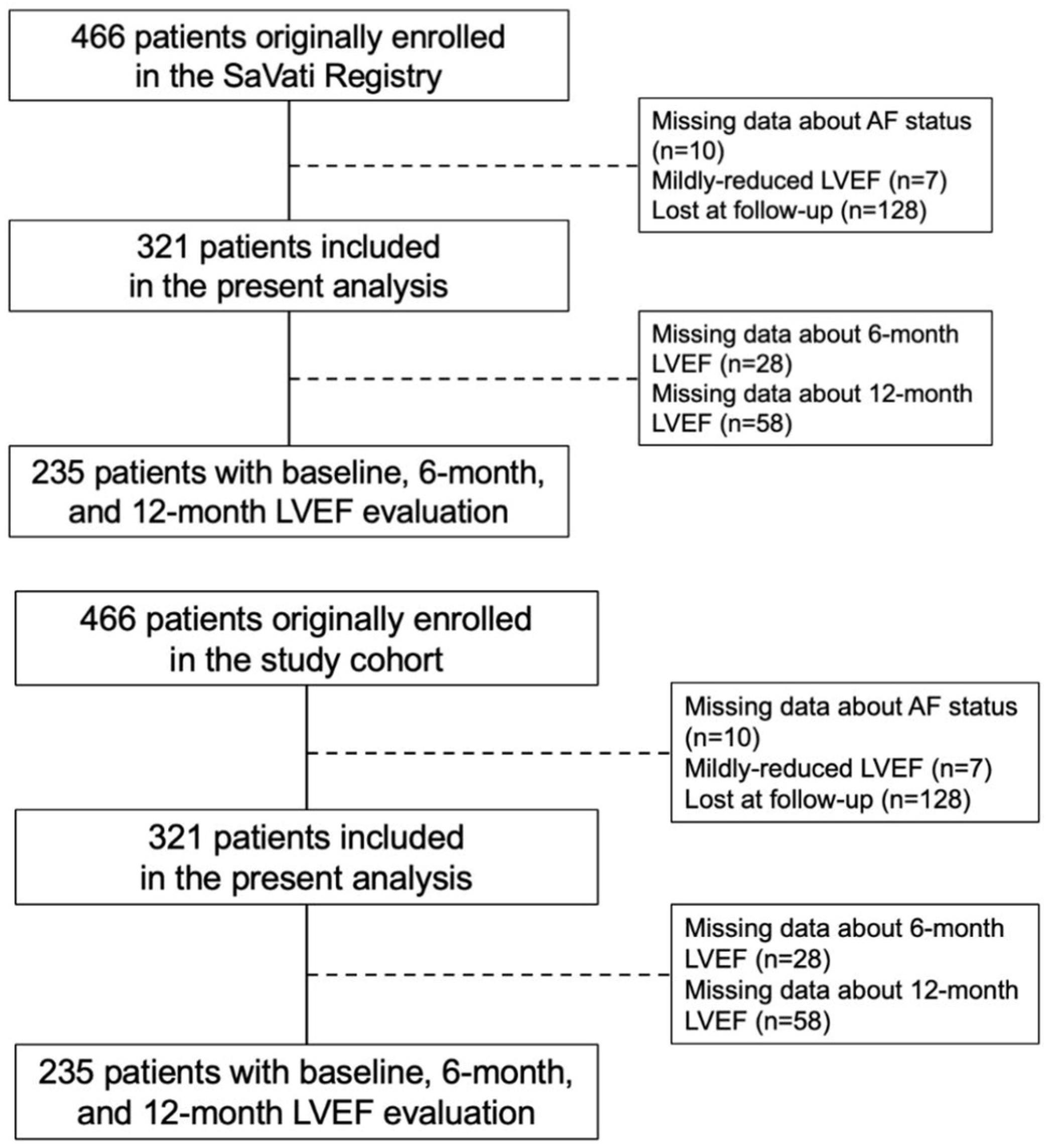
3.1. Study Population
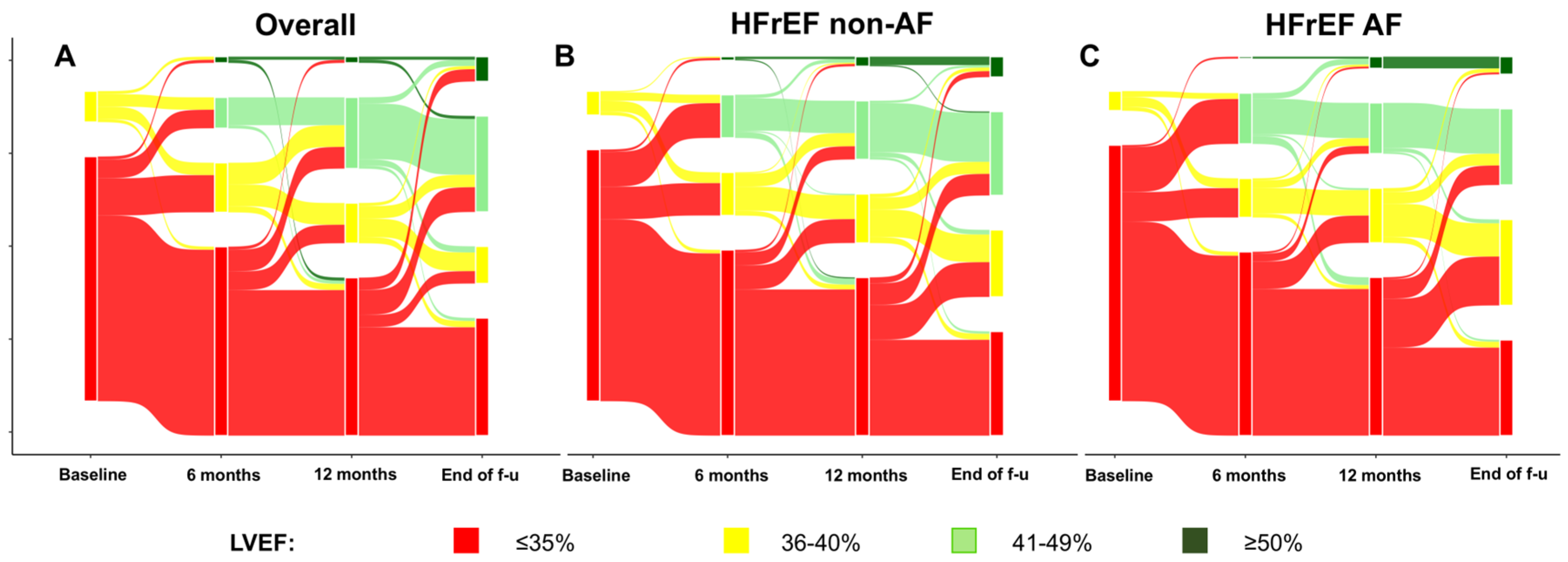
3.2. Follow-Up Changes in Renal Function and LVEF
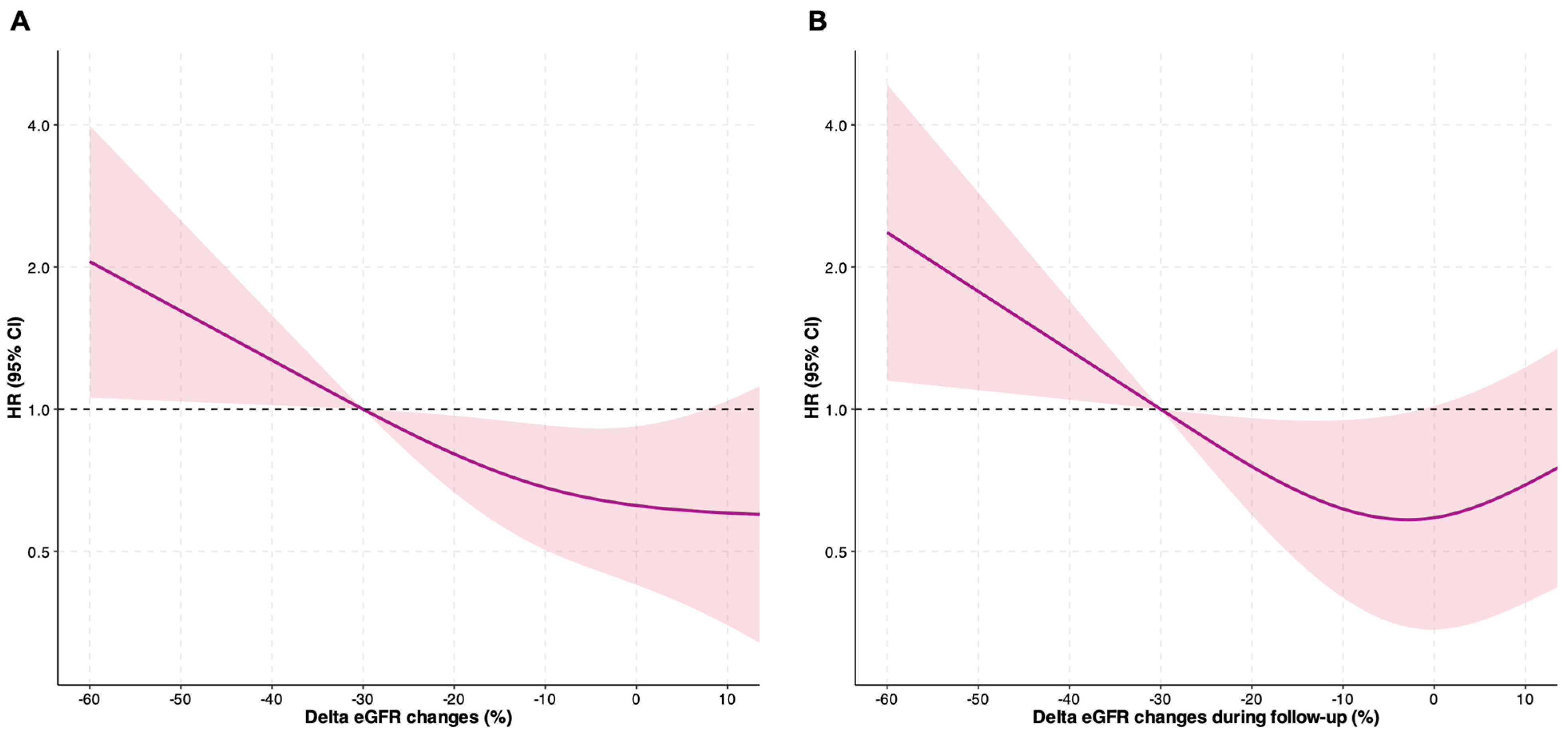
3.3. Risk of Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| ARNi | Angiotensin receptor–neprilysin inhibitor |
| CKD | Chronic kidney disease |
| eGFR | Estimated glomerular filtration rate |
| GDMT | Guideline-directed medical therapy |
| HFrEF | Heart failure with reduced ejection fraction |
| hHF | Hospitalization for heart failure |
| LVEF | Left ventricular ejection fraction |
| NYHA | New York Heart Association |
| RAASis | Renin–angiotensin system inhibitors |
| RDW | Red cell distribution width |
| SGLT2is | Sodium–glucose transport protein 2 inhibitors |
References
- Khan, M.S.; Shahid, I.; Bennis, A.; Rakisheva, A.; Metra, M.; Butler, J. Global epidemiology of heart failure. Nat. Rev. Cardiol. 2024, 21, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; He, J.; Han, Y.; Han, S.; Li, P.; Liao, H.; Guo, J. Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2021. Europace 2024, 26, euae195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeitler, E.P.; Johnson, A.E.; Cooper, L.B.; Steinberg, B.A.; Houston, B.A. Atrial Fibrillation and Heart Failure with Reduced Ejection Fraction: New Assessment of an Old Problem. JACC Heart Fail. 2024, 12, 1528–1539. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Lassen, M.C.H.; Ostrominski, J.W.; Claggett, B.L.; Packer, M.; Zile, M.; Desai, A.S.; Shah, A.M.; Cikes, M.; Merkely, B.; Gori, M.; et al. Cardiovascular-kidney-metabolic overlap in heart failure with preserved ejection fraction: Cardiac structure and function, clinical outcomes, and response to sacubitril/valsartan in PARAGON-HF. Eur. J. Heart Fail. 2024, 26, 1762–1774. [Google Scholar] [CrossRef] [PubMed]
- Sfairopoulos, D.; Liu, T.; Zhang, N.; Tse, G.; Bazoukis, G.; Letsas, K.; Goudis, C.; Milionis, H.; Vrettos, A.; Korantzopoulos, P. Association between sodium-glucose cotransporter-2 inhibitors and incident atrial fibrillation/atrial flutter in heart failure patients with reduced ejection fraction: A meta-analysis of randomized controlled trials. Heart Fail. Rev. 2023, 28, 925–936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, Y.; Chen, L.; Sun, S.; Ran, X. Sodium-Glucose Transporter 2 Inhibitors in Heart Failure: An Overview of Systematic Reviews. J. Cardiovasc. Dev. Dis. 2024, 11, 198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devadoss, R.; Dhillon, G.; Sharma, P.; Verma, R.K.; Munjal, R.; Kashyap, R. Heartfelt Breakthroughs: Elevating Quality of Life with Cutting-Edge Advances in Heart Failure Treatment. J. Cardiovasc. Dev. Dis. 2024, 11, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Newman, J.D.; O’Meara, E.; Böhm, M.; Savarese, G.; Kelly, P.R.; Vardeny, O.; Allen, L.A.; Lancellotti, P.; Gottlieb, S.S.; Samad, Z.; et al. Implications of Atrial Fibrillation for Guideline-Directed Therapy in Patients with Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2024, 83, 932–950. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Testani, J. Cardiorenal interactions in heart failure: Insights from recent therapeutic advances. Cardiovasc. Res. 2024, 120, 1372–1384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verstreken, S.; Beles, M.; Oeste, C.L.; Moya, A.; Masuy, I.; Dierckx, R.; Heggermont, W.; Dauw, J.; Hens, D.; Bartunek, J.; et al. eGFR slope as predictor of mortality in heart failure patients. ESC Heart Fail. 2024, 12, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, V.L.; Proietti, M.; Spagni, S.; Valenti, A.C.; Battista, A.; Pettorelli, D.; Colella, J.; Vitolo, M.; Lip, G.Y.; Boriani, G. Usefulness of Red Cells Distribution Width to Predict Worse Outcomes in Patients with Atrial Fibrillation. Am. J. Cardiol. 2019, 124, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Okoh, N.; Ang, S.P.; Rodriguez, C.A.; Chia, J.E.; Levine, J.S. Short-Term Mortality in Hospitalized Patients with Congestive Heart Failure: Markers of Thrombo-Inflammation Are Independent Risk Factors and Only Weakly Associated with Renal Insufficiency and Co-Morbidity Burden. J. Cardiovasc. Dev. Dis. 2024, 11, 93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sramko, M.; Melenovsky, V.; Wichterle, D.; Franekova, J.; Clemens, M.; Kautzner, J. Impact of Atrial Fibrillation on Natriuretic Peptides: An Invasive Atrial Hemodynamic Study. JACC Clin. Electrophysiol. 2018, 4, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Chatur, S.; Claggett, B.L.; McCausland, F.R.; Rouleau, J.; Zile, M.R.; Packer, M.; Pfeffer, M.A.; Lefkowitz, M.; McMurray, J.J.V.; Solomon, S.D.; et al. Variation in Renal Function Following Transition to Sacubitril/Valsartan in Patients with Heart Failure. J. Am. Coll. Cardiol. 2023, 81, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Ettrup-Christensen, A.; Butt, J.H.; Andersen, M.P.; Sessa, M.; Polcwiartek, C.; Fosbøl, E.L.; Rørth, R.; Kristensen, S.L.; Torp-Pedersen, C.; Køber, L.; et al. Trends in Medical and Device Therapies Following Incident Heart Failure in Denmark during 1996–2019: A Nationwide Register-Based Follow-Up Study. J. Cardiovasc. Dev. Dis. 2023, 10, 362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, H.Y.; Feng, A.N.; Fong, M.C.; Hsueh, C.W.; Lai, W.T.; Huang, K.C.; Chong, E.; Chen, C.N.; Chang, H.C.; Yin, W.H. Sacubitril/valsartan in heart failure with reduced ejection fraction patients: Real world experience on advanced chronic kidney disease, hypotension, and dose escalation. J. Cardiol. 2019, 74, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, R.; Lu, C.; Chen, Q.; Xu, T.; Li, D. Effects of the Angiotensin-Receptor Neprilysin Inhibitor on Cardiac Reverse Remodeling: Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e012272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dzeshka, M.S.; Lip, G.Y.; Snezhitskiy, V.; Shantsila, E. Cardiac Fibrosis in Patients with Atrial Fibrillation: Mechanisms and Clinical Implications. J. Am. Coll. Cardiol. 2015, 66, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Bonini, N.; Proietti, M.; Romiti, G.F.; Vitolo, M.; Fawzy, A.M.; Ding, W.Y.; Imberti, J.F.; Fauchier, L.; Marin, F.; Nabauer, M.; et al. Optimal Medical Therapy for Heart Failure and Integrated Care in Patients with Atrial Fibrillation: A Report From the ESC-EHRA EORP Atrial Fibrillation Long-Term General Registry. J. Am. Heart Assoc. 2025, 14, e030499. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Mei, D.A.; Vitolo, M.; Imberti, J.F. The 2024 ESC guidelines on atrial fibrillation: Essential updates for everyday clinical practice. Intern. Emerg. Med. 2025, 20, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.A.; Romiti, G.F.; Vitolo, M.; Imberti, J.F.; Corica, B.; Mantovani, M.; Bonini, N.; Marin, F.; Diemberger, I.; Dan, G.A.; et al. Atrial fibrillation and female sex: Use of oral anticoagulants in a large European cohort and residual risk of thromboembolism and stroke. Eur. Heart J. Qual. Care Clin. Outcomes 2025, qcaf075. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Corradi, D.; Dobrev, D.; Aguinaga, L.; Cabrera, J.A.; Chugh, S.S.; de Groot, J.R.; Soulat-Dufour, L.; Fenelon, G.; Hatem, S.N.; et al. Atrial cardiomyopathy revisited-evolution of a concept: A clinical consensus statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS). Europace 2024, 26, euae204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boriani, G.; Gerra, L.; Mantovani, M.; Tartaglia, E.; Mei, D.A.; Imberti, J.F.; Vitolo, M.; Bonini, N. Atrial cardiomyopathy: An entity of emerging interest in the clinical setting. Eur. J. Intern. Med. 2023, 118, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, M.; De Mitri, G.; Imberti, J.F.; Gerra, L.; Mei, D.A.; Birtolo, C.; Tartaglia, E.; Casali, E.; Bonini, N.; Vitolo, M.; et al. Left and right atrial echocardiographic parameters and outcome in patients with atrial fibrillation. Kardiol. Pol. 2025, 83, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.C.; Moura, B.; Metra, M.; Böhm, M.; Bauersachs, J.; Ben Gal, T.; Adamopoulos, S.; Abdelhamid, M.; Bistola, V.; Čelutkienė, J.; et al. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Laroche, C.; Diemberger, I.; Popescu, M.I.; Rasmussen, L.H.; Petrescu, L.; Crijns, H.J.G.M.; Tavazzi, L.; Maggioni, A.P.; Lip, G.Y.H. Glomerular filtration rate in patients with atrial fibrillation and 1-year outcomes. Sci. Rep. 2016, 6, 30271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boriani, G.; Mei, D.A.; Bonini, N.; Vitolo, M.; Imberti, J.F.; Romiti, G.F.; Corica, B.; Diemberger, I.; Dan, G.A.; Potpara, T.; et al. Chronic kidney disease classification according to different formulas and impact on adverse outcomes in patients with atrial fibrillation: A report from a prospective observational European registry. Eur. J. Intern. Med. 2025, 136, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Damman, K.; Testani, J.M.; Martens, P.; Mueller, C.; Lassus, J.; Tang, W.H.W.; Skouri, H.; Verbrugge, F.H.; Orso, F.; et al. Evaluation of kidney function throughout the heart failure trajectory-a position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 584–603. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.D.; Saczynski, J.S.; Ward, J.A.; Jaggi, K.; Bourrell, P.; Darling, C.; Goldberg, R.J. The Relationship Between Atrial Fibrillation and Chronic Kidney Disease: Epidemiologic and Pathophysiologic Considerations for a Dual Epidemic. J. Atr. Fibrillation 2012, 5, 442. [Google Scholar] [PubMed] [PubMed Central]
- Kim, S.M.; Jeong, Y.; Kim, Y.L.; Kang, M.; Kang, E.; Ryu, H.; Kim, Y.; Han, S.S.; Ahn, C.; Oh, K.H. Association of Chronic Kidney Disease with Atrial Fibrillation in the General Adult Population: A Nationwide Population-Based Study. J. Am. Heart Assoc. 2023, 12, e028496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsukamoto, S.; Uehara, T.; Azushima, K.; Wakui, H.; Tamura, K. Updates for Cardio-Kidney Protective Effects by Angiotensin Receptor-Neprilysin Inhibitor: Requirement for Additional Evidence of Kidney Protection. J. Am. Heart Assoc. 2023, 12, e029565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zafrir, B.; Lund, L.H.; Laroche, C.; Ruschitzka, F.; Crespo-Leiro, M.G.; Coats, A.J.S.; Anker, S.D.; Filippatos, G.; Seferovic, P.M.; Maggioni, A.P.; et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: A report from 14 964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur. Heart J. 2018, 39, 4277–4284. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, M.; Bucci, T.; Imberti, J.F.; Lam, S.H.M.; Kotalczyk, A.; Boriani, G.; Guo, Y.; Lip, G.Y.H. Patterns of comorbidities, clinical course, and impact of the ABC Pathway for Integrated Care in patients with atrial fibrillation: A report from the prospective Optimal Thromboprophylaxis in Elderly Chinese Patients with Atrial Fibrillation (ChiOTEAF) Registry. Eur. Heart J. Qual. Care Clin. Outcomes 2025, qcaf014. [Google Scholar] [CrossRef] [PubMed]
- Krisai, P.; Johnson, L.S.B.; Moschovitis, G.; Benz, A.; Ramasundarahettige, C.; McIntyre, W.F.; Wong, J.A.; Conen, D.; Sticherling, C.; Connolly, S.J.; et al. Incidence and Predictors of Heart Failure in Patients with Atrial Fibrillation. CJC Open 2021, 3, 1482–1489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Savarese, G.; Lindberg, F.; Cannata, A.; Chioncel, O.; Stolfo, D.; Musella, F.; Tomasoni, D.; Abdelhamid, M.; Banerjee, D.; Bayes-Genis, A.; et al. How to tackle therapeutic inertia in heart failure with reduced ejection fraction. A scientific statement of the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2024, 26, 1278–1297. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.J.; Morrow, D.A.; DeVore, A.D.; Duffy, C.I.; Ambrosy, A.P.; McCague, K.; Rocha, R.; Braunwald, E.; Investigators, P.-H. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N. Engl. J. Med. 2019, 380, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.C.; Savarese, G. Implementing an earlier and more intensive follow-up in acute heart failure: The STRONG-HF and COACH trials. Nat. Rev. Cardiol. 2023, 20, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Imberti, J.F.; Vitolo, M. Atrial fibrillation and remote monitoring through cardiac implantable electronic devices in heart failure patients. Eur. J. Heart Fail. 2020, 22, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Romiti, G.F.; Proietti, M.; Vitolo, M.; Bonini, N.; Fawzy, A.M.; Ding, W.Y.; Fauchier, L.; Marin, F.; Nabauer, M.; Dan, G.A.; et al. Clinical complexity and impact of the ABC (Atrial fibrillation Better Care) pathway in patients with atrial fibrillation: A report from the ESC-EHRA EURObservational Research Programme in AF General Long-Term Registry. BMC Med. 2022, 20, 326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, W.Y.; Proietti, M.; Romiti, G.F.; Vitolo, M.; Fawzy, A.M.; Boriani, G.; Marin, F.; Blomström-Lundqvist, C.; Potpara, T.S.; Fauchier, L.; et al. Impact of ABC (Atrial Fibrillation Better Care) pathway adherence in high-risk subgroups with atrial fibrillation: A report from the ESC-EHRA EORP-AF long-term general registry. Eur. J. Intern. Med. 2023, 107, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Rillig, A.; Magnussen, C.; Ozga, A.K.; Suling, A.; Brandes, A.; Breithardt, G.; Camm, A.J.; Crijns, H.J.G.M.; Eckardt, L.; Elvan, A.; et al. Early Rhythm Control Therapy in Patients with Atrial Fibrillation and Heart Failure. Circulation 2021, 144, 845–858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Proietti, M.; Vitolo, M.; Harrison, S.L.; Lane, D.A.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.S.; Dan, G.A.; Boriani, G.; et al. Real-world applicability and impact of early rhythm control for European patients with atrial fibrillation: A report from the ESC-EHRA EORP-AF Long-Term General Registry. Clin. Res. Cardiol. 2022, 111, 70–84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bidaoui, G.; Assaf, A.; Marrouche, N. Atrial Fibrillation in Heart Failure: Novel Insights, Challenges, and Treatment Opportunities. Curr. Heart Fail. Rep. 2024, 22, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Janota, O.; Mantovani, M.; Kwiendacz, H.; Irlik, K.; Bucci, T.; Lam, S.H.M.; Huang, B.; Alam, U.; Boriani, G.; Hendel, M.; et al. Metabolically “extremely unhealthy” obese and non-obese people with diabetes and the risk of cardiovascular adverse events: The Silesia Diabetes-Heart Project. Cardiovasc. Diabetol. 2024, 23, 326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Marzo, V.; Savarese, G.; Porto, I.; Metra, M.; Ameri, P. Efficacy of SGLT2-inhibitors across different definitions of heart failure with preserved ejection fraction. J. Cardiovasc. Med. 2023, 24, 537–543. [Google Scholar] [CrossRef] [PubMed]

| Overall | No AF | AF | p Value | |
|---|---|---|---|---|
| N (%) | 321 (100) | 187 (58.3%) | 134 (41.7%) | |
| Female sex, n (%) | 62 (19.3) | 37 (19.8) | 25 (18.7) | 0.913 |
| Age (years) median [IQR] | 67 [58–74] | 64 [56–71] | 72 [65–78] | <0.001 |
| BMI (kg/m2), median [IQR] | 27.68 [24.77–30.49] | 28.05 [24.88–30.86] | 27.17 [24.69–30.47] | 0.266 |
| NYHA class, median [IQR] | 2 [2–3] | 2 [2–2] | 2 [2–3] | 0.202 |
| Ischemic etiology, n (%) | 153 (47.8) | 93 (49.7) | 60 (45.1) | 0.483 |
| LVEF (%), median [IQR] | 31 [29–35] | 30 [28–35] | 32 [30–35] | 0.011 |
| Hypertension, n (%) | 215 (67.0) | 124 (66.3) | 91 (67.9) | 0.857 |
| Systolic BP (mmHg), median [IQR] | 120 [110–130] | 120 [110–130] | 120 [110–130] | 0.204 |
| Diastolic BP (mmHg), median [IQR] | 75 [70–80] | 75 [70–80] | 75 [70–80] | 0.419 |
| Diabetes, n (%) | 69 (21.5) | 46 (24.6) | 23 (17.2) | 0.144 |
| Dyslipidemia, n (%) | 228 (71.0) | 137 (73.3) | 91 (67.9) | 0.359 |
| Smoking, n (%) | 0.083 | |||
| Never | 159 (49.7) | 86 (46.0) | 73 (54.9) | |
| Former | 114 (35.6) | 67 (35.8) | 47 (35.3) | |
| Current | 47 (14.7) | 34 (18.2) | 13 (9.8) | |
| PAD, n (%) | 20 (7.5) | 9 (5.6) | 11 (10.4) | 0.230 |
| Carotid artery disease, n (%) | 23 (7.2) | 10 (5.3) | 13 (9.7) | 0.203 |
| TE events, n (%) | 19 (5.9) | 11 (5.9) | 8 (6.0) | 1.000 |
| Previous ACS, n (%) | 105 (32.7) | 66 (35.3) | 39 (29.1) | 0.296 |
| Device, n (%) | 0.285 | |||
| No | 94 (29.7) | 52 (28.0) | 42 (32.3) | |
| ICD | 153 (48.4) | 93 (50.0) | 60 (46.2) | |
| CRT-D | 62 (19.6) | 39 (21.0) | 23 (17.7) | |
| CRT-P | 7 (2.2) | 2 (1.1) | 5 (3.8) | |
| CKD, n (%) | 80 (26.7) | 32 (18.5) | 48 (37.8) | <0.001 |
| eGFR (mL/min/1.73 m2), median [IQR] | 73.00 [59.38–87.43] | 76.50 [64.20–91.00] | 69.00 [55.05–81.65] | <0.001 |
| Kalemia (mEq/L), median [IQR] | 4.30 [3.90–4.60] | 4.30 [4.00–4.60] | 4.40 [3.90–4.70] | 0.495 |
| Hb (g/dL), median [IQR] | 13.80 [12.90–15.10] | 13.90 [13.05–15.10] | 13.50 [12.60–15.15] | 0.239 |
| RDW (%), median [IQR] | 14.10 [13.40–15.40] | 13.80 [13.20–14.60] | 14.80 [13.62–15.75] | <0.001 |
| BNP (ng/L), median [IQR] | 291 [135–570] | 192 [96–453] | 366 [227–642] | 0.004 |
| Treatments | ||||
| ARNi, n (%) | 241 (75.1) | 150 (80.2) | 91 (67.9) | 0.017 |
| ACEi, n (%) | 26 (8.1) | 14 (7.5) | 12 (8.9) | 0.427 |
| ARB, n (%) | 42 (13.1) | 22 (11.8) | 20 (14.9) | 0.473 |
| MRA, n (%) | 267 (83.2) | 153 (81.8) | 114 (85.1) | 0.537 |
| Betablocker, n (%) | 311 (96.9) | 180 (96.3) | 131 (97.8) | 0.660 |
| SLGT2i, n (%) | 124 (38.6) | 64 (34.2) | 60 (44.8) | 0.072 |
| Diuretics, n (%) | 272 (85.0) | 154 (82.8) | 118 (88.1) | 0.253 |
| OAC, n (%) | 158 (49.7) | 31 (16.8) | 127 (94.8) | <0.001 |
| SAPT, n (%) | 114 (35.7) | 94 (50.8) | 20 (14.9) | <0.001 |
| Long DAPT, n (%) | 11 (3.5) | 10 (5.5) | 1 (0.8) | 0.052 |
| Overall | No AF | AF | p Value | |
|---|---|---|---|---|
| Estimated glomerular filtration rate | ||||
| eGFR (mL/min/1.73 m2), median [IQR] | 73.00 [59.38–87.43] | 76.50 [64.20–91.00] | 69.00 [55.05–81.65] | <0.001 |
| eGFR 6-month FU, median [IQR] | 70.83 [54.44–85.32] | 77.15 [58.06–87.31] | 63.96 [50.04–81.70] | 0.002 |
| eGFR 12-month FU, median [IQR] | 69.17 [55.58–85.12] | 75.17 [61.28–88.14] | 63.01 [50.23–79.07] | 0.001 |
| eGFR last available FU, median [IQR] | 64.42 [52.05–79.79] | 68.94 [56.05–83.79] | 57.55 [47.49–74.53] | <0.001 |
| Delta eGFR 6-month, median [IQR] | −2.66 [−9.99–4.06] | −2.51 [−9.75–4.79] | −3.21 [−10.34–2.61] | 0.708 |
| Delta eGFR 12-month, median [IQR] | −2.86 [−12.17–3.15] | −2.72 [−12.28–2.63] | −3.64 [−11.95–4.11] | 0.944 |
| Delta eGFR overall, median [IQR] | −10.29 [−20.82–1.56] | −10.43 [−19.44–(−0.05)] | −9.88 [-26.81–3.17] | 0.945 |
| WeGFR, n (%) | 42 (14.6) | 20 (11.9) | 22 (18.3) | 0.176 |
| Left ventricular ejection fraction | ||||
| LVEF (%), median [IQR] | 31 [29–35] | 30 [28–35] | 32 [30–35] | 0.011 |
| LVEF 6-month FU, median [IQR] | 35 [30–40] | 35 [30–40] | 35 [30–40] | 0.858 |
| LVEF 12-month FU, median [IQR] | 35 [30–40] | 35 [30–40] | 35 [30–43] | 0.752 |
| LVEF last available FU, median [IQR] | 40 [35–45] | 40 [35–45] | 39 [34–47] | 0.759 |
| Delta LVEF 6-month, median [IQR] | 0 [0–8] | 0 [0–9] | 0 [0–6] | 0.103 |
| Delta LVEF 12-month, median [IQR] | 2 [0–9] | 3 [0–10] | 1 [0–7] | 0.155 |
| Delta LVEF overall, median [IQR] | 7 [0–15] | 8 [2–15] | 5 [0–15] | 0.206 |
| LVEF ≥ 10% from baseline, n (%) | 190 (63.3) | 121 (68.4) | 69 (56.1) | 0.041 |
| ImpLVEF, n (%) | 187 (62.3) | 114 (64.4) | 73 (59.3) | 0.443 |
| Event Count | IR Per 100 Person-Year (95% CI) | uHR (95% CI) | aHR * (95% CI) | aHR° (95% CI) | |
|---|---|---|---|---|---|
| Primary outcome | 60 (18.7) | ||||
| No AF | 28 (15.0) | 5.65 (3.76–8.17) | Ref. | Ref. | Ref. |
| AF | 32 (23.9) 0.061 | 11.57 (7.92–16.34) | 2.03 (1.22–3.38) | 1.96 (1.07–3.58) | 2.12 (1.16–3.86) |
| All-cause death | 26 (8.1) | ||||
| No AF | 13 (7.0) | 2.46 (1.31–4.20) | Ref. | Ref. | Ref. |
| AF | 13 (9.7) 0.495 | 4.01 (2.13–6.85) | 1.69 (0.78–3.65) | 0.87 (0.35–2.12) | 1.06 (0.42–2.67) |
| hHF | 46 (14.3) | ||||
| No AF | 19 (10.2) | 3.83 (2.31–5.98) | Ref. | Ref. | Ref. |
| AF | 27 (20.1) 0.018 | 9.75 (6.43–14.19) | 2.46 (1.37–4.44) | 2.66 (1.35–5.26) | 2.80 (1.44–5.46) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonini, N.; Mantovani, M.; Vitolo, M.; Serafini, K.; Tartaglia, E.; Rampini, F.; Grossule, F.; Cherubini, B.; Mastronardi, M.L.; Trapanese, P.; et al. Real-World Use of ARNI Within GDMT in HFrEF Patients with and Without Atrial Fibrillation: A Retrospective Analysis of Cardiac and Renal Functions and Clinical Outcomes. J. Cardiovasc. Dev. Dis. 2025, 12, 328. https://doi.org/10.3390/jcdd12090328
Bonini N, Mantovani M, Vitolo M, Serafini K, Tartaglia E, Rampini F, Grossule F, Cherubini B, Mastronardi ML, Trapanese P, et al. Real-World Use of ARNI Within GDMT in HFrEF Patients with and Without Atrial Fibrillation: A Retrospective Analysis of Cardiac and Renal Functions and Clinical Outcomes. Journal of Cardiovascular Development and Disease. 2025; 12(9):328. https://doi.org/10.3390/jcdd12090328
Chicago/Turabian StyleBonini, Niccolò, Marta Mantovani, Marco Vitolo, Kevin Serafini, Enrico Tartaglia, Francesca Rampini, Francesca Grossule, Benedetta Cherubini, Maria Laura Mastronardi, Paola Trapanese, and et al. 2025. "Real-World Use of ARNI Within GDMT in HFrEF Patients with and Without Atrial Fibrillation: A Retrospective Analysis of Cardiac and Renal Functions and Clinical Outcomes" Journal of Cardiovascular Development and Disease 12, no. 9: 328. https://doi.org/10.3390/jcdd12090328
APA StyleBonini, N., Mantovani, M., Vitolo, M., Serafini, K., Tartaglia, E., Rampini, F., Grossule, F., Cherubini, B., Mastronardi, M. L., Trapanese, P., Imberti, J. F., Mei, D. A., & Boriani, G. (2025). Real-World Use of ARNI Within GDMT in HFrEF Patients with and Without Atrial Fibrillation: A Retrospective Analysis of Cardiac and Renal Functions and Clinical Outcomes. Journal of Cardiovascular Development and Disease, 12(9), 328. https://doi.org/10.3390/jcdd12090328









