Blood Pressure Variability in Hypertension: A Rehabilitation Perspective
Abstract
1. Introduction
2. Types of Blood Pressure Variability
Classification and Clinical Implications of BPV
- Short-term BPV
- Definition: fluctuations occurring over minutes, hours, or 24 h periods (beat-to-beat or diurnal changes);
- Causes: physical activity, stress, autonomic dysfunction, and diurnal rhythms;
- Measurement: assessed via ambulatory blood pressure monitoring (ABPM) or continuous BP devices;
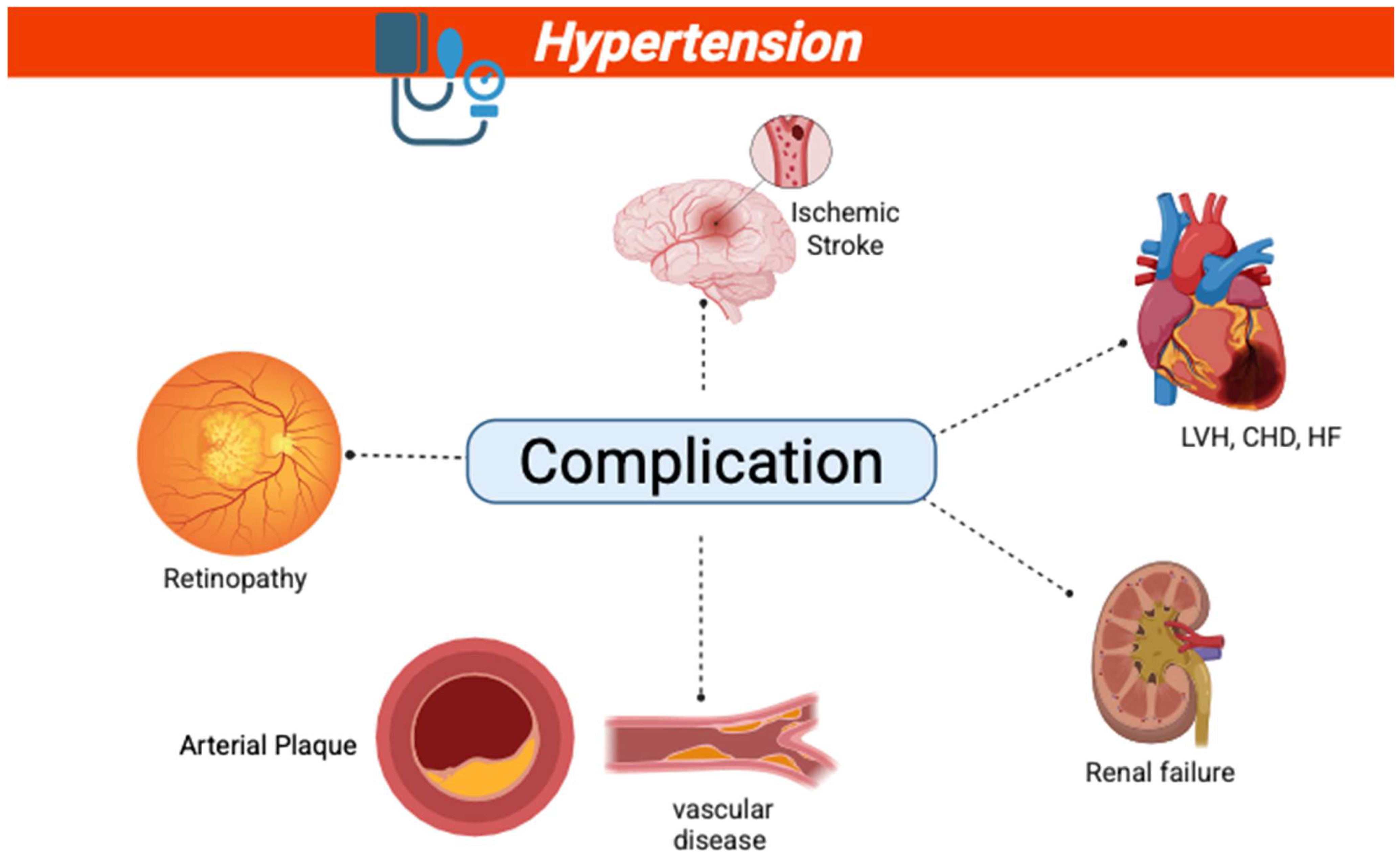
- Clinical Relevance: Elevated short-term BPV is associated with cardiovascular morbidity, particularly in individuals with high variability. It is also linked to target organ damage, such as left ventricular hypertrophy and arterial stiffness [11].
- Long-term BPV
- Definition: variations spanning weeks, months, or years, reflecting instability in BP control;
- Measurement: quantified through repeated clinic visits or serial ABPM over extended periods;
- Clinical Relevance: increased long-term BPV predicts stroke, coronary events, and all-cause mortality, especially in hypertensive patients [4].
- Visit-to-Visit BPV
- Definition: variability in BP levels between consecutive clinical visits;
- Drivers: disease severity, clinical conditions, medication adherence, and white-coat effect;
- Clinical Relevance: increased visit-to-visit BPV is an independent predictor of cardiovascular morbidity and mortality, particularly in the elderly and those with pre-existing cardiovascular disease [12].
- Normal vs. Altered BPV: Thresholds and Clinical Significance
3. Mechanisms Affecting Blood Pressure Variability
4. Impact of Blood Pressure Variability on Cardiovascular Structure and Function
4.1. Effects on Cardiac and Arterial Structures
4.2. Role in Left Ventricular Hypertrophy and Remodeling
4.3. Left Ventricular Stiffness and Blood Pressure Variability
5. Mechanisms Linking BPV to Left Ventricular Hypertrophy
6. Arterial Stiffness and Blood Pressure Variability
7. Arterial Elasticity and Vascular Aging
8. Clinical Studies Correlating BPV and Arterial Stiffness
9. Clinical Implications in Hypertension Management
10. Targeting BPV to Reduce Cardiovascular Risks
11. Cardiovascular Rehabilitation and Its Role in Managing BPV and Cardiovascular Stiffness
12. Future Directions and Research Gap
13. Interventions Targeting BPV to Reduce Stiffness
14. Conclusions
Funding
Conflicts of Interest
References
- Mena, L.J.; Felix, V.G.; Melgarejo, J.D.; Maestre, G.E. 24-Hour Blood Pressure Variability Assessed by Average Real Variability: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e006895. [Google Scholar] [CrossRef]
- Parati, G.; Stergiou, G.S.; Dolan, E.; Bilo, G. Blood pressure variability: Clinical relevance and application. J. Clin. Hypertens. 2018, 20, 1133–1137. [Google Scholar] [CrossRef]
- Lee, R.; Dickhout, J.; Sandow, S. Vascular structural and functional changes: Their association with causality in hypertension: Models, remodeling and relevance. Hypertens. Res. 2017, 40, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Howard, S.C.; Dolan, E.; O’Brien, E.; Dobson, J.E.; Dahlöf, B.; Sever, P.S.; Poulter, N.R. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010, 375, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Baicu, C.F.; Zile, M.R.; Aurigemma, G.P.; Gaasch, W.H. Left Ventricular Systolic Performance, Function, and Contractility in Patients With Diastolic Heart Failure. Circulation 2005, 111, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F.; Hwang, S.J.; Vasan, R.S.; Larson, M.G.; Pencina, M.J.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J. Arterial stiffness and cardiovascular events: The Framingham Heart Study. Circulation 2010, 121, 505–511. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Kim, H.L. Arterial stiffness and hypertension. Clin. Hypertens. 2023, 29, 31. [Google Scholar] [CrossRef]
- Höcht, C. Blood Pressure Variability: Prognostic Value and Therapeutic Implications. Int. Sch. Res. Not. 2013, 2013, 398485. [Google Scholar] [CrossRef]
- Rosei, E.A.; Chiarini, G.; Rizzoni, D. How important is blood pressure variability? Eur. Heart J. Suppl. 2020, 22 (Suppl. E), E1–E6. [Google Scholar] [CrossRef]
- Mancia, G.; Facchetti, R.; Parati, G.; Zanchetti, A. Visit-to-visit blood pressure variability, carotid atherosclerosis, and cardiovascular events in the European Lacidipine Study on Atherosclerosis. Circulation 2012, 126, 569–578. [Google Scholar] [CrossRef]
- Stevens, S.L.; Wood, S.; Koshiaris, C.; Law, K.; Glasziou, P.; Stevens, R.J.; McManus, R.J. Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. BMJ 2016, 354, i4098. [Google Scholar] [CrossRef]
- Chang, T.I.; Flythe, J.E.; Brunelli, S.M.; Muntner, P.; Greene, T.; Cheung, A.K.; Chertow, G.M. Visit-to-visit systolic blood pressure variability and outcomes in hemodialysis. J. Hum. Hypertens. 2014, 28, 18–24. [Google Scholar] [CrossRef]
- Muntner, P.; Shimbo, D.; Tonelli, M.; Reynolds, K.; Arnett, D.K.; Oparil, S. The Relationship Between Visit-to-Visit Variability in Systolic Blood Pressure and All-Cause Mortality in the General Population. Hypertension 2011, 57, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Shlipak, M.G.; Stawski, R.S.; Peralta, C.A.; Psaty, B.M.; Harris, T.B.; Satterfield, S.; Shiroma, E.J.; Newman, A.B.; Odden, M.C.; et al. Visit-to-Visit Blood Pressure Variability and Mortality and Cardiovascular Outcomes Among Older Adults: The Health, Aging, and Body Composition Study. Am. J. Hypertens. 2017, 30, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Paton, J.F.R. The sympathetic nervous system and blood pressure in humans: Implications for hypertension. J. Hum. Hypertens. 2012, 26, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Bombelli, M.; Seravalle, G.; Dell’Oro, R.; Quarti-Trevano, F. Diurnal blood pressure variation and sympathetic activity. Hypertens. Res. 2010, 33, 381–385. [Google Scholar] [CrossRef]
- Joyner, M.J.; Charkoudian, N.; Wallin, B.G. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp. Physiol. 2008, 93, 715–724. [Google Scholar] [CrossRef]
- Joyner, M.J.; Charkoudian, N.; Wallin, B.G. Sympathetic Nervous System and Blood Pressure in Humans. Hypertension 2010, 56, 10–16. [Google Scholar] [CrossRef]
- Ferrario, C.M. Role of Angiotensin II in Cardiovascular Disease—Therapeutic Implications of More Than a Century of Research. J. Renin Angiotensin Aldosterone Syst. 2006, 7, 3–14. [Google Scholar] [CrossRef]
- Hall, J.E. Control of blood pressure by the renin-angiotensin-aldosterone system. Clin. Cardiol. 1991, 14, 6–21. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E.; Czarzasta, K.; Cudnoch-Jedrzejewska, A. Dysregulation of the Renin-Angiotensin System and the Vasopressinergic System Interactions in Cardiovascular Disorders. Curr. Hypertens. Rep. 2018, 20, 19. [Google Scholar] [CrossRef]
- Zhou, T.L.; Henry, R.M.A.; Stehouwer, C.D.A.; van Sloten, T.T.; Reesink, K.D.; Kroon, A.A. Blood Pressure Variability, Arterial Stiffness, and Arterial Remodeling. Hypertension 2018, 72, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Pathiravasan, C.; Ukonu, N.; Rong, J.; Benjamin, E.; McManus, D.; Larson, M.; Vasan, R.; Hamburg, N.; et al. Association of Arterial Stiffness With Mid- to Long-Term Home Blood Pressure Variability in the Electronic Framingham Heart Study: Cohort Study. JMIR Cardio 2024, 8, e54801. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Katsahian, S.; Fassot, C.; Tropeano, A.I.; Gautier, I.; Laloux, B.; Boutouyrie, P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke 2003, 34, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, G.; Bilo, G.; Pucci, G.; Laurent, S.; Macquin-Mavier, I.; Boutouyrie, P.; Battista, F.; Settimi, L.; Desamericq, G.; Dolbeau, G.; et al. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: Findings from 2 large databases. Hypertension 2012, 60, 369–377. [Google Scholar] [CrossRef]
- Burchfield, J.S.; Xie, M.; Hill, J.A. Pathological Ventricular Remodeling. Circulation 2013, 128, 388–400. [Google Scholar] [CrossRef]
- Renna, N.F.; de las Heras, N.; Miatello, R.M. Pathophysiology of Vascular Remodeling in Hypertension. Int. J. Hypertens. 2013, 2013, 808353. [Google Scholar] [CrossRef]
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left ventricular hypertrophy and hypertension. Prog. Cardiovasc. Dis. 2020, 63, 10–21. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wang, J.G. Blood Pressure Variability and Left Ventricular Diastolic Dysfunction. Am. J. Hypertens. 2024, 37, 163–164. [Google Scholar] [CrossRef]
- Grossman, W. Cardiac hypertrophy: Useful. adaptation or pathologic process? Am. J. Med. 1980, 69, 576–584. [Google Scholar] [CrossRef]
- Perrino, C.; Prasad, S.V.N.; Mao, L.; Noma, T.; Yan, Z.; Kim, H.S.; Smithies, O.; Rockman, H.A. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J. Clin. Investig. 2006, 116, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- Selvetella, G.; Lembo, G. Mechanisms of Cardiac Hypertrophy. Heart Fail. Clin. 2005, 1, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Brilla, C.G.; Campbell, S.E. Regulatory Mechanisms of Myocardial Hypertrophy and Fibrosis: Results of in vivo Studies. Cardiology 2008, 81, 266–273. [Google Scholar] [CrossRef] [PubMed]
- González, A.; López, B.; Díez, J. Fibrosis in hypertensive heart disease: Role of the renin-angiotensin-aldosterone system. Med. Clin. N. Am. 2004, 88, 83–97. [Google Scholar] [CrossRef]
- Rababa’h, A.M.; Guillory, A.N.; Mustafa, R.; Hijjawi, T. Oxidative Stress and Cardiac Remodeling: An Updated Edge. Curr. Cardiol. Rev. 2018, 14, 53–59. [Google Scholar] [CrossRef]
- Schirone, L.; Forte, M.; Palmerio, S.; Yee, D.; Nocella, C.; Angelini, F.; Pagano, F.; Schiavon, S.; Bordin, A.; Carrizzo, A.; et al. A Review of the Molecular Mechanisms Underlying the Development and Progression of Cardiac Remodeling. Oxidative Med. Cell. Longev. 2017, 2017, 3920195. [Google Scholar] [CrossRef]
- Isabelle, M.; Chimenti, S.; Beaussier, H.; Gransagne, D.; Villeneuve, N.; Safar, M.E.; Duchatelle, V.; Vilaine, J.-P.; Vayssettes-Courchay, C.; Bézie, Y. SBP, DBP, and pulse blood pressure variability are temporally associated with the increase in pulse wave velocity in a model of aortic stiffness. J. Hypertens. 2016, 34, 666. [Google Scholar] [CrossRef]
- Silva, M.A.V.; Resende, L.A.P.R.; Vieira, M.M.; Jajah, C.B.F.; Berzotti, L.A.; Rambourg, N.C.; Pierson, I.D.d.S.; Achkar, J.L.C.; Vieira, L.M.; Moreira, G.M.; et al. Correlation between short-term blood pressure variability parameters with mobil-O-graph pulse wave velocity. Clin. Hypertens. 2022, 28, 5. [Google Scholar] [CrossRef]
- Ogoh, S. What is important for aging-induced arterial stiffening, autonomic dysfunction, vascular characteristics or both? Hypertens. Res. 2017, 40, 434–435. [Google Scholar] [CrossRef]
- Shirwany, N.A.; Zou, M.-h. Arterial stiffness: A brief review. Acta Pharmacol. Sin. 2010, 31, 1267–1276. [Google Scholar] [CrossRef]
- Clement, D.L.; De Buyzere, M.L.; Duprez, D.A. Hypertension in peripheral arterial disease. Curr. Pharm. Des. 2004, 10, 3615–3620. [Google Scholar] [CrossRef]
- Duprez, D.A.; De Buyzere, M.L.; De Backer, T.L.; Van De Veire, N.; Clement, D.L.; Cohn, J.N. Relationship between arterial elasticity indices and carotid artery intima-media thickness. Am. J. Hypertens. 2000, 13, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, G.C.; DAntoni, V.D.A.; Morsella, V.M.; Torti, M.T.; Grassini, P.G.; Vacca, L.V.; Stocchi, F.; Selli, S.; Volterrani, M. Correlation Between Systolic Blood Pressure Variability and Global Longitudinal Strain in Patients with Parkinson’s Disease and Dysautonomia. J. Cardiovasc. Dis. Res. 2020, 11, 1–3. [Google Scholar] [CrossRef]
- Shin, S.H.; Jang, J.H.; Baek, Y.S.; Kwon, S.W.; Park, S.D.; Woo, S.I.; Kim, D.; Kwan, J. Relation of blood pressure variability to left ventricular function and arterial stiffness in hypertensive patients. Singap. Med. J. 2019, 60, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Ruilope, L.M.; McInnes, G.T.; Waeber, B.; Weber, M.A. Angiotensin-II receptor blockers: Benefits beyond blood pressure reduction? J. Hum. Hypertens. 2005, 19, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Ochoa, J.E. Blood Pressure Variability. In Prehypertension and Cardiometabolic Syndrome; Zimlichman, R., Julius, S., Mancia, G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 395–417. [Google Scholar] [CrossRef]
- Faiss, R.; Girard, O.; Millet, G.P. Advancing hypoxic training in team sports: From intermittent hypoxic training to repeated sprint training in hypoxia. Br. J. Sports Med. 2013, 47 (Suppl. S1), i45–i50. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Smart, N.A. Exercise training for blood pressure: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004473. [Google Scholar] [CrossRef]
- Ambrosetti, M.; Abreu, A.; Corrà, U.; Davos, C.H.; Hansen, D.; Frederix, I.; Iliou, M.C.; Pedretti, R.F.E.; Schmid, J.-P.; Vigorito, C.; et al. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2021, 28, 460–495. [Google Scholar] [CrossRef]
- Lopes, S.; Mesquita-Bastos, J.; Garcia, C.; Leitão, C.; Ribau, V.; Teixeira, M.; Bertoquini, S.; Ribeiro, I.P.; de Melo, J.B.; Oliveira, J.; et al. Aerobic exercise improves central blood pressure and blood pressure variability among patients with resistant hypertension: Results of the EnRicH trial. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2023, 46, 1547–1557. [Google Scholar] [CrossRef]
- Caminiti, G.; Iellamo, F.; Mancuso, A.; Cerrito, A.; Montano, M.; Manzi, V.; Volterrani, M. Effects of 12 weeks of aerobic versus combined aerobic plus resistance exercise training on short-term blood pressure variability in patients with hypertension. J. Appl. Physiol. 2021, 130, 1085–1092. [Google Scholar] [CrossRef]
- Matias, L.A.S.; Mariano, I.M.; Batista, J.P.; de Souza, T.C.F.; Amaral, A.L.; Dechichi, J.G.C.; Rodrigues, M.d.L.; Carrijo, V.H.V.; Cunha, T.M.; Puga, G.M. Acute and chronic effects of combined exercise on ambulatory blood pressure and its variability in hypertensive postmenopausal women. Chin. J. Physiol. 2020, 63, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, K.S.; Yoon, E.S.; Sharman, J.E.; Davies, J.E.; Shih, Y.T.; Chen, C.H.; Fernhall, B.; Jae, S.Y. Resistance exercise training reduces arterial reservoir pressure in older adults with prehypertension and hypertension. Hypertens. Res. 2013, 36, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Araújo, T.d.P.; Borges, L.G.S.; Barroso, W.K.S.; Brandão, A.A.; Barbosa, E.C.D.; Feitosa, A.D.M.; Malachias, M.V.B.; Gomes, M.M.; Amodeo, C.; Povoa, R.M.d.S.; et al. Factors associated with uncontrolled blood pressure in hypertensive Brazilians. J. Clin. Hypertens. 2022, 24, 814–824. [Google Scholar] [CrossRef]
- Brandão Rondon, M.U.P.; Alves, M.J.N.N.; Braga, A.M.F.W.; Teixeira, O.T.U.N.; Barretto, A.C.P.; Krieger, E.M.; Negrão, C.E. Postexercise blood pressure reduction in elderly hypertensive patients. J. Am. Coll. Cardiol. 2002, 39, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, G.; Iellamo, F.; Perrone, M.A.; Marazzi, G.; Gismondi, A.; Cerrito, A.; Franchini, A.; Volterrani, M. Concurrent Aerobic Plus Resistance Training Elicits Different Effects on Short-Term Blood Pressure Variability of Hypertensive Patients in Relation to Their Nocturnal Blood Pressure Pattern. Medicina 2022, 58, 1682. [Google Scholar] [CrossRef]
- Diaz, K.M.; Feairheller, D.L.; Sturgeon, K.M.; Williamson, S.T.; Veerabhadrappa, P.; Kretzschmar, J.; Perkins, A.; Ling, C.; Crabbe, D.L.; Brown, M.D. Abstract P135: The Effects of Aerobic Exercise Training on Visit-to-Visit and Ambulatory Blood Pressure Variability in Non-Hypertensive and Hypertensive African Americans. Circulation 2012, 125 (Suppl. S10), AP135. [Google Scholar] [CrossRef]
- Taylor, K.A.; Wiles, J.D.; Coleman, D.A.; Leeson, P.; Sharma, R.; O’Driscoll, J.M. Neurohumoral and ambulatory haemodynamic adaptations following isometric exercise training in unmedicated hypertensive patients. J. Hypertens. 2019, 37, 827. [Google Scholar] [CrossRef]
- MartinezAguirre-Betolaza, A.; Mujika, I.; Fryer, S.M.; Corres, P.; Gorostegi-Anduaga, I.; Arratibel-Imaz, I.; Pérez-Asenjo, J.; Maldonado-Martín, S. Effects of different aerobic exercise programs on cardiac autonomic modulation and hemodynamics in hypertension: Data from EXERDIET-HTA randomized trial. J. Hum. Hypertens. 2020, 34, 709–718. [Google Scholar] [CrossRef]
- Seidel, M.; Pagonas, N.; Seibert, F.S.; Bauer, F.; Rohn, B.; Vlatsas, S.; Mühlberger, D.; Nina, B.; Westhoff, T.H. The differential impact of aerobic and isometric handgrip exercise on blood pressure variability and central aortic blood pressure. J. Hypertens. 2021, 39, 1269. [Google Scholar] [CrossRef]
- Batista, J.P.; Tavares, J.B.; Gonçalves, L.F.; de Souza, T.C.F.; Mariano, I.M.; Amaral, A.L.; Rodrigues, M.d.L.; Matias, L.A.S.; Resende, A.P.M.; Puga, G.M. Mat Pilates training reduces blood pressure in both well-controlled hypertensive and normotensive postmenopausal women: A controlled clinical trial study. Clin. Exp. Hypertens. 2022, 44, 548–556. [Google Scholar] [CrossRef]
- Mariano, I.M.; Dechichi, J.G.C.; Matias, L.A.S.; Rodrigues, M.d.L.; Batista, J.P.; de Souza, T.C.F.; Amaral, A.L.; Carrijo, V.H.V.; Puga, G.M. Ambulatory blood pressure variability and combined exercise training: Comparison between hypertensive and normotensive postmenopausal women. Blood Press. Monit. 2020, 25, 338–345. [Google Scholar] [CrossRef]
- Kollias, A.; Kyriakoulis, K.G.; Stergiou, G.S. Evidence on the clinical relevance of short-term blood pressure variability? Untying the Gordian knot. Eur. J. Prev. Cardiol. 2022, 29, 1375–1376. [Google Scholar] [CrossRef]
- Steinsaltz, D.; Patten, H.; Bester, D.W.; Rehkopf, D.H. Short-Term and Mid-Term Blood Pressure Variability and Long-Term Mortality: Evidence from the Third National Health and Nutrition Examination Study. 2023. Available online: http://medrxiv.org/lookup/doi/10.1101/2023.12.18.23300161 (accessed on 5 December 2024).
- Parati, G.; Croce, A.; Bilo, G. Blood pressure variability: No longer a mASCOT for research nerds. Eur. Heart J. 2024, 45, 1170–1172. [Google Scholar] [CrossRef]
- Zhazykbayeva, S.; Pabel, S.; Mügge, A.; Sossalla, S.; Hamdani, N. The molecular mechanisms associated with the physiological responses to inflammation and oxidative stress in cardiovascular diseases. Biophys. Rev. 2020, 12, 947–968. [Google Scholar] [CrossRef] [PubMed]
| Scheme | Population | Study Type | Age (Years) | Rehabilitation/Exercise Intervention | BPV Measurement and Results | Main Outcomes | Reference |
|---|---|---|---|---|---|---|---|
| Susana Lopes et al. (2023) | 60/HT | RCT | 40–75 | 12-week aerobic training | ↓ Central SBP variability by −2.85 mmHg (p = 0.008) | Reduced central SBP | [52] |
| Giuseppe Caminiti et al. (2021) | 55/HT | RCT | ≥45 | 12-week aerobic vs. combined (aerobic + resistance) training | ↓ 24 h SBP ARV: Combined: 8.8 to 7.1 mmHg; Aerobic: 8.4 to 7.6 mmHg | Combined training is more effective than aerobic alone in reducing BPV | [53] |
| Matias et al. (2020) | 14/HT | SACT | 50 and 70 | Acute and 10-week combined training | Acute: ↓ SBP-SD by ~2 mmHg; Chronic: no significant BPV change | Acute sessions reduce BPV; long-term effect on BP but not BPV | [54] |
| Brandão Rondon et al. (2002) | 24/HT | RCT | 68.9 ± 1.5 | Low-intensity bicycle exercise | ↓ Short-term BPV ↓ CO, SV, LVDV | Suggested sex-specific responsiveness to BPV modulation | [57] |
| Heffernan et al. (2013) | 21/HT | RCT | 61 ± 1 | 3 sessions/week, 12 weeks, aerobic or resistance exercise | ↓ Central BP | Resistance training alone is effective in BP and BPV control | [55] |
| Araújo et al. (2022) | 2643/HT | Cross-sectional study, Multicenter study | 61.6 ± 11.9 | 10 weeks combined resistance + aerobic | ↓ Ambulatory 24 h SBP-SD from 12.3 to 10.7 mmHg | Combined training lowered both BP and variability | [56] |
| Caminiti et al. (2022) | 72/HT | SACT | 66.1 ± 12.7 | 3 sessions/week, 12 weeks, combined exercise | ↓ Daytime BPV and 24 h mean SBP | HIIT walking was safe and beneficial for BP control | [58] |
| Diaz et al. (2012) | 14/HT | SACT | NA | 3 sessions/week, 24 weeks, aerobic exercise | ↓ Systolic BPV (SBPV) | CV, SD, ARV, ASV | [59] |
| Taylor et al. (2019) | 24/HT | RCT | 43.8 ± 7.3 | 3 sessions/week, 4 weeks, isometric exercise | BP significantly reduced (12.4/6.2 to 11.8/5.6 mmHg) (p < 0.001) | ARV | [60] |
| MartinezAguirre-Betolaza et al. (2020) | 249/HT | RCT | 54.2 ± 7.2 53.1 ± 8.6 54.4 ± 7.2 52.9 ± 8.5 67.6 ± 6.6 | MICT HVHIIT LVHIIT/2 session/week for 16 weeks | Improvements in BP and ANS | Combined training lowered both BP and ANS control | [61] |
| Seidel et al. (2021) | 66/HT | RCT | 60.7 ± 9.9 | 5 sessions/week, 12 weeks, aerobic, handgrip | ↓ Systolic daytime variability (12.1 ± 2.5 to 10.3 ± 2.8, p = 0.04) ↓ Central SBP from 145 ± 15 to 134 ± 19 mmHg (p = 0.01) | Combined training lowered both BP and variability | [62] |
| Batista et al. (2022) | 47/HT | SACT | 58.0 ± 5.0 | 3 sessions/week, 12 weeks, Mat Pilates | ↓ BP, BPV, and HRV | Pilates training lowered both BP and variability | [63] |
| Mariano et al. (2020) | 13/HT | SACT | 30 sessions/week, 10 weeks, combined | BP reductions in hypertensive | Acute sessions reduce BPV; long-term effect on BP but not BPV | [64] |
| Category | Highlights |
|---|---|
| BPV Impact | Elevated BPV increases the risk of stroke, organ damage, and death. Acute exercise reduces short-term BPV, but long-term BPV control is crucial to prevent adverse outcomes. |
| Rehabilitation Impact | Exercise interventions: aerobic, resistance, combined training, HIIT, isometric exercise, and Pilates consistently lower BPV and improve overall BP control. They also positively affect ANS function and vascular health. |
| Exercise Type | Combining aerobic and resistance training is more effective in lowering BP than aerobic training alone. Resistance training alone also benefits BP and BP control. |
| Duration and Frequency | Both acute and sustained rehabilitation programs reduce BPV, but acute exercise provides quicker reductions, while long-term exercise contributes to sustained BP improvements. |
| Population Benefits | Population benefits are observed in hypertensive populations of all ages, including the elderly and those with pre-existing cardiovascular risk factors. Rehabilitation should be tailored to individual patients. |
| Future direction | Future Research Directions: Optimal exercise doses for BPV reduction, digital health interventions for sustained BPV control, and the combination of exercise and medication. Mechanisms of BPV Reduction: Investigate the mechanisms underlying exercise-induced BPV reduction and its association with cardiovascular events and mortality. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raju, M.; Perrone, M.A.; Alashram, A.R.; Iellamo, F. Blood Pressure Variability in Hypertension: A Rehabilitation Perspective. J. Cardiovasc. Dev. Dis. 2025, 12, 317. https://doi.org/10.3390/jcdd12080317
Raju M, Perrone MA, Alashram AR, Iellamo F. Blood Pressure Variability in Hypertension: A Rehabilitation Perspective. Journal of Cardiovascular Development and Disease. 2025; 12(8):317. https://doi.org/10.3390/jcdd12080317
Chicago/Turabian StyleRaju, Manikandan, Marco Alfonso Perrone, Anas R. Alashram, and Ferdinando Iellamo. 2025. "Blood Pressure Variability in Hypertension: A Rehabilitation Perspective" Journal of Cardiovascular Development and Disease 12, no. 8: 317. https://doi.org/10.3390/jcdd12080317
APA StyleRaju, M., Perrone, M. A., Alashram, A. R., & Iellamo, F. (2025). Blood Pressure Variability in Hypertension: A Rehabilitation Perspective. Journal of Cardiovascular Development and Disease, 12(8), 317. https://doi.org/10.3390/jcdd12080317