Late Gadolinium Enhancement Variation in Asymptomatic Individuals: Comparison with Dilated Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
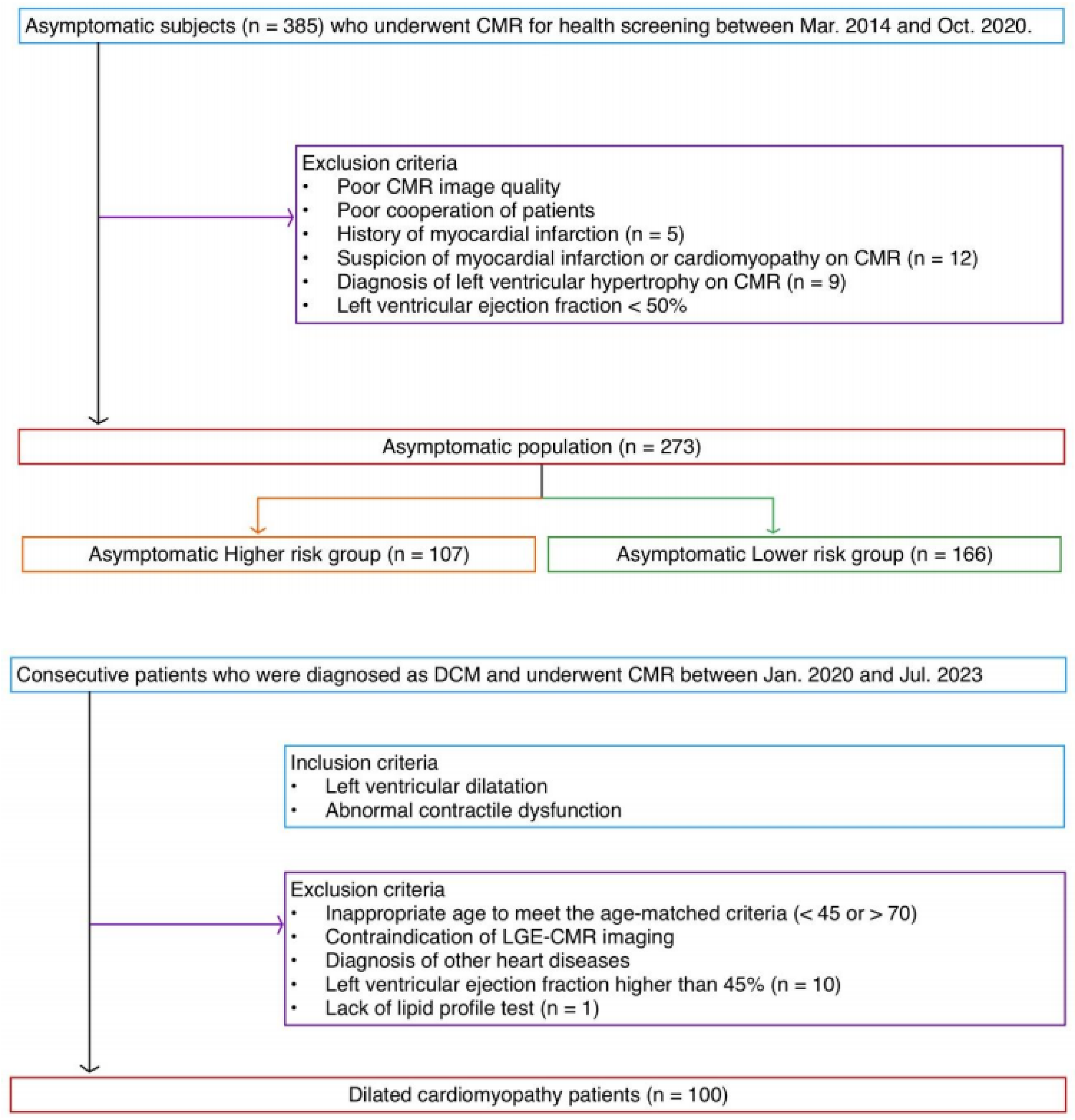
2.1. Population
2.2. CMR Technique
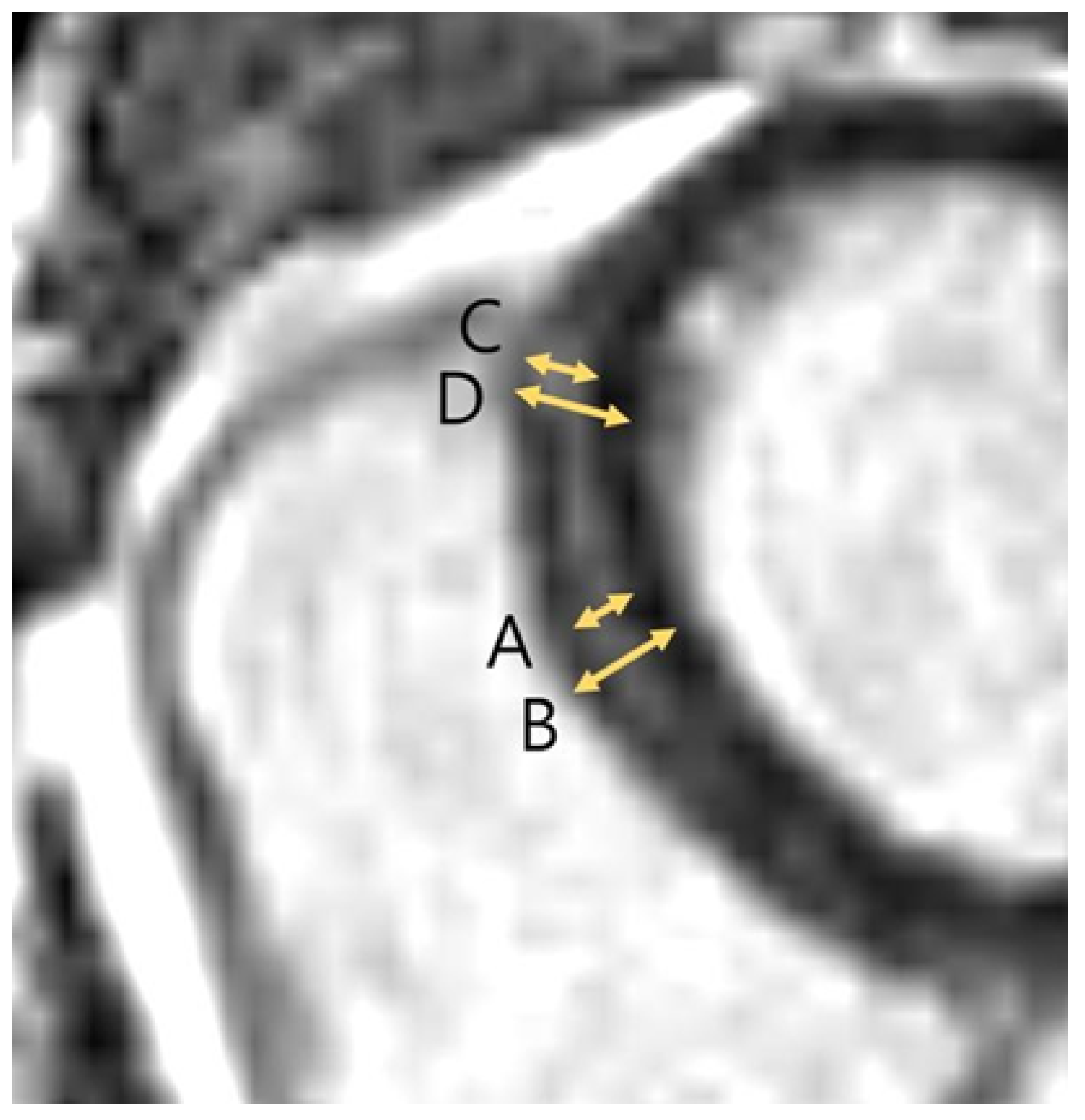
2.3. Image Analysis
2.4. Clinical Information
2.5. Statistical Analysis
3. Results
3.1. Demographics
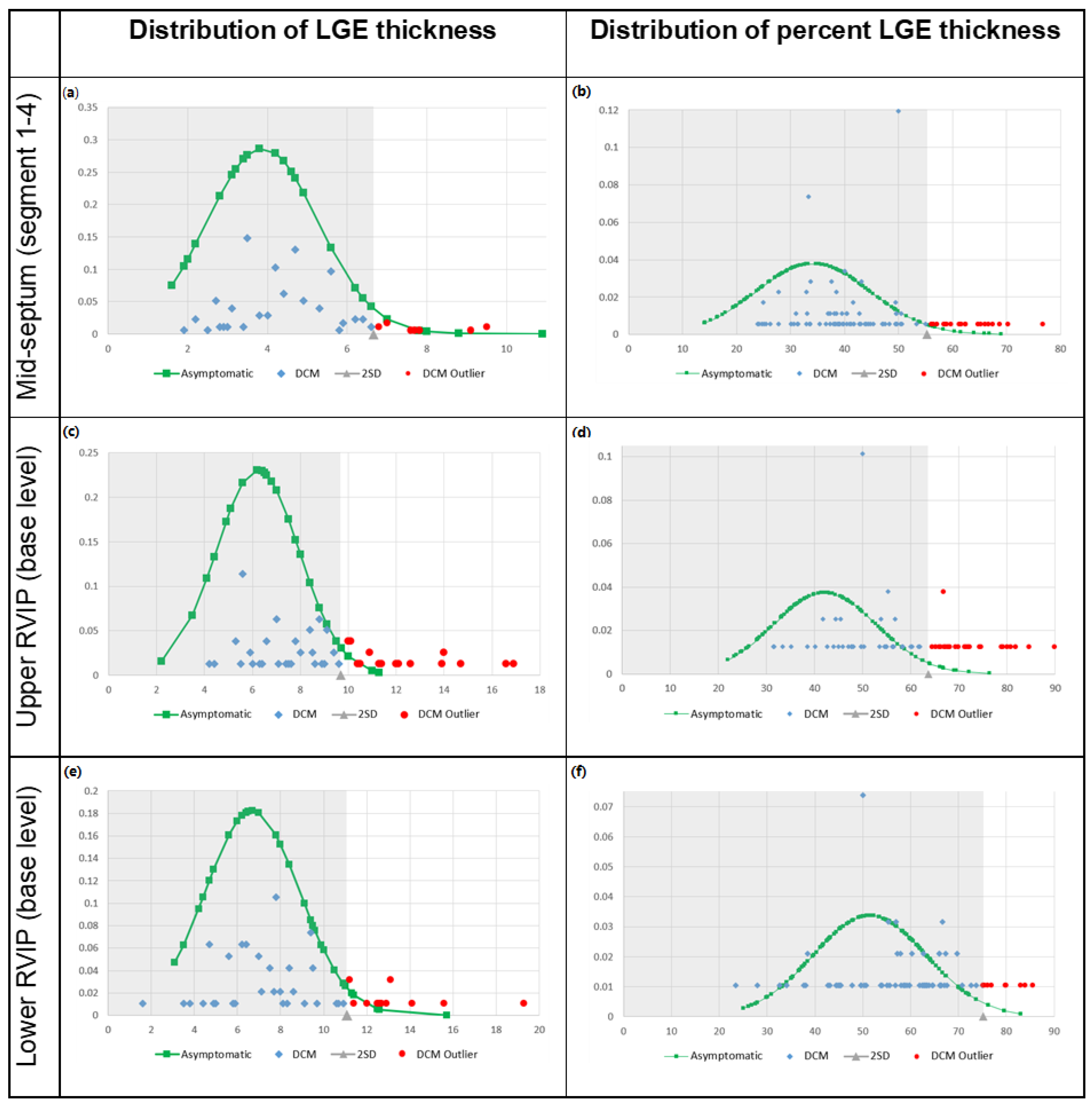
3.2. LGE Characteristics
3.3. Overlap Percentage of LGE Extent
3.4. Cut-Off Value for Pathological LGE
3.5. ASCVD Risk Score
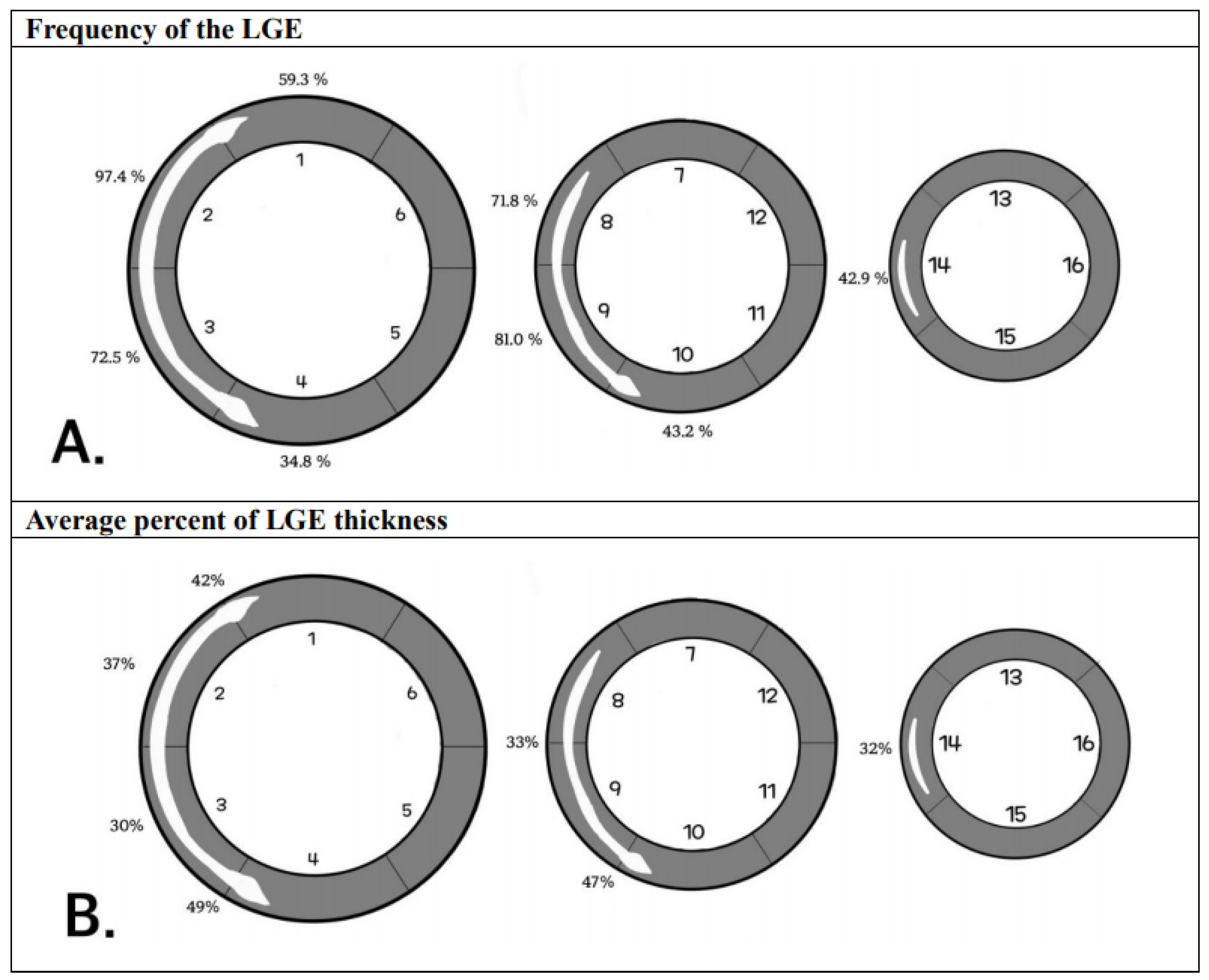
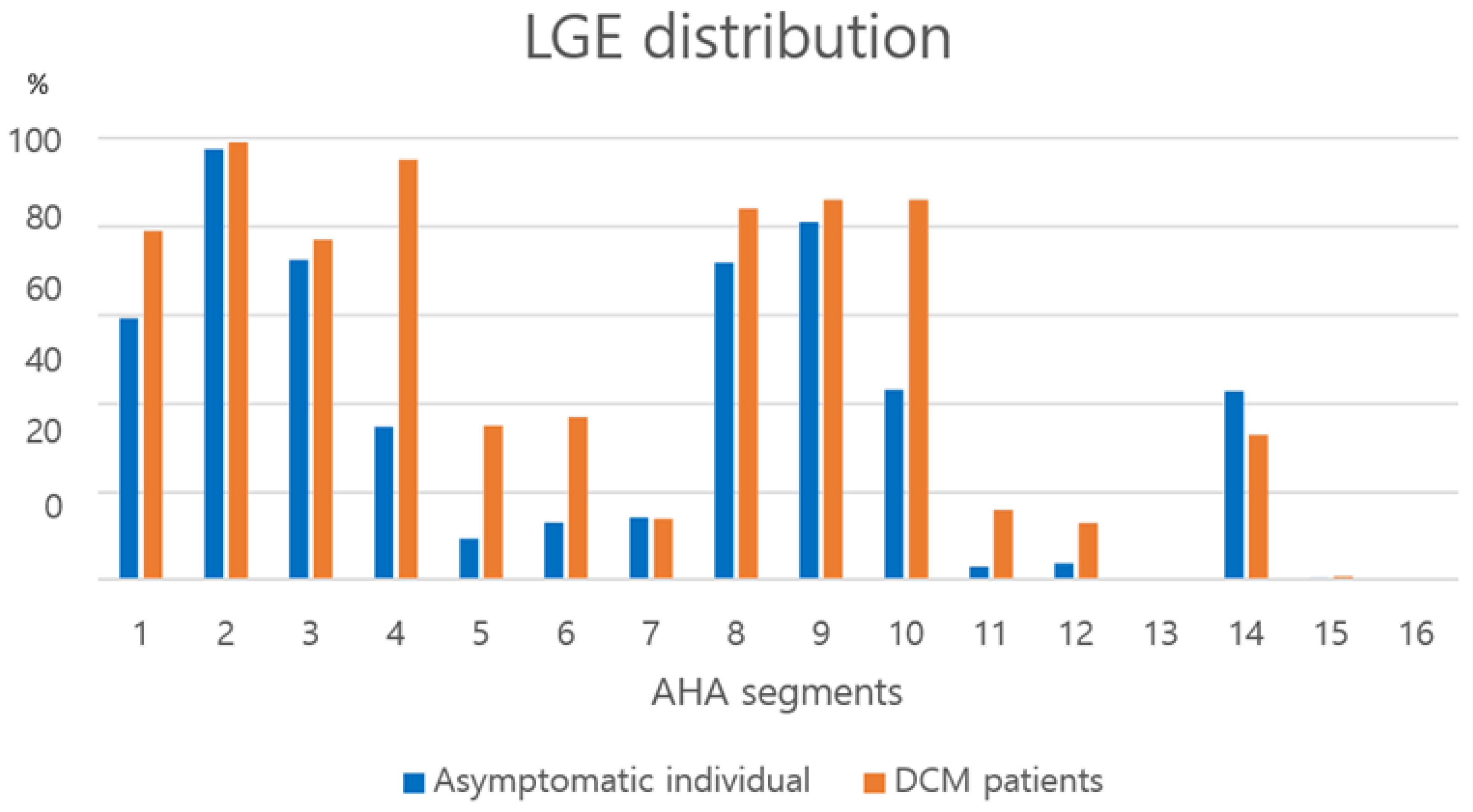
3.6. LGE Distribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHA | American Heart Association |
| ASCVD | Atherosclerotic cardiovascular disease |
| CMR | Cardiac magnetic resonance |
| DCM | Dilated cardiomyopathy |
| LGE | Late gadolinium enhancement |
| LVEF | Left ventricular ejection fraction |
| RVIP | Right ventricular insertion point |
References
- Becker, M.A.J.; van der Lingen, A.C.J.; Cornel, J.H.; van de Ven, P.M.; van Rossum, A.C.; Allaart, C.P.; Germans, T. Septal Midwall Late Gadolinium Enhancement in Ischemic Cardiomyopathy and Nonischemic Dilated Cardiomyopathy-Characteristics and Prognosis. Am. J. Cardiol. 2023, 201, 294–301. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Lazzeroni, D.; Palmisano, A.; Gigli, L.; Esposito, A.; De Cobelli, F.; Camici, P.G.; Mazzone, P.; Basso, C.; et al. Septal Late Gadolinium Enhancement and Arrhythmic Risk in Genetic and Acquired Non-Ischaemic Cardiomyopathies. Heart Lung Circ. 2020, 29, 1356–1365. [Google Scholar] [CrossRef]
- Anagnostopoulos, I.; Kousta, M.; Kossyvakis, C.; Lakka, E.; Paraskevaidis, N.T.; Schizas, N.; Alexopoulos, N.; Deftereos, S.; Giannopoulos, G. The Prognostic Role of Late Gadolinium Enhancement on Cardiac Magnetic Resonance in Patients with Nonischemic Cardiomyopathy and Reduced Ejection Fraction, Implanted with Cardioverter Defibrillators for Primary Prevention. A Systematic Review and Meta-Analysis. J. Interv. Card. Electrophysiol. 2022, 63, 523–530. [Google Scholar]
- Theerasuwipakorn, N.; Chokesuwattanaskul, R.; Phannajit, J.; Marsukjai, A.; Thapanasuta, M.; Klem, I.; Chattranukulchai, P. Impact of Late Gadolinium-Enhanced Cardiac Mri on Arrhythmic and Mortality Outcomes in Nonischemic Dilated Cardiomyopathy: Updated Systematic Review and Meta-Analysis. Sci. Rep. 2023, 13, 13775. [Google Scholar] [CrossRef] [PubMed]
- Alba, A.C.; Gaztañaga, J.; Foroutan, F.; Thavendiranathan, P.; Merlo, M.; Alonso-Rodriguez, D.; Vallejo-García, V.; Vidal-Perez, R.; Corros-Vicente, C.; Barreiro-Pérez, M.; et al. Prognostic Value of Late Gadolinium Enhancement for the Prediction of Cardiovascular Outcomes in Dilated Cardiomyopathy: An International, Multi-Institutional Study of the Minicor Group. Circ. Cardiovasc. Imaging 2020, 13, e010105. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.Y.; He, W.F.; Zhang, T.Y.; Wang, L.L.; Yang, F.; Feng, Y.L.; Li, C.P.; Li, R. Association between Late Gadolinium Enhancement and Outcome in Dilated Cardiomyopathy: A Meta-Analysis. World J. Radiol. 2023, 15, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Infante, A.N.; Koo, C.C.Y.; Yip, A.; Lim, Y.H.; Yeo, W.T.; Quek, S.T.; Lim, T.W.; Seow, S.C.; Chai, P.; Ong, C.C.; et al. Magnetic Resonance Imaging of Dilated Cardiomyopathy: Prognostic Benefit of Identifying Late Gadolinium Enhancement in Asian Patients. Singap. Med. J. 2021, 62, 347–352. [Google Scholar] [CrossRef]
- Barison, A.; Aimo, A.; Mirizzi, G.; Castiglione, V.; Ripoli, A.; Panchetti, L.; Rossi, A.; Giannoni, A.; Startari, U.; Aquaro, G.D.; et al. The Extent and Location of Late Gadolinium Enhancement Predict Defibrillator Shock and Cardiac Mortality in Patients with Non-Ischaemic Dilated Cardiomyopathy. Int. J. Cardiol. 2020, 307, 180–186. [Google Scholar] [CrossRef]
- Kim, E.K.; Lee, G.Y.; Jang, S.Y.; Chang, S.A.; Kim, S.M.; Park, S.J.; Choi, J.O.; Park, S.W.; Choe, Y.H.; Lee, S.C.; et al. The Extent of Late Gadolinium Enhancement Can Predict Adverse Cardiac Outcomes in Patients with Non-Ischemic Cardiomyopathy with Reduced Left Ventricular Ejection Fraction: A Prospective Observational Study. Korean J. Radiol. 2021, 22, 324–333. [Google Scholar] [CrossRef]
- Behera, D.R.; V K, A.K.; K K, N.N.; S, S.; Nair, K.K.M.; G, S.; T, R.K.; Gopalakrishnan, A.; S, H. Prognostic Value of Late Gadolinium Enhancement in Cardiac Mri of Non-Ischemic Dilated Cardiomyopathy Patients. Indian Heart J. 2020, 72, 362–368. [Google Scholar] [CrossRef]
- Sano, M.; Satoh, H.; Suwa, K.; Saotome, M.; Urushida, T.; Katoh, H.; Hayashi, H.; Saitoh, T. Intra-Cardiac Distribution of Late Gadolinium Enhancement in Cardiac Sarcoidosis and Dilated Cardiomyopathy. World J. Cardiol. 2016, 8, 496–503. [Google Scholar] [CrossRef]
- Liu, J.M.; Liu, A.; Leal, J.; McMillan, F.; Francis, J.; Greiser, A.; Rider, O.J.; Myerson, S.; Neubauer, S.; Ferreira, V.M.; et al. Measurement of Myocardial Native T1 in Cardiovascular Diseases and Norm in 1291 Subjects. J. Cardiovasc. Magn. Reson. 2017, 19, 74. [Google Scholar] [CrossRef]
- Kim, M.Y.; Cho, S.J.; Kim, H.J.; Kim, S.M.; Lee, S.C.; Paek, M.; Choe, Y.H. T1 Values and Extracellular Volume Fraction in Asymptomatic Subjects: Variations in Left Ventricular Segments and Correlation with Cardiovascular Risk Factors. Sci. Rep. 2022, 12, 12544. [Google Scholar] [CrossRef]
- Lund, G.K.; Leptin, S.; Ragab, H.; Sinn, M.R.; Fierenz, A.; Cavus, E.; Muellerleile, K.; Chen, H.; Erley, J.; Harms, P.; et al. Prognostic Relevance of Ischemic Late Gadolinium Enhancement in Apparently Healthy Endurance Athletes: A Follow-up Study over 5 Years. Sports Med. Open 2024, 10, 13. [Google Scholar] [CrossRef]
- Ragab, H.; Lund, G.K.; Breitsprecher, L.; Sinn, M.R.; Muellerleile, K.; Cavus, E.; Stehning, C.; Tahir, E.; Blankenberg, S.; Patten, M.; et al. Prevalence and Pattern of Focal and Potential Diffuse Myocardial Fibrosis in Male and Female Marathon Runners Using Contrast-Enhanced Cardiac Magnetic Resonance. Eur. Radiol. 2023, 33, 4648–4656. [Google Scholar] [CrossRef]
- Tahir, E.; Scherz, B.; Starekova, J.; Muellerleile, K.; Fischer, R.; Schoennagel, B.; Warncke, M.; Stehning, C.; Cavus, E.; Bohnen, S.; et al. Acute impact of an endurance race on cardiac function and biomarkers of myocardial injury in triathletes with and without myocardial fibrosis. Eur. J. Prev. Cardiol. 2020, 27, 94–104. [Google Scholar] [CrossRef]
- Domenech-Ximenos, B.; Sanz-de la Garza, M.; Prat-González, S.; Sepúlveda-Martínez, A.; Crispi, F.; Duran-Fernandez, K.; Perea, R.J.; Bijnens, B.; Sitges, M. Prevalence and Pattern of Cardiovascular Magnetic Resonance Late Gadolinium Enhancement in Highly Trained Endurance Athletes. J. Cardiovasc. Magn. Reson. 2020, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; et al. Proposal for a Revised Definition of Dilated Cardiomyopathy, Hypokinetic Non-Dilated Cardiomyopathy, and Its Implications for Clinical Practice: A Position Statement of the Esc Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J. Cardiovasc. Magn. Reson. 2020, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Grigoratos, C.; Pantano, A.; Meschisi, M.; Gaeta, R.; Ait-Ali, L.; Barison, A.; Todiere, G.; Festa, P.; Sinagra, G.; Aquaro, G.D. Clinical Importance of Late Gadolinium Enhancement at Right Ventricular Insertion Points in Otherwise Normal Hearts. Int. J. Cardiovasc. Imaging 2020, 36, 913–920. [Google Scholar] [CrossRef]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Assenza, G.E.; Haas, T.S.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; Rowin, E.; et al. Significance of Late Gadolinium Enhancement at Right Ventricular Attachment to Ventricular Septum in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2015, 116, 436–441. [Google Scholar] [CrossRef]
- Cabanis, P.; Magat, J.; Rodriguez-Padilla, J.; Ramlugun, G.; Yon, M.; Bihan-Poudec, Y.; Pallares-Lupon, N.; Vaillant, F.; Pasdois, P.; Jais, P.; et al. Cardiac Structure Discontinuities Revealed by Ex-Vivo Microstructural Characterization. A Focus on the Basal Inferoseptal Left Ventricle Region. J. Cardiovasc. Magn. Reson. 2023, 25, 78. [Google Scholar] [CrossRef]
- Sato, T.; Tsujino, I.; Ohira, H.; Oyama-Manabe, N.; Nishimura, M. Paradoxical Motion of the Interventricular Septum as a Primary Mechanism of Late Gadolinium Enhancement at Ventricular Insertion Points. Int. J. Cardiol. 2012, 158, 156–157. [Google Scholar] [CrossRef]
- Swift, A.J.; Rajaram, S.; Capener, D.; Elliot, C.; Condliffe, R.; Wild, J.M.; Kiely, D.G. LGE Patterns in Pulmonary Hypertension Do Not Impact Overall Mortality. JACC Cardiovasc. Imaging 2014, 7, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, B.A.; Vandenberk, B.; Dykstra, S.; Labib, D.; Chew, D.S.; Lydell, C.; Howarth, A.; Heydari, B.; Fine, N.; Howlett, J.; et al. Patients with Non-Ischemic Cardiomyopathy and Mid-Wall Striae Have Similar Arrhythmic Outcomes as Ischemic Cardiomyopathy. Int. J. Cardiovasc. Imaging 2023, 39, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Halliday, B.P.; Baksi, A.J.; Gulati, A.; Ali, A.; Newsome, S.; Izgi, C.; Arzanauskaite, M.; Lota, A.; Tayal, U.; Vassiliou, V.S.; et al. Outcome in Dilated Cardiomyopathy Related to the Extent, Location, And pattern of Late Gadolinium Enhancement. JACC Cardiovasc. Imaging 2019, 12, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Mulla, W.; Segev, A.; Novak, A.; Yogev, D.; Abu-Much, A.; Fardman, A.; Massalha, E.; Goietin, O.; Kuperstein, R.; Matetzky, S.; et al. Significance of Anteroseptal Late Gadolinium Enhancement among Patients with Acute Myocarditis. Am. J. Cardiol. 2023, 199, 18–24. [Google Scholar] [CrossRef]
- Nakamura, M.; Kido, T.; Hirai, K.; Tabo, K.; Tanabe, Y.; Kawaguchi, N.; Kurata, A.; Kido, T.; Yamaguchi, O.; Mochizuki, T. What Is the Mid-Wall Linear High Intensity “Lesion” on Cardiovascular Magnetic Resonance Late Gadolinium Enhancement? J. Cardiovasc. Magn. Reson. 2020, 22, 66. [Google Scholar] [CrossRef]
- Ma, P.; Shang, Y.; Hu, Y.; Liu, J.; Zhou, X.; Wang, J. Linear Late Gadolinium Enhancement in the Basal Anterior Septum and Lateral Wall May Represent the Contrast Enhancement of Vessels: A CMR and CCTA Comparison Study. J. Cardiol. 2022, 79, 581–587. [Google Scholar] [CrossRef]

| Characteristics | Asymptomatic Individuals (n = 273) | DCM Patients (n = 100) | p-Value |
|---|---|---|---|
| Age (Y) | 54.3 ± 5.8 | 55.3 ± 4.9 | 0.107 |
| Age range (Y) | [44, 82] | [47, 70] | |
| Male (%) | 209 (76.6%) | 73 (73%) | 0.216 |
| BMI (kg/m2) | 24.5 ± 2.8 | 23.1 ± 3.8 | 0.001 |
| Hypertension | 103 (37.7%) | 37 (37%) | 0.948 |
| Diabetes | 57 (20.9%) | 33 (33%) | 0.008 |
| Hypercholesterolemia | 98 (35.9%) | 22 (22%) | 0.002 |
| Smoking | 152 (55.7%) | 59 (59%) | 0.567 |
| Alcohol use | 212 (77.7%) | 46 (46%) | <0.001 |
| Atrial fibrillation | 0 | 14 (14%) | |
| LBBB | 0 | 7 (7%) | |
| ASCVD risk score | 7.6 ± 6.2 | 8.4 ± 6.5 | 0.292 |
| Low risk (0–4.9) | 112 (41.0%) | 41 (41%) | |
| Borderline risk (5–7.4) | 54 (19.8%) | 14 (14%) | |
| Intermediate risk (7.5–20) | 93 (34.1%) | 38 (38%) | |
| High risk (>20) | 14 (5.1%) | 7 (7%) | |
| LVEF (%) | 66.0 ± 5.5 | 26.5 ± 9.6 | <0.001 |
| LVESVi (mL/m2) | 68.5 ± 10.0 | 167.0 ± 117.8 | <0.001 |
| LVEDVi (mL/m2) | 23.5 ± 5.9 | 116.8 ± 43.8 | <0.001 |
| LVMi (g/m2) | 58.0 ± 10.9 | 104.4 ± 100.3 | <0.001 |
| RVEF (%) | 58.9 ± 6.3 | 41.7 ± 15.0 | <0.001 |
| NT-proBNP (mg/dL) | 27.2 ± 24.5 | 2578.8 ± 3804.8 | <0.001 |
| LGE presence (%) | 99.267 | 100 |
| LGE Characteristics | Asymptomatic Individuals (n = 273) | DCM Patients (n = 100) | p-Value |
|---|---|---|---|
| Average number of LGE segments | 5.5 ± 1.7 | 7.6 ± 2.2 | <0.001 |
| Maximal thickness of upper RVIP LGE stripes | 6.1 ± 1.9 mm (n = 239) | 8.7 ± 2.7 mm (n = 80) | <0.001 |
| % thickness of upper RVIP basal LGE stripes | 42.2 ± 10.6% (n = 237) | 57.5 ± 13.0% (n = 79) | <0.001 |
| % thickness of upper RVIP middle LGE stripes | 44.3 ± 10.1% (n = 39) | 59.4 ± 15.8% (n = 14) | 0.005 |
| % thickness of upper RVIP LGE stripes | 42.5 ± 10.6% | 57.8 ± 13.4% | <0.001 |
| Maximal thickness of mid-septal LGE stripes | 4.5 ± 1.3 mm (n = 270) | 5.7 ± 1.8 mm (n = 100) | <0.001 |
| % thickness of mid-septal segment 1, 2 stripes | 37.0 ± 9.5% (n = 267) | 44.3 ± 10.4% (n = 99) | <0.001 |
| % thickness of mid-septal segment 3, 4 stripes | 29.5 ± 9.3% (n = 197) | 39.9 ± 9.9% (n = 77) | <0.001 |
| % thickness of mid-septal segment 8, 9 stripes | 32.9 ± 9.8% (n = 239) | 44.2 ± 12.2% (n = 87) | <0.001 |
| % thickness of mid-septal segment 14 stripes | 32.2 ± 7.9% (n = 117) | 42.6 ± 11.7% (n = 33) | <0.001 |
| % thickness of mid-septal stripes | 33.3 ± 9.8% | 42.9 ± 11.1% | <0.001 |
| Maximal thickness of lower RVIP LGE stripes | 6.4 ± 2.3 mm (n = 266) | 8.6 ± 2.8 mm (n = 99) | <0.001 |
| % thickness of lower RVIP basal LGE stripes | 49.4 ± 12.6% (n = 252) | 58.5 ± 12.3% (n = 95) | <0.001 |
| % thickness of lower RVIP middle LGE stripes | 47.1 ± 12.5% (n = 248) | 56.3 ± 13.0% (n = 86) | <0.001 |
| % thickness of lower RVIP LGE stripes | 48.2 ± 12.6% | 57.5 ± 12.6% | <0.001 |
| LGE Thickness | % LGE Thickness | |
|---|---|---|
| Mid-septum | 80.7% | 82.1% |
| Segment 1, 2 (n = 99) | 94.9% (94/99) | 100% (99/99) |
| Segment 3, 4 (n = 77) | 85.7% (66/77) | 72.7% (56/77) |
| Segment 8, 9 (n = 87) | 74.7% (65/87) | 78.2% (68/87) |
| Segment 14 (n = 33) | 42.4% (14/33) | 60.6% (20/33) |
| Upper RVIP | 75.3% | 63.4% |
| Upper RVIP base level (n = 79) | 73.4% (58/79) | 64.6% (51/79) |
| Upper RVIP middle level (n = 14) | 85.7% (12/14) | 57.1% (8/14) |
| Lower RVIP | 80.7% | 88.4% |
| Lower RVIP base level (n = 95) | 84.2% (80/95) | 89.5% (85/95) |
| Lower RVIP middle level (n = 86) | 76.7% (66/86) | 87.2% (75/86) |
| Overall | 79.8% | 81.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Cho, S.J.; Kim, S.M.; Kim, M.Y.; Choe, Y.H. Late Gadolinium Enhancement Variation in Asymptomatic Individuals: Comparison with Dilated Cardiomyopathy. J. Cardiovasc. Dev. Dis. 2025, 12, 312. https://doi.org/10.3390/jcdd12080312
Park S, Cho SJ, Kim SM, Kim MY, Choe YH. Late Gadolinium Enhancement Variation in Asymptomatic Individuals: Comparison with Dilated Cardiomyopathy. Journal of Cardiovascular Development and Disease. 2025; 12(8):312. https://doi.org/10.3390/jcdd12080312
Chicago/Turabian StylePark, Seoyeon, Soo Jin Cho, Sung Mok Kim, Moon Young Kim, and Yeon Hyeon Choe. 2025. "Late Gadolinium Enhancement Variation in Asymptomatic Individuals: Comparison with Dilated Cardiomyopathy" Journal of Cardiovascular Development and Disease 12, no. 8: 312. https://doi.org/10.3390/jcdd12080312
APA StylePark, S., Cho, S. J., Kim, S. M., Kim, M. Y., & Choe, Y. H. (2025). Late Gadolinium Enhancement Variation in Asymptomatic Individuals: Comparison with Dilated Cardiomyopathy. Journal of Cardiovascular Development and Disease, 12(8), 312. https://doi.org/10.3390/jcdd12080312






