Dysfunctional Electron Transport Chain Assembly in COXPD8
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Case Presentation
- Exon/Intron 22 c.2872c>T; p.Arg 958Ter, a heterozygous maternal, autosomal recessive mutation, consistent with that previously reported in a family of three siblings with hypertrophic cardiomyopathy [6]). The mutation was classified as likely pathogenic.
- Exon/Intron 12 c.1738C>G; p.Arg580Gly, heterozygous paternal, autosomal recessive, with the same expected phenotype though not previously reported. Different missense mutations at this amino acid have been associated with fatal infantile cardiomyopathy, although the mutation was classified as of uncertain significance.
2.3. Comparison Infant Patients
2.4. Tissue Biobank
2.5. Isolation of Mitochondria
2.6. Oxygen Consumption
2.7. Electrophoresis and Immunoblotting
2.8. Enzyme Assays
3. Results
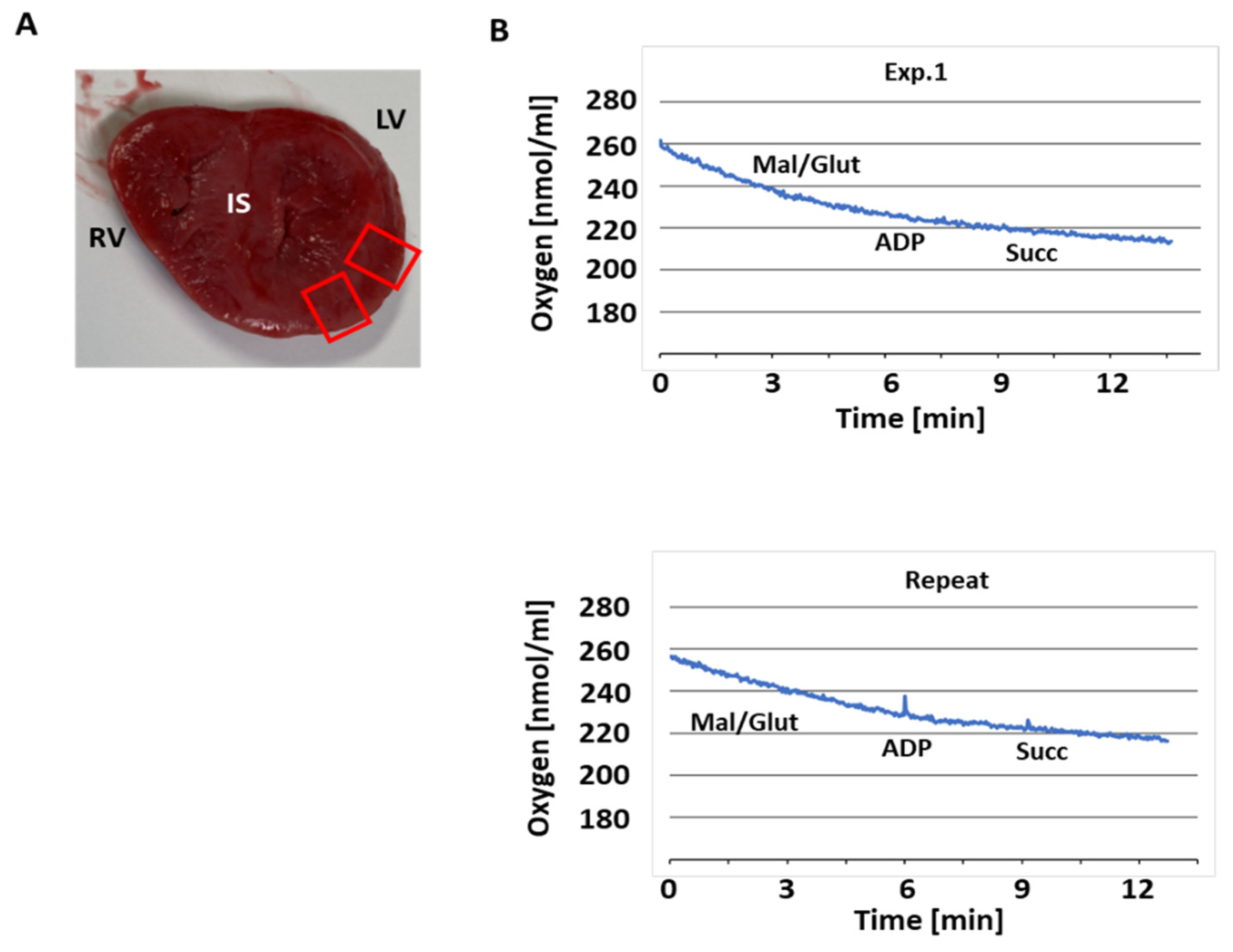
3.1. Sample Processing
3.2. Respiratory Activity
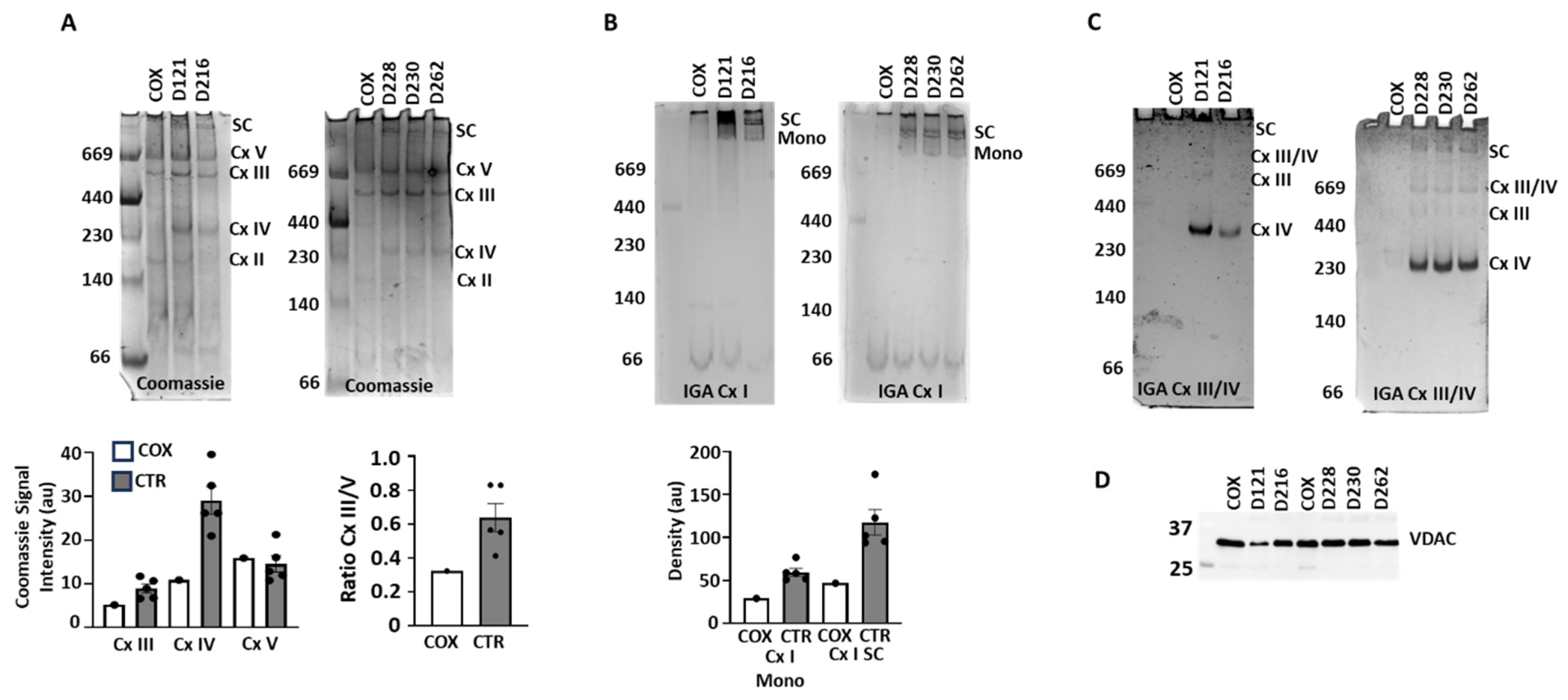
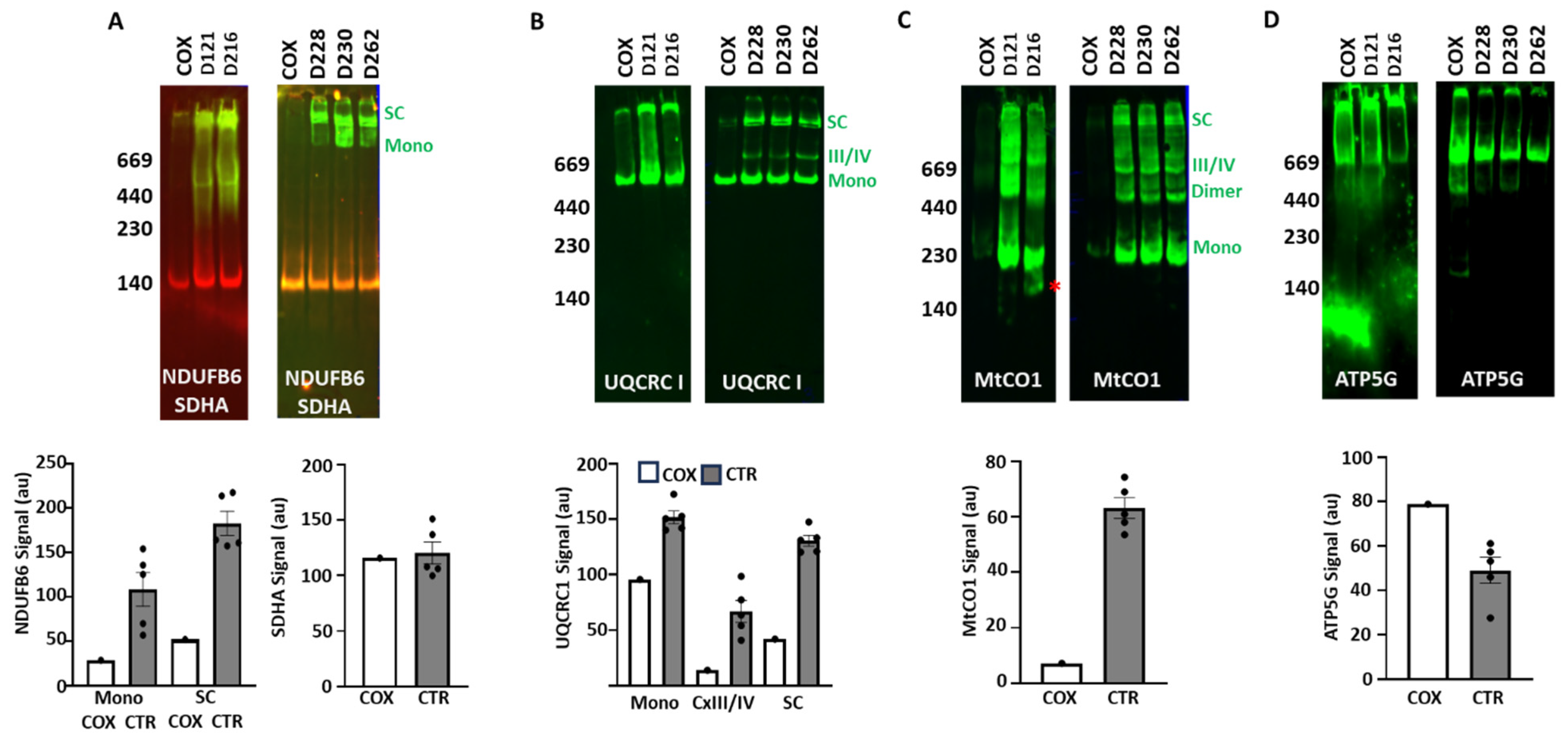
3.3. ETC Complex and Supercomplex Assembly
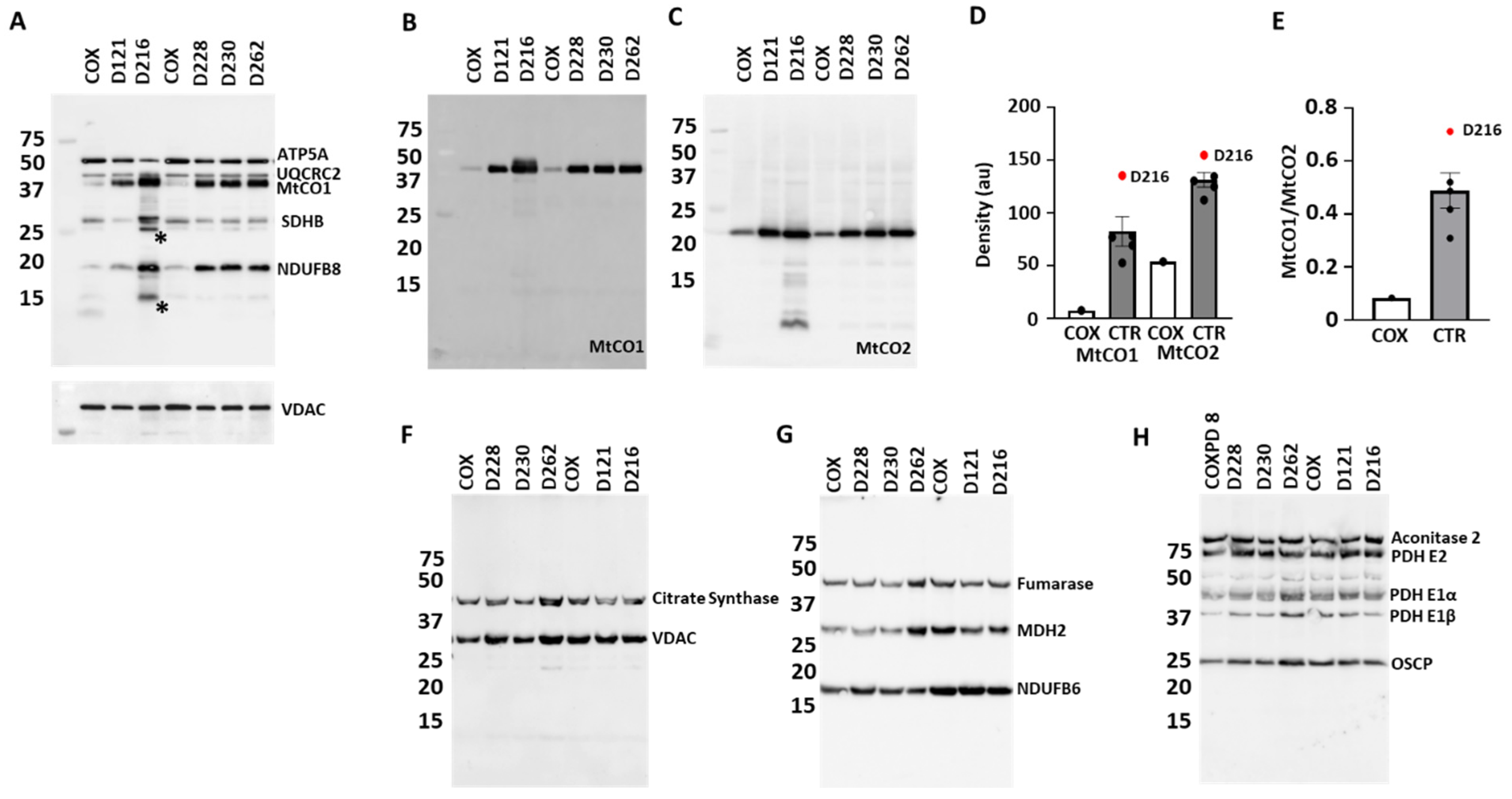
3.4. Protein Expression
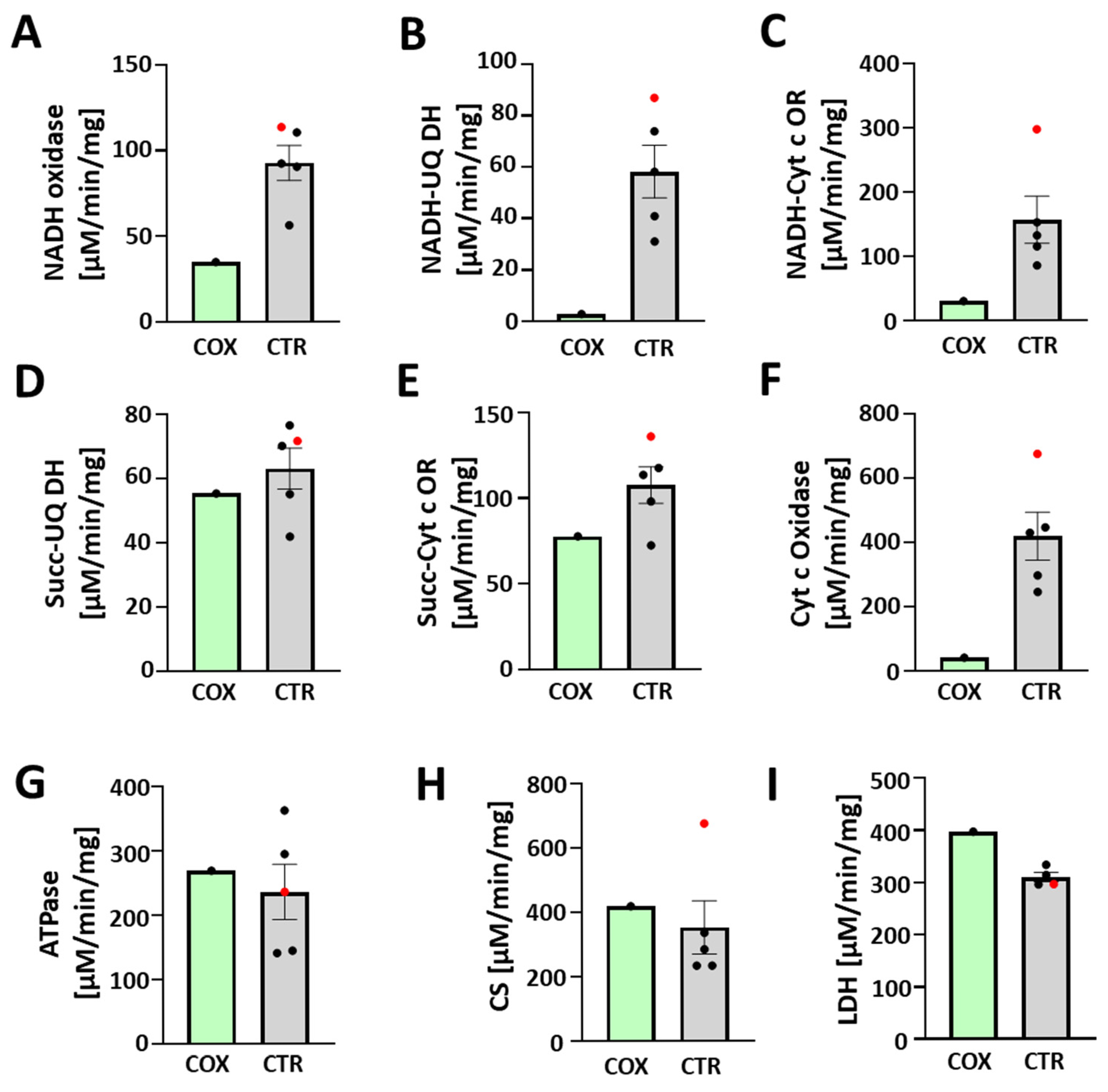
3.5. Enzymatic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AARS2 | Mitochondrial alanyl-tRNA synthetase gene |
| BRINDL | BioRepository for Investigation of Neonatal Diseases of the Lung |
| COXPD8 | Combined oxidative phosphorylation deficiency type 8 |
| Cx | ETC complex |
| DOL | Day of life |
| ECMO | Extracorporeal membrane oxygenation |
| ETC | Electron transport chain |
| g | Gram |
| HIE | Hypoxic–ischemic encephalopathy |
| IGA | In-gel assay |
| LDH | Lactate dehydrogenase |
| OXPHOS | Oxidative phosphorylation |
| PAGE | Polyacrylamide gel electrophoresis |
| RAC | Radial alveolar counts |
| SC | Supercomplexes |
| TCA | Tricarboxylic cycle |
| V0 | Basal respiration |
| Vmax | Maximal respiration |
References
- Götz, A.; Tyynismaa, H.; Euro, L.; Ellonen, P.; Hyötyläinen, T.; Ojala, T.; Hämäläinen, R.H.; Tommiska, J.; Raivio, T.; Oresic, M.; et al. Exome sequencing identifies mitochondrial alanyl-tRNA synthetase mutations in infantile mitochondrial cardiomyopathy. Am. J. Hum. Genet. 2011, 88, 635–642. [Google Scholar] [CrossRef]
- Sissler, M.; González-Serrano, L.E.; Westhof, E. Recent Advances in Mitochondrial Aminoacyl-tRNA Synthetases and Disease. Trends Mol. Med. 2017, 23, 693–708. [Google Scholar] [CrossRef]
- Taanman, J.W. The mitochondrial genome: Structure, transcription, translation and replication. Biochim. Biophys. Acta 1999, 1410, 103–123. [Google Scholar] [CrossRef]
- Sommerville, E.W.; Zhou, X.-L.; Oláhová, M.; Jenkins, J.; Euro, L.; Konovalova, S.; Hilander, T.; Pyle, A.; He, L.; Habeebu, S.; et al. Instability of the mitochondrial alanyl-tRNA synthetase underlies fatal infantile-onset cardiomyopathy. Hum. Mol. Genet. 2019, 28, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Lapuente-Brun, E.; Moreno-Loshuertos, R.; Acín-Pérez, R.; Latorre-Pellicer, A.; Colás, C.; Balsa, E.; Perales-Clemente, E.; Quirós, P.M.; Calvo, E.; Rodríguez-Hernández, M.A.; et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 2013, 340, 1567–1570. [Google Scholar] [CrossRef]
- van Helden, R.W.; Birket, M.J.; Freund, C.; Arendzen, C.H.; Mikkers, H.M.; Orlova, V.; de Coo, R.I.; Mummery, C.L.; Bellin, M. Generation of three human induced pluripotent stem cell lines, LUMCi024-A, LUMCi025-A, and LUMCi026-A, from two patients with combined oxidative phosphorylation deficiency 8 and a related control. Stem Cell Res. 2021, 53, 102374. [Google Scholar] [CrossRef]
- Acín-Pérez, R.; Bayona-Bafaluy, M.P.; Fernández-Silva, P.; Moreno-Loshuertos, R.; Pérez-Martos, A.; Bruno, C.; Moraes, C.T.; A Enríquez, J. Respiratory complex III is required to maintain complex I in mammalian mitochondria. Mol. Cell 2004, 13, 805–815. [Google Scholar] [CrossRef]
- Pryhuber, G.S. Donor Acceptance Criteria for URMC HTC SenNet Inclusion; Protocolsio: Berkeley, CA, USA, 2023. [Google Scholar] [CrossRef]
- Pryhuber, G.S.; Huyck, H.; Krenitsky, D. URMC HTC Formalin-Inflated, Paraffin-Embedded Human Lung Tissue; Protocolsio: Berkeley, CA, USA, 2022. [Google Scholar] [CrossRef]
- Pryhuber, G.S.; Huyck, H. URMC_HTC_Lung and Lobe Processing for SenNet; Protocolsio: Berkeley, CA, USA, 2023. [Google Scholar] [CrossRef]
- Pryhuber, G.S.; Poole, C. HTC_H&E Stain (Paraffin or Cryosections); Protocolsio: Berkeley, CA, USA, 2022. [Google Scholar] [CrossRef]
- Cooney, T.P.; Thurlbeck, W.M. The radial alveolar count method of Emery and Mithal: A reappraisal 1—Postnatal lung growth. Thorax 1982, 37, 572–579. [Google Scholar] [CrossRef]
- Bandyopadhyay, G.; Jehrio, M.G.; Baker, C.; Bhattacharya, S.; Misra, R.S.; Huyck, H.L.; Chu, C.; Myers, J.R.; Ashton, J.; Polter, S.; et al. Bulk RNA sequencing of human pediatric lung cell populations reveals unique transcriptomic signature associated with postnatal pulmonary development. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 326, L604–L617. [Google Scholar] [CrossRef] [PubMed]
- Beutner, G.; Burris, J.R.; Collins, M.P.; Kulkarni, C.A.; Nadtochiy, S.M.; Bentley, K.L.d.M.; Cohen, E.D.; Brookes, P.S.; Porter, G.A. Coordinated metabolic responses to cyclophilin D deletion in the developing heart. iScience 2024, 27, 109157. [Google Scholar] [CrossRef]
- Beutner, G.; Porter, G.A., Jr. Analyzing Supercomplexes of the Mitochondrial Electron Transport Chain with Native Electrophoresis, In-gel Assays, and Electroelution. J. Vis. Exp. 2017, 124, 55738. [Google Scholar]
- Wittig, I.; Carrozzo, R.; Santorelli, F.M.; Schägger, H. Functional assays in high-resolution clear native gels to quantify mitochondrial complexes in human biopsies and cell lines. Electrophoresis 2007, 28, 3811–3820. [Google Scholar] [CrossRef]
- Beutner, G.; Porter, G.A., Jr. Native Gel Electrophoresis and Immunoblotting to Analyze Electron Transport Chain Complexes. Methods Mol. Biol. 2021, 2276, 103–112. [Google Scholar]
- Kirby, D.M.; Thorburn, D.R.; Turnbull, D.M.; Taylor, R.W. Biochemical assays of respiratory chain complex activity. Methods Cell Biol. 2007, 80, 93–119. [Google Scholar]
- Spinazzi, M.; Casarin, A.; Pertegato, V.; Salviati, L.; Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012, 7, 1235–1246. [Google Scholar] [CrossRef]
- Pryce, J.W.; Bamber, A.R.; Ashworth, M.T.; Kiho, L.; Malone, M.; Sebire, N.J. Reference ranges for organ weights of infants at autopsy: Results of >1,000 consecutive cases from a single centre. BMC Clin. Pathol. 2014, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Drose, S.; Stepanova, A.; Galkin, A. Ischemic A/D transition of mitochondrial complex I and its role in ROS generation. Biochim. Biophys. Acta 2016, 1857, 946–957. [Google Scholar] [CrossRef]
- Galkin, A.; Abramov, A.Y.; Frakich, N.; Duchen, M.R.; Moncada, S. Lack of oxygen deactivates mitochondrial complex I: Implications for ischemic injury? J. Biol. Chem. 2009, 284, 36055–36061. [Google Scholar] [CrossRef]
- Xu, X.; Jin, K.; Bais, A.S.; Zhu, W.; Yagi, H.; Feinstein, T.N.; Nguyen, P.K.; Criscione, J.D.; Liu, X.; Beutner, G.; et al. Uncompensated mitochondrial oxidative stress underlies heart failure in an iPSC-derived model of congenital heart disease. Cell Stem Cell 2022, 29, 840–855.e7. [Google Scholar] [CrossRef] [PubMed]
- Euro, L.; Konovalova, S.; Asin-Cayuela, J.; Tulinius, M.; Griffin, H.; Horvath, R.; Taylor, R.W.; Chinnery, P.F.; Schara, U.; Thorburn, D.R.; et al. Structural modeling of tissue-specific mitochondrial alanyl-tRNA synthetase (AARS2) defects predicts differential effects on aminoacylation. Front. Genet. 2015, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schrøder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef]
- Reisch, A.S.; Elpeleg, O. Biochemical assays for mitochondrial activity: Assays of TCA cycle enzymes and PDHc. Methods Cell Biol. 2007, 80, 199–222. [Google Scholar]
- Rahman, S. Mitochondrial disease in children. J. Intern. Med. 2020, 287, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Gorman, G.S.; Schaefer, A.M.; Ng, Y.; Gomez, N.; Blakely, E.L.; Alston, C.L.; Feeney, C.; Horvath, R.; Yu-Wai-Man, P.; Chinnery, P.F.; et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 2015, 77, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Lamont, P.J.; Surtees, R.; Woodward, C.E.; Leonard, J.V.; Wood, N.W.; Harding, A.E. Clinical and laboratory findings in referrals for mitochondrial DNA analysis. Arch. Dis. Child. 1998, 79, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Kristensen, J.M.; Stride, N.; Wojtaszewski, J.F.P.; Helge, J.W.; Dela, F. Skeletal muscle mitochondrial respiration in AMPKalpha2 kinase-dead mice. Acta Physiol. 2012, 205, 314–320. [Google Scholar] [CrossRef]
- Leong, D.W.; Komen, J.C.; Hewitt, C.A.; Arnaud, E.; McKenzie, M.; Phipson, B.; Bahlo, M.; Laskowski, A.; Kinkel, S.A.; Davey, G.M.; et al. Proteomic and metabolomic analyses of mitochondrial complex I-deficient mouse model generated by spontaneous B2 short interspersed nuclear element (SINE) insertion into NADH dehydrogenase (ubiquinone) Fe-S protein 4 (Ndufs4) gene. J. Biol. Chem. 2012, 287, 20652–20663. [Google Scholar] [CrossRef]
| ID | Gestational Age (Weeks) | Sex | Age at Death (Days) | Weight (Percentile) | Cause of Death | WIT/CIT * (Hours) |
|---|---|---|---|---|---|---|
| D228 | 37 | M | 1 | 27 | Bladder outlet obstruction, pulmonary hypoplasia, hypertensive changes in pulmonary arteries | 2/21 |
| D262 | 33 | M | 1 | 13 | Anencephaly, normal lung | 3/19 |
| D230 | 37 | M | 10 | <3 | Anencephaly, normal lung | 4/27 |
| D121 | 40 | F | 35 | <3 | Hydranencephaly due to in utero vascular accident, normal lung | 6/20 |
| D455 | 38 | M | 51 | 5 | COXPD8, pulmonary hypoplasia, hypertensive changes in pulmonary arteries | 3/31 |
| D216 | 36 | M | 157 | 90 | Neonatal abstinence, NICU × 11 days; + respiratory virus, severe and persistent metabolic acidosis, normal lung with patchy lymphocytic inflammation | 0/30 |
| Antibody | Dilution | Source | Catalogue Number # | Comments |
|---|---|---|---|---|
| Aconitase 2 | 1:1000 | Thermo Fisher Scientific (Waltham, MA, USA) | PA5-29960 | |
| ATP5G | 1:2000 | Abcam (Hong Kong, China) | Ab180149 | |
| Citrate Synthase | 1:1000 | Cell Signaling Technology (Danvers, MA, USA) | 14309 | |
| Fumarase | 1:500 | Thermo Fisher Scientific | PA5-82899 | |
| MtCO1 | 1:2000 | Thermo Fisher Scientific | 459600 | |
| MtCO2 | 1:2000 | Thermo Fisher Scientific | MA5-57085 | |
| NDUFB6 | 1:1000 | Abcam | Ab110244 | |
| OxPhos Cocktail | 1:1000 | Abcam | Ab110413 | Mixture of anti-NDUFB8, SDHB, UQCRC2, MtCO1, and ATP5A |
| PDH | 1:1000 | Thermo Fisher Scientific | 456799 | Mixture of anti-PDH E1β, PDH E1α, PDH E2 and OSCP |
| SDHA | 1:4000 | Thermo Fisher Scientific | 459200 | |
| UQCRC1 | 1:1000 | Thermo Fisher Scientific | 459140 | |
| VDAC | 1:2000 | Abcam | Ab154856 |
| ID | Heart Weight Observed (g) | Heart Weight Expected (g) 50%ile (5–95%ile) | RAC | Expected RAC |
|---|---|---|---|---|
| D228 | 17.3 | 20 (13–30) | 3.3 | 4 |
| D262 | 10.7 | 20 (13–30) | 3.2 | 3 |
| D230 | 7.5 | 22 (15–32) | 4.2 | 4 |
| D121 | 17.9 | 26 (19–36) | 5.0 | 5 |
| D455 | 73.4 | 28 (20–38) | 3.5 | 5–6 |
| D216 | 49.4 | 38 (28–48) | 7.9 | 7–8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beutner, G.; Huyck, H.L.; Deutsch, G.; Pryhuber, G.S.; Porter Jr., G.A. Dysfunctional Electron Transport Chain Assembly in COXPD8. J. Cardiovasc. Dev. Dis. 2025, 12, 318. https://doi.org/10.3390/jcdd12080318
Beutner G, Huyck HL, Deutsch G, Pryhuber GS, Porter Jr. GA. Dysfunctional Electron Transport Chain Assembly in COXPD8. Journal of Cardiovascular Development and Disease. 2025; 12(8):318. https://doi.org/10.3390/jcdd12080318
Chicago/Turabian StyleBeutner, Gisela, Heidie L. Huyck, Gail Deutsch, Gloria S. Pryhuber, and George A. Porter Jr. 2025. "Dysfunctional Electron Transport Chain Assembly in COXPD8" Journal of Cardiovascular Development and Disease 12, no. 8: 318. https://doi.org/10.3390/jcdd12080318
APA StyleBeutner, G., Huyck, H. L., Deutsch, G., Pryhuber, G. S., & Porter Jr., G. A. (2025). Dysfunctional Electron Transport Chain Assembly in COXPD8. Journal of Cardiovascular Development and Disease, 12(8), 318. https://doi.org/10.3390/jcdd12080318








