Cardiac Magnetic Resonance Imaging in the Evaluation and Prognosis of Infiltrative Cardiomyopathies
Abstract
1. Introduction
2. Basics of Cardiac Magnetic Resonance Imaging
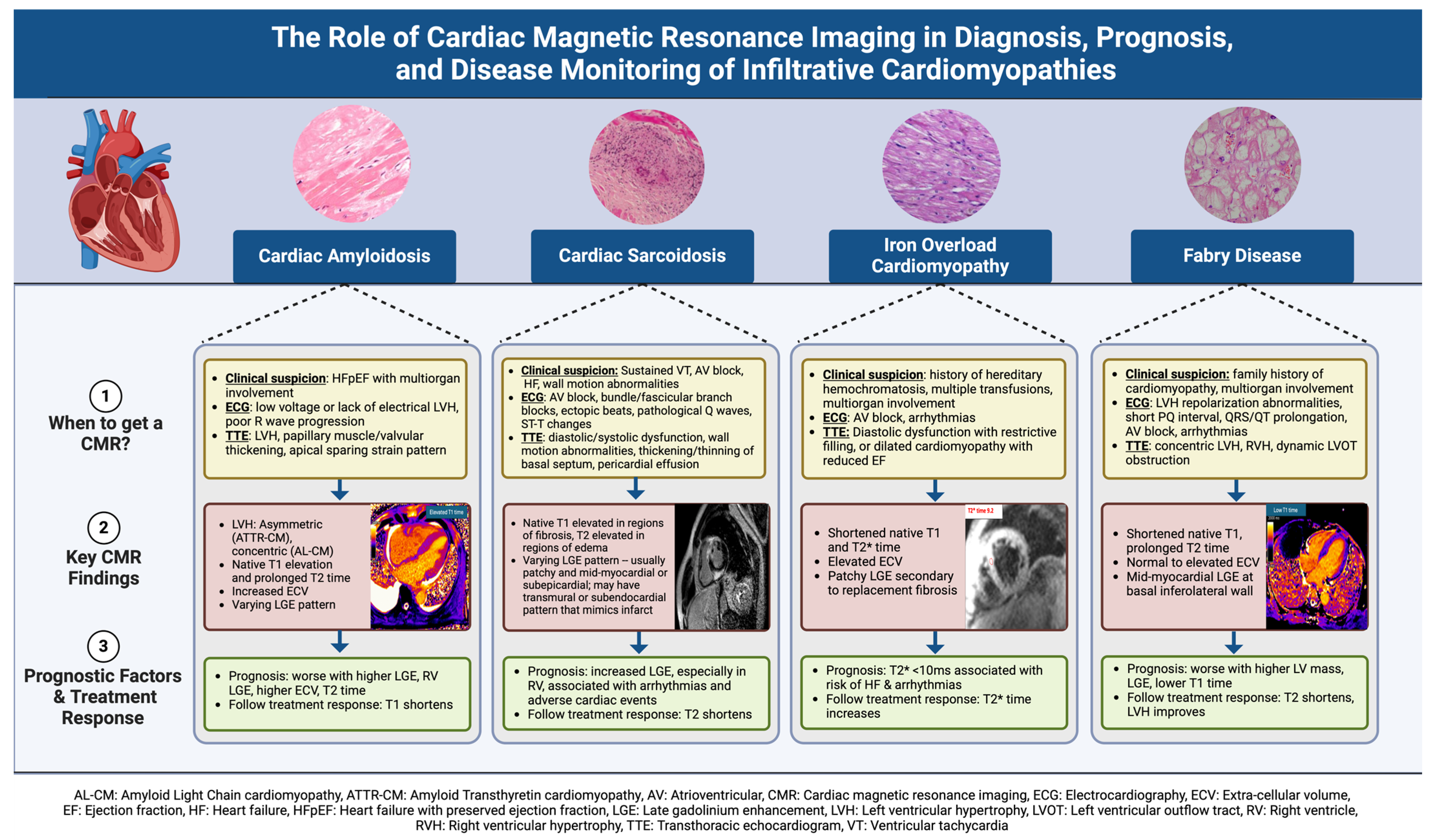
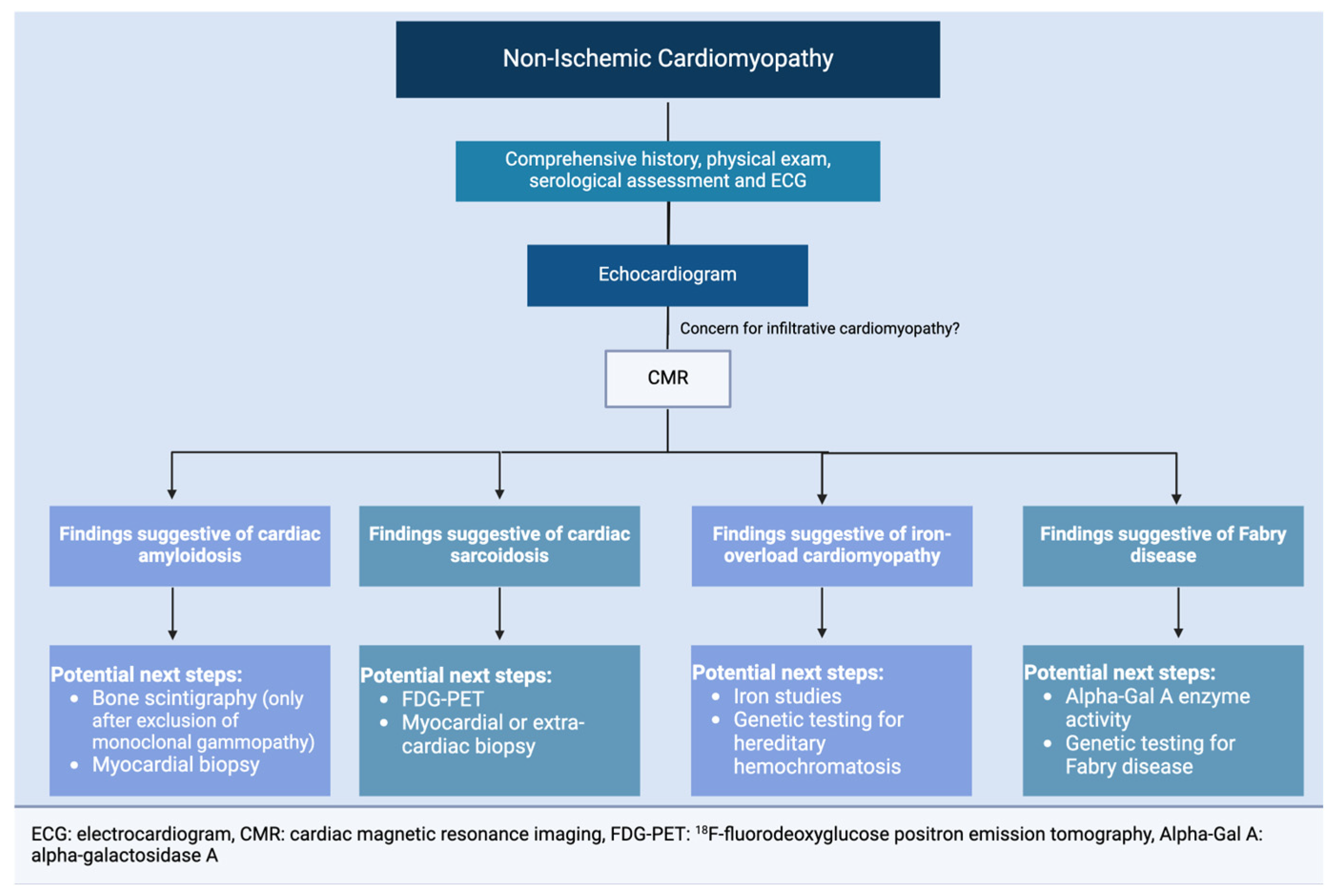
3. Cardiac Amyloidosis
3.1. Pathophysiology and Clinical Presentation
3.2. ECG Findings
3.3. Echocardiography Findings
3.4. CMR Findings
3.5. The Role of CMR in Prognosis and Disease Monitoring
4. Fabry Disease
4.1. Pathophysiology and Clinical Presentation
4.2. ECG Findings
4.3. Echocardiography Findings
4.4. CMR Findings
4.5. The Role of CMR in Prognosis and Disease Monitoring
5. Iron Overload Cardiomyopathy
5.1. Pathophysiology and Clinical Presentation
5.2. ECG Findings
5.3. Echocardiography Findings
5.4. CMR Findings
5.5. The Role of CMR in Prognosis and Disease Monitoring
6. Cardiac Sarcoidosis
6.1. Pathophysiology and Clinical Presentation
6.2. ECG Findings
6.3. Echocardiography Findings
6.4. CMR Findings
6.5. The Role of CMR in Prognosis and Disease Monitoring
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | American College of Cardiology |
| AHA | American Heart Association |
| AL-CM | Amyloid Light Chain Cardiomyopathy |
| alpha-Gal A | alpha-galactosidase A enzyme |
| ATTR-CM | Transthyretin-derived Cardiomyopathy |
| AV | Atrioventricular |
| CM | Cardiomyopathy |
| CMR | Cardiac Magnetic Resonance |
| CS | Cardiac Sarcoidosis |
| ECG | Electrocardiography |
| ECV | Extra-cellular volume |
| EF | Ejection fraction |
| ESC | European Society of Cardiology |
| FD | Fabry Disease |
| FDG-PET | 18F-fluorodeoxyglucose positron emission tomography |
| Gb3 | Globotriaosylceramide |
| Gd | Gadolinium |
| GLS | Global longitudinal strain |
| HCM | Hypertrophic cardiomyopathy |
| HFSA | Heart Failure Society of America |
| IOC | Iron overload cardiomyopathy |
| LGE | Late gadolinium enhancement |
| LV | Left ventricle |
| LVH | Left ventricular hypertrophy |
| LVOT | Left ventricular outflow tract |
| RV | Right Ventricle |
| SSFP | Steady-State Free Precession |
References
- Pereira, N.L.; Grogan, M.; Dec, G.W. Spectrum of Restrictive and Infiltrative Cardiomyopathies. J. Am. Coll. Cardiol. 2018, 71, 1130–1148. [Google Scholar] [CrossRef] [PubMed]
- Bejar, D.; Colombo, P.C.; Latif, F.; Yuzefpolskaya, M. Infiltrative Cardiomyopathies. Clin. Med. Insights Cardiol. 2015, 9, CMC.S19706. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kuhl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2007, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Penugonda, N. Cardiac MRI in Infiltrative Disorders: A Concise Review. Curr. Cardiol. Rev. 2010, 6, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.-Y.I.; Su, M.-Y.M.; Tseng, Y.-H.E. Introduction to Cardiovascular Magnetic Resonance: Technical Principles and Clinical Applications. Acta Cardiol. Sin. 2016, 32, 129–144. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Writing Committee; Kittleson, M.M.; Ruberg, F.L.; Ambardekar, A.V.; Brannagan, T.H.; Cheng, R.K.; Clarke, J.O.; Dember, L.M.; Frantz, J.G.; Hershberger, R.E.; et al. 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient with Cardiac Amyloidosis: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2023, 81, 1076–1126. [Google Scholar] [CrossRef]
- Murtagh, B.; Hammill, S.C.; Gertz, M.A.; Kyle, R.A.; Tajik, A.J.; Grogan, M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am. J. Cardiol. 2005, 95, 535–537. [Google Scholar] [CrossRef]
- Falk, R.H.; Plehn, J.F.; Deering, T.; Schick, E.C.; Boinay, P.; Rubinow, A.; Skinner, M.; Cohen, A.S. Sensitivity and specificity of the echocardiographic features of cardiac amyloidosis. Am. J. Cardiol. 1987, 59, 418–422. [Google Scholar] [CrossRef]
- Sun, J.P.; Stewart, W.J.; Yang, X.S.; Donnell, R.O.; Leon, A.R.; Felner, J.M.; Thomas, J.D.; Merlino, J.D. Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am. J. Cardiol. 2009, 103, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Phelan, D.; Collier, P.; Thavendiranathan, P.; Popović, Z.B.; Hanna, M.; Plana, J.C.; Marwick, T.H.; Thomas, J.D. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart Br. Card. Soc. 2012, 98, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Ternacle, J.; Bodez, D.; Guellich, A.; Audureau, E.; Rappeneau, S.; Lim, P.; Radu, C.; Guendouz, S.; Couetil, J.-P.; Benhaiem, N.; et al. Causes and Consequences of Longitudinal LV Dysfunction Assessed by 2D Strain Echocardiography in Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2016, 9, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Cappelli, F.; Chacko, L.; Restrepo-Cordoba, M.A.; Lopez-Sainz, A.; Giannoni, A.; Aimo, A.; Baggiano, A.; Martinez-Naharro, A.; Whelan, C.; et al. Multiparametric Echocardiography Scores for the Diagnosis of Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2020, 13, 909–920. [Google Scholar] [CrossRef]
- Scheel, P.J.; Mukherjee, M.; Hays, A.G.; Vaishnav, J. Multimodality Imaging in the Evaluation and Prognostication of Cardiac Amyloidosis. Front. Cardiovasc. Med. 2022, 9, 787618. [Google Scholar] [CrossRef]
- de Gregorio, C.; Trimarchi, G.; Faro, D.C.; De Gaetano, F.; Campisi, M.; Losi, V.; Zito, C.; Tamburino, C.; Di Bella, G.; Monte, I.P. Myocardial Work Appraisal in Transthyretin Cardiac Amyloidosis and Nonobstructive Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2023, 208, 173–179. [Google Scholar] [CrossRef]
- Monte, I.P.; Faro, D.C.; Trimarchi, G.; de Gaetano, F.; Campisi, M.; Losi, V.; Teresi, L.; Di Bella, G.; Tamburino, C.; de Gregorio, C. Left Atrial Strain Imaging by Speckle Tracking Echocardiography: The Supportive Diagnostic Value in Cardiac Amyloidosis and Hypertrophic Cardiomyopathy. J. Cardiovasc. Dev. Dis. 2023, 10, 261. [Google Scholar] [CrossRef]
- Martinez-Naharro, A.; Treibel, T.A.; Abdel-Gadir, A.; Bulluck, H.; Zumbo, G.; Knight, D.S.; Kotecha, T.; Francis, R.; Hutt, D.F.; Rezk, T.; et al. Magnetic Resonance in Transthyretin Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2017, 70, 466–477. [Google Scholar] [CrossRef]
- Martinez-Naharro, A.; Kotecha, T.; Norrington, K.; Boldrini, M.; Rezk, T.; Quarta, C.; Treibel, T.A.; Whelan, C.J.; Knight, D.S.; Kellman, P.; et al. Native T1 and Extracellular Volume in Transthyretin Amyloidosis. JACC Cardiovasc. Imaging 2019, 12, 810–819. [Google Scholar] [CrossRef]
- Kotecha, T.; Martinez-Naharro, A.; Treibel, T.A.; Francis, R.; Nordin, S.; Abdel-Gadir, A.; Knight, D.S.; Zumbo, G.; Rosmini, S.; Maestrini, V.; et al. Myocardial Edema and Prognosis in Amyloidosis. J. Am. Coll. Cardiol. 2018, 71, 2919–2931. [Google Scholar] [CrossRef]
- Fontana, M.; Chung, R.; Hawkins, P.N.; Moon, J.C. Cardiovascular magnetic resonance for amyloidosis. Heart Fail. Rev. 2015, 20, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Sado, D.M.; Flett, A.S.; Banypersad, S.M.; White, S.K.; Maestrini, V.; Quarta, G.; Lachmann, R.H.; Murphy, E.; Mehta, A.; Hughes, D.A.; et al. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart 2012, 98, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Banypersad, S.M.; Sado, D.M.; Flett, A.S.; Gibbs, S.D.J.; Pinney, J.H.; Maestrini, V.; Cox, A.T.; Fontana, M.; Whelan, C.J.; Wechalekar, A.D.; et al. Quantification of Myocardial Extracellular Volume Fraction in Systemic AL Amyloidosis. Circ. Cardiovasc. Imaging 2013, 6, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.K.; Forero, J.F.; Popovic, Z.B.; Phelan, D.; Delgado, D.; Rakowski, H.; Wintersperger, B.J.; Thavendiranathan, P. Patterns of CMR measured longitudinal strain and its association with late gadolinium enhancement in patients with cardiac amyloidosis and its mimics. J. Cardiovasc. Magn. Reson. 2017, 19, 61. [Google Scholar] [CrossRef]
- Wan, K.; Sun, J.; Han, Y.; Luo, Y.; Liu, H.; Yang, D.; Cheng, W.; Zhang, Q.; Zeng, Z.; Chen, Y. Right ventricular involvement evaluated by cardiac magnetic resonance imaging predicts mortality in patients with light chain amyloidosis. Heart Vessels 2018, 33, 170–179. [Google Scholar] [CrossRef]
- Fontana, M.; Pica, S.; Reant, P.; Abdel-Gadir, A.; Treibel, T.A.; Banypersad, S.M.; Maestrini, V.; Barcella, W.; Rosmini, S.; Bulluck, H.; et al. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation 2015, 132, 1570–1579. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Appelbaum, E.; Davidoff, R.; Ozonoff, A.; Kissinger, K.V.; Harrigan, C.; Skinner, M.; Manning, W.J. Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in light-chain cardiac amyloidosis. Am. J. Cardiol. 2009, 103, 544–549. [Google Scholar] [CrossRef]
- Martinez-Naharro, A.; Abdel-Gadir, A.; Treibel, T.A.; Zumbo, G.; Knight, D.S.; Rosmini, S.; Lane, T.; Mahmood, S.; Sachchithanantham, S.; Whelan, C.J.; et al. CMR-Verified Regression of Cardiac AL Amyloid After Chemotherapy. JACC Cardiovasc. Imaging 2018, 11, 152–154. [Google Scholar] [CrossRef]
- Germain, D.P. Fabry disease. Orphanet J. Rare Dis. 2010, 5, 30. [Google Scholar] [CrossRef]
- Mehta, A.; Ricci, R.; Widmer, U.; Dehout, F.; Garcia de Lorenzo, A.; Kampmann, C.; Linhart, A.; Sunder-Plassmann, G.; Ries, M.; Beck, M. Fabry disease defined: Baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur. J. Clin. Investig. 2004, 34, 236–242. [Google Scholar] [CrossRef]
- Nakao, S.; Takenaka, T.; Maeda, M.; Kodama, C.; Tanaka, A.; Tahara, M.; Yoshida, A.; Kuriyama, M.; Hayashibe, H.; Sakuraba, H. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N. Engl. J. Med. 1995, 333, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Chong, K.-W.; Hsu, J.-H.; Yu, H.-C.; Shih, C.-C.; Huang, C.-H.; Lin, S.-J.; Chen, C.-H.; Chiang, C.-C.; Ho, H.-J.; et al. High Incidence of the Cardiac Variant of Fabry Disease Revealed by Newborn Screening in the Taiwan Chinese Population. Circ. Cardiovasc. Genet. 2009, 2, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, B.; Takenaka, T.; Teraguchi, H.; Tei, C.; Lee, P.; McKenna, W.J.; Elliott, P.M. Prevalence of Anderson-Fabry Disease in Male Patients with Late Onset Hypertrophic Cardiomyopathy. Circulation 2002, 105, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.; Pieroni, M.; Morgante, E.; Antuzzi, D.; Russo, A.; Russo, M.A.; Maseri, A.; Frustaci, A. Prevalence of Fabry Disease in Female Patients with Late-Onset Hypertrophic Cardiomyopathy. Circulation 2004, 110, 1047–1053. [Google Scholar] [CrossRef]
- Waldek, S.; Patel, M.R.; Banikazemi, M.; Lemay, R.; Lee, P. Life expectancy and cause of death in males and females with Fabry disease: Findings from the Fabry Registry. Genet. Med. Off. J. Am. Coll. Med. Genet. 2009, 11, 790–796. [Google Scholar] [CrossRef]
- Linhart, A.; Kampmann, C.; Zamorano, J.L.; Sunder-Plassmann, G.; Beck, M.; Mehta, A.; Elliott, P.M. European FOS Investigators Cardiac manifestations of Anderson-Fabry disease: Results from the international Fabry outcome survey. Eur. Heart J. 2007, 28, 1228–1235. [Google Scholar] [CrossRef]
- Calcagnino, M.; O’Mahony, C.; Coats, C.; Cardona, M.; Garcia, A.; Janagarajan, K.; Mehta, A.; Hughes, D.; Murphy, E.; Lachmann, R.; et al. Exercise-Induced Left Ventricular Outflow Tract Obstruction in Symptomatic Patients with Anderson-Fabry Disease. J. Am. Coll. Cardiol. 2011, 58, 88–89. [Google Scholar] [CrossRef]
- Niemann, M.; Breunig, F.; Beer, M.; Herrmann, S.; Strotmann, J.; Hu, K.; Emmert, A.; Voelker, W.; Ertl, G.; Wanner, C.; et al. The right ventricle in Fabry disease: Natural history and impact of enzyme replacement therapy. Heart Br. Card. Soc. 2010, 96, 1915–1919. [Google Scholar] [CrossRef]
- Labombarda, F.; Saloux, E.; Milesi, G.; Bienvenu, B. Loss of base-to-apex circumferential strain gradient: A specific pattern of Fabry cardiomyopathy? Echocardiography 2017, 34, 504–510. [Google Scholar] [CrossRef]
- Perry, R.; Shah, R.; Saiedi, M.; Patil, S.; Ganesan, A.; Linhart, A.; Selvanayagam, J.B. The Role of Cardiac Imaging in the Diagnosis and Management of Anderson-Fabry Disease. JACC Cardiovasc. Imaging 2019, 12 Pt 1, 1230–1242. [Google Scholar] [CrossRef]
- Esposito, R.; Santoro, C.; Mandoli, G.E.; Cuomo, V.; Sorrentino, R.; La Mura, L.; Pastore, M.C.; Bandera, F.; D’Ascenzi, F.; Malagoli, A.; et al. Cardiac Imaging in Anderson-Fabry Disease: Past, Present and Future. J. Clin. Med. 2021, 10, 1994. [Google Scholar] [CrossRef] [PubMed]
- Ponsiglione, A.; Gambardella, M.; Green, R.; Cantoni, V.; Nappi, C.; Ascione, R.; De Giorgi, M.; Cuocolo, R.; Pisani, A.; Petretta, M.; et al. Cardiovascular magnetic resonance native T1 mapping in Anderson-Fabry disease: A systematic review and meta-analysis. J. Cardiovasc. Magn. Reson. 2022, 24, 31. [Google Scholar] [CrossRef] [PubMed]
- Pica, S.; Sado, D.M.; Maestrini, V.; Fontana, M.; White, S.K.; Treibel, T.; Captur, G.; Anderson, S.; Piechnik, S.K.; Robson, M.D.; et al. Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2014, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Sado, D.M.; White, S.K.; Piechnik, S.K.; Banypersad, S.M.; Treibel, T.; Captur, G.; Fontana, M.; Maestrini, V.; Flett, A.S.; Robson, M.D.; et al. Identification and Assessment of Anderson-Fabry Disease by Cardiovascular Magnetic Resonance Noncontrast Myocardial T1 Mapping. Circ. Cardiovasc. Imaging 2013, 6, 392–398. [Google Scholar] [CrossRef]
- Deva, D.P.; Hanneman, K.; Li, Q.; Ng, M.Y.; Wasim, S.; Morel, C.; Iwanochko, R.M.; Thavendiranathan, P.; Crean, A.M. Cardiovascular magnetic resonance demonstration of the spectrum of morphological phenotypes and patterns of myocardial scarring in Anderson-Fabry disease. J. Cardiovasc. Magn. Reson. 2016, 18, 14. [Google Scholar] [CrossRef]
- Hanneman, K.; Karur, G.R.; Wasim, S.; Wald, R.M.; Iwanochko, R.M.; Morel, C.F. Left Ventricular Hypertrophy and Late Gadolinium Enhancement at Cardiac MRI Are Associated with Adverse Cardiac Events in Fabry Disease. Radiology 2020, 294, 42–49. [Google Scholar] [CrossRef]
- Orsborne, C.; Bradley, J.; Bonnett, L.J.; Pleva, L.A.; Naish, J.H.; Clark, D.G.; Abidin, N.; Woolfson, P.; Nucifora, G.; Schmitt, M.; et al. Validated Model for Prediction of Adverse Cardiac Outcome in Patients with Fabry Disease. J. Am. Coll. Cardiol. 2022, 80, 982–994. [Google Scholar] [CrossRef]
- Kampmann, C.; Perrin, A.; Beck, M. Effectiveness of agalsidase alfa enzyme replacement in Fabry disease: Cardiac outcomes after 10 years’ treatment. Orphanet J. Rare Dis. 2015, 10, 125. [Google Scholar] [CrossRef]
- Desnick, R.J.; Brady, R.; Barranger, J.; Collins, A.J.; Germain, D.P.; Goldman, M.; Grabowski, G.; Packman, S.; Wilcox, W.R. Fabry Disease, an Under-Recognized Multisystemic Disorder: Expert Recommendations for Diagnosis, Management, and Enzyme Replacement Therapy. Ann. Intern. Med. 2003, 138, 338–346. [Google Scholar] [CrossRef]
- Martins, A.M.; D’Almeida, V.; Kyosen, S.O.; Takata, E.T.; Delgado, A.G.; Gonçalves, Â.M.B.F.; Filho, C.C.B.; Filho, D.M.; Biagini, G.; Pimentel, H.; et al. Guidelines to Diagnosis and Monitoring of Fabry Disease and Review of Treatment Experiences. J. Pediatr. 2009, 155, S19–S31. [Google Scholar] [CrossRef]
- Linhart, A.; Germain, D.P.; Olivotto, I.; Akhtar, M.M.; Anastasakis, A.; Hughes, D.; Namdar, M.; Pieroni, M.; Hagège, A.; Cecchi, F.; et al. An expert consensus document on the management of cardiovascular manifestations of Fabry disease. Eur. J. Heart Fail. 2020, 22, 1076–1096. [Google Scholar] [CrossRef] [PubMed]
- McLaren, G.D.; Muir, W.A.; Kellermeyer, R.W. Iron overload disorders: Natural history, pathogenesis, diagnosis, and therapy. Crit. Rev. Clin. Lab. Sci. 1983, 19, 205–266. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C. Cardiac iron across different transfusion-dependent diseases. Blood Rev. 2008, 22 (Suppl. 2), S14–S21. [Google Scholar] [CrossRef]
- Noetzli, L.J.; Carson, S.M.; Nord, A.S.; Coates, T.D.; Wood, J.C. Longitudinal analysis of heart and liver iron in thalassemia major. Blood 2008, 112, 2973–2978. [Google Scholar] [CrossRef] [PubMed]
- Buja, L.M.; Roberts, W.C. Iron in the heart: Etiology and clinical significance. Am. J. Med. 1971, 51, 209–221. [Google Scholar] [CrossRef]
- Kremastinos, D.T.; Farmakis, D. Iron Overload Cardiomyopathy in Clinical Practice. Circulation 2011, 124, 2253–2263. [Google Scholar] [CrossRef]
- Schmitt, B.; Golub, R.M.; Green, R. Screening primary care patients for hereditary hemochromatosis with transferrin saturation and serum ferritin level: Systematic review for the American College of Physicians. Ann. Intern. Med. 2005, 143, 522–536. [Google Scholar] [CrossRef]
- Detterich, J.; Noetzli, L.; Dorey, F.; Bar-Cohen, Y.; Harmatz, P.; Coates, T.; Wood, J. Electrocardiographic consequences of cardiac iron overload in thalassemia major. Am. J. Hematol. 2012, 87, 139–144. [Google Scholar] [CrossRef]
- Aronow, W.S.; Meister, L.; Kent, J.R. Atrioventricular block in familial hemochromatosis treated by permanent synchronous pacemaker. Arch. Intern. Med. 1969, 123, 433–435. [Google Scholar] [CrossRef]
- Spirito, P.; Lupi, G.; Melevendi, C.; Vecchio, C. Restrictive diastolic abnormalities identified by Doppler echocardiography in patients with thalassemia major. Circulation 1990, 82, 88–94. [Google Scholar] [CrossRef]
- Vichinsky, E.P.; MacKlin, E.A.; Waye, J.S.; Lorey, F.; Olivieri, N.F. Changes in the epidemiology of thalassemia in North America: A new minority disease. Pediatrics 2005, 116, e818–e825. [Google Scholar] [CrossRef] [PubMed]
- Narayana, G.; Ramagopal, G.; Sutay, N.R.; Duggal, B. Assessment of Regional and Global Myocardial Systolic Function by 2D Longitudinal Speckle Tracking in Children with Beta Thalassemia Major. J. Clin. Prev. Cardiol. 2017, 6, 137. [Google Scholar] [CrossRef]
- Zimmerman, M.J.; Rosing, D.R.; Shizukuda, Y. Advancement of echocardiography for surveillance of iron overload cardiomyopathy: Comparison to cardiac magnetic resonance imaging. J. Echocardiogr. 2021, 19, 141–149. [Google Scholar] [CrossRef]
- Hahalis, G.; Manolis, A.S.; Apostolopoulos, D.; Alexopoulos, D.; Vagenakis, A.G.; Zoumbos, N.C. Right ventricular cardiomyopathy in β-thalassaemia major. Eur. Heart J. 2002, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C. Magnetic resonance imaging measurement of iron overload. Curr. Opin. Hematol. 2007, 14, 183–190. [Google Scholar] [CrossRef]
- Carpenter, J.-P.; He, T.; Kirk, P.; Roughton, M.; Anderson, L.J.; de Noronha, S.V.; Sheppard, M.N.; Porter, J.B.; Walker, J.M.; Wood, J.C.; et al. On T2* Magnetic Resonance and Cardiac Iron. Circulation 2011, 123, 1519–1528. [Google Scholar] [CrossRef]
- Anderson, L.J.; Holden, S.; Davis, B.; Prescott, E.; Charrier, C.C.; Bunce, N.H.; Firmin, D.N.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef]
- Torlasco, C.; Cassinerio, E.; Roghi, A.; Faini, A.; Capecchi, M.; Abdel-Gadir, A.; Giannattasio, C.; Parati, G.; Moon, J.C.; Cappellini, M.D.; et al. Role of T1 mapping as a complementary tool to T2* for non-invasive cardiac iron overload assessment. PLoS ONE 2018, 13, e0192890. [Google Scholar] [CrossRef]
- Meloni, A.; Martini, N.; Positano, V.; De Luca, A.; Pistoia, L.; Sbragi, S.; Spasiano, A.; Casini, T.; Bitti, P.P.; Allò, M.; et al. Myocardial iron overload by cardiovascular magnetic resonance native segmental T1 mapping: A sensitive approach that correlates with cardiac complications. J. Cardiovasc. Magn. Reson. 2021, 23, 70. [Google Scholar] [CrossRef]
- Pepe, A.; Positano, V.; Capra, M.; Maggio, A.; Pinto, C.L.; Spasiano, A.; Forni, G.; Derchi, G.; Favilli, B.; Rossi, G.; et al. Myocardial scarring by delayed enhancement cardiovascular magnetic resonance in thalassaemia major. Heart Br. Card. Soc. 2009, 95, 1688–1693. [Google Scholar] [CrossRef]
- Meloni, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Cecinati, V.; Ricchi, P.; Gerardi, C.; Restaino, G.; Righi, R.; et al. Multi-Parametric Cardiac Magnetic Resonance for Prediction of Heart Failure Death in Thalassemia Major. Diagnostics 2023, 13, 890. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.; Roughton, M.; Porter, J.B.; Walker, J.M.; Tanner, M.A.; Patel, J.; Wu, D.; Taylor, J.; Westwood, M.A.; Anderson, L.J.; et al. Cardiac T2* Magnetic Resonance for Prediction of Cardiac Complications in Thalassemia Major. Circulation 2009, 120, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Aessopos, A.; Fragodimitri, C.; Karabatsos, F.; Hatziliami, A.; Yousef, J.; Giakoumis, A.; Dokou, A.; Gotsis, E.D.; Berdoukas, V.; Karagiorga, M. Cardiac magnetic resonance imaging R2* assessments and analysis of historical parameters in patients with transfusion-dependent thalassemia. Haematologica 2007, 92, 131–132. [Google Scholar] [CrossRef]
- Marsella, M.; Borgna-Pignatti, C.; Meloni, A.; Caldarelli, V.; Dell’Amico, M.C.; Spasiano, A.; Pitrolo, L.; Cracolici, E.; Valeri, G.; Positano, V.; et al. Cardiac iron and cardiac disease in males and females with transfusion-dependent thalassemia major: A T2* magnetic resonance imaging study. Haematologica 2011, 96, 515–520. [Google Scholar] [CrossRef]
- Iannuzzi, M.C.; Rybicki, B.A.; Teirstein, A.S. Sarcoidosis. N. Engl. J. Med. 2007, 357, 2153–2165. [Google Scholar] [CrossRef]
- Drent, M.; Crouser, E.D.; Grunewald, J. Challenges of Sarcoidosis and Its Management. N. Engl. J. Med. 2021, 385, 1018–1032. [Google Scholar] [CrossRef]
- Rybicki, B.A.; Major, M.; Popovich, J., Jr.; Maliank, M.J.; Lannuzzi, M.C. Racial Differences in Sarcoidosis Incidence: A 5-Year Study in a Health Maintenance Organization. Am. J. Epidemiol. 1997, 145, 234–241. [Google Scholar] [CrossRef]
- Newman, L.S.; Rose, C.S.; Maier, L.A. Sarcoidosis. N. Engl. J. Med. 1997, 336, 1224–1234. [Google Scholar] [CrossRef]
- Arkema, E.V.; Cozier, Y.C. Sarcoidosis epidemiology: Recent estimates of incidence, prevalence and risk factors. Curr. Opin. Pulm. Med. 2020, 26, 527. [Google Scholar] [CrossRef]
- Silverman, K.J.; Hutchins, G.M.; Bulkley, B.H. Cardiac sarcoid: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978, 58, 1204–1211. [Google Scholar] [CrossRef]
- Iwai, K.; Tachibana, T.; Takemura, T.; Matsui, Y.; Kitalchi, M.; Kawabata, Y. Pathological studies on sarcoidosis autopsy. I. Epidemiological features of 320 cases in Japan. Acta Pathol. Jpn. 1993, 43, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Birnie, D.H.; Nery, P.B.; Ha, A.C.; Beanlands, R.S.B. Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 2016, 68, 411–421. [Google Scholar] [CrossRef]
- Gilotra, N.A.; Griffin, J.M.; Pavlovic, N.; Houston, B.A.; Chasler, J.; Goetz, C.; Chrispin, J.; Sharp, M.; Kasper, E.K.; Chen, E.S.; et al. Sarcoidosis-Related Cardiomyopathy: Current Knowledge, Challenges, and Future Perspectives State-of-the-Art Review. J. Card. Fail. 2022, 28, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, H.; Yuwai, K.; Hiroe, M. Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disord. 2007, 27, 89–102. [Google Scholar]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Cosedis Nielsen, J.; et al. HRS Expert Consensus Statement on the Diagnosis and Management of Arrhythmias Associated with Cardiac Sarcoidosis. Heart Rhythm. 2014, 11, 1304–1323. [Google Scholar] [CrossRef]
- Wand, A.L.; Chrispin, J.; Saad, E.; Mukherjee, M.; Hays, A.G.; Gilotra, N.A. Current State and Future Directions of Multimodality Imaging in Cardiac Sarcoidosis. Front. Cardiovasc. Med. 2021, 8, 785279. [Google Scholar] [CrossRef]
- Joyce, E.; Ninaber, M.K.; Katsanos, S.; Debonnaire, P.; Kamperidis, V.; Bax, J.J.; Taube, C.; Delgado, V.; Ajmone Marsan, N. Subclinical left ventricular dysfunction by echocardiographic speckle-tracking strain analysis relates to outcome in sarcoidosis. Eur. J. Heart Fail. 2015, 17, 51–62. [Google Scholar] [CrossRef]
- Lehtonen, J.; Uusitalo, V.; Pöyhönen, P.; Mäyränpää, M.I.; Kupari, M. Cardiac sarcoidosis: Phenotypes, diagnosis, treatment, and prognosis. Eur. Heart J. 2023, 44, 1495–1510. [Google Scholar] [CrossRef]
- Burstow, D.J.; Tajik, A.J.; Bailey, K.R.; DeRemee, R.A.; Taliercio, C.P. Two-dimensional echocardiographic findings in systemic sarcoidosis. Am. J. Cardiol. 1989, 63, 478–482. [Google Scholar] [CrossRef]
- Ayyala, U.S.; Nair, A.P.; Padilla, M.L. Cardiac Sarcoidosis. Clin. Chest Med. 2008, 29, 493–508. [Google Scholar] [CrossRef]
- Blankstein, R.; Waller, A.H. Evaluation of Known or Suspected Cardiac Sarcoidosis. Circ. Cardiovasc. Imaging 2016, 9, e000867. [Google Scholar] [CrossRef] [PubMed]
- Greulich, S.; Kitterer, D.; Latus, J.; Aguor, E.; Steubing, H.; Kaesemann, P.; Patrascu, A.; Greiser, A.; Groeninger, S.; Mayr, A.; et al. Comprehensive Cardiovascular Magnetic Resonance Assessment in Patients with Sarcoidosis and Preserved Left Ventricular Ejection Fraction. Circ. Cardiovasc. Imaging 2016, 9, e005022. [Google Scholar] [CrossRef] [PubMed]
- Tonegawa-Kuji, R.; Oyama-Manabe, N.; Aoki, R.; Nagayoshi, S.; Pawhay, C.M.H.; Kusano, K.; Nakajima, T. T2-weighted short-tau-inversion-recovery imaging reflects disease activity of cardiac sarcoidosis. Open Heart 2021, 8, e001728. [Google Scholar] [CrossRef]
- Watanabe, E.; Kimura, F.; Nakajima, T.; Hiroe, M.; Kasai, Y.; Nagata, M.; Kawana, M.; Hagiwara, N. Late Gadolinium Enhancement in Cardiac Sarcoidosis: Characteristic Magnetic Resonance Findings and Relationship with Left Ventricular Function. J. Thorac. Imaging 2013, 28, 60–66. [Google Scholar] [CrossRef]
- Mahrholdt, H.; Wagner, A.; Judd, R.M.; Sechtem, U.; Kim, R.J. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur. Heart J. 2005, 26, 1461–1474. [Google Scholar] [CrossRef]
- Vita, T.; Okada, D.R.; Veillet-Chowdhury, M.; Bravo, P.E.; Mullins, E.; Hulten, E.; Agrawal, M.; Madan, R.; Taqueti, V.R.; Steigner, M.; et al. Complementary Value of Cardiac Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in the Assessment of Cardiac Sarcoidosis. Circ. Cardiovasc. Imaging 2018, 11, e007030. [Google Scholar] [CrossRef]
- Okasha, O.; Kazmirczak, F.; Chen, K.A.; Farzaneh-Far, A.; Shenoy, C. Myocardial Involvement in Patients with Histologically Diagnosed Cardiac Sarcoidosis: A Systematic Review and Meta-Analysis of Gross Pathological Images from Autopsy or Cardiac Transplantation Cases. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2019, 8, e011253. [Google Scholar] [CrossRef]
- Greulich, S.; Deluigi, C.C.; Gloekler, S.; Wahl, A.; Zürn, C.; Kramer, U.; Nothnagel, D.; Bültel, H.; Schumm, J.; Grün, S.; et al. CMR Imaging Predicts Death and Other Adverse Events in Suspected Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2013, 6, 501–511. [Google Scholar] [CrossRef]
- Hulten, E.; Agarwal, V.; Cahill, M.; Cole, G.; Vita, T.; Parrish, S.; Bittencourt, M.S.; Murthy, V.L.; Kwong, R.; Di Carli, M.F.; et al. Presence of Late Gadolinium Enhancement by Cardiac Magnetic Resonance Among Patients with Suspected Cardiac Sarcoidosis Is Associated with Adverse Cardiovascular Prognosis: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2016, 9, e005001. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Hosadurg, N.; Iwanaga, Y.; Chen, Y.; Liu, W.; Wan, K.; Patel, A.R.; Wicks, E.C.; Gkoutos, G.V.; et al. Prognostic Value of RV Abnormalities on CMR in Patients with Known or Suspected Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2023, 16, 361–372. [Google Scholar] [CrossRef]
| CMR Parameter | Key CMR Findings | Illustrative Example |
|---|---|---|
| Cardiac Structure and Function |
| 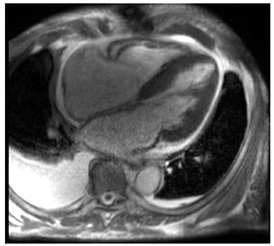 |
| T1 time | Elevated native T1 time | 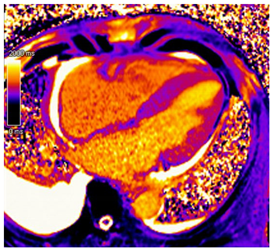 |
| T2 Time | Possibly elevated T2 time but not as high as reported in myocarditis or myocardial infarction | 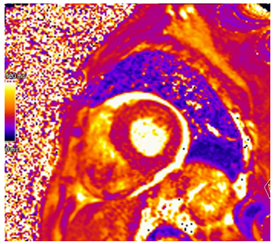 |
| LGE | Diffuse patchy LGE of the left ventricle LGE of the atria and the right ventricle may be present | 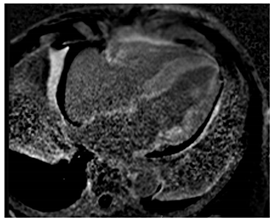 |
| ECV | Markedly elevated ECV | 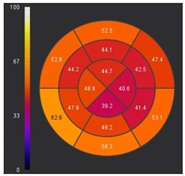 |
| CMR Parameter | Key CMR Findings | Illustrative Example |
|---|---|---|
| Cardiac Structure and Function |
|  |
| T1 time | Short native T1 time | 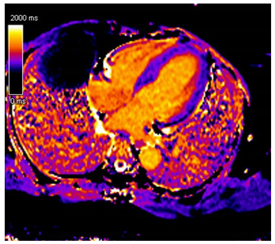 |
| T2 Time | Possibly elevated T2 time in the basal inferolateral wall or in areas of LGE | 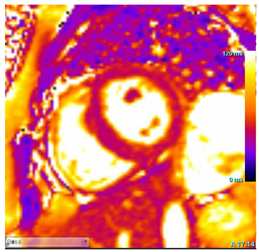 |
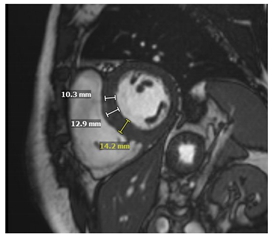
| LGE | Mid-myocardium LGE of the basal inferolateral wall | 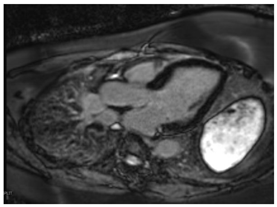 |
| CMR Parameter | Key CMR Findings | Illustrative Example |
|---|---|---|
| Cardiac Structure and Function | Dilated ventricular chambers with reduced systolic function in later stages | 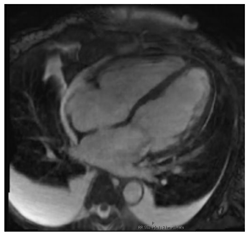 |
| T1 time | Short native T1 time |  |
| T2 Time | Short T2 time | 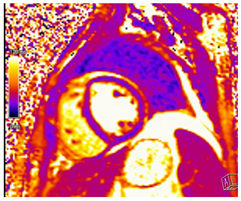 |
| T2* Time | Short T2* time, which inversely correlates with myocardial iron content
| 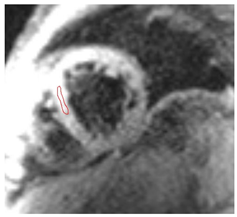 |
| LGE | Patchy areas of LGE can be seen secondary to replacement fibrosis | 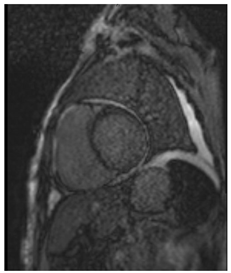 |
| CMR Parameter | Key CMR Findings | Illustrative Example |
|---|---|---|
| Cardiac Structure and Function |
| 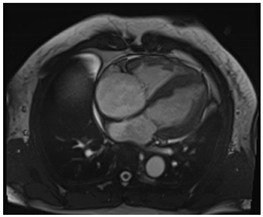 |
| T1 time | Native T1 time may be focally increased in regions of fibrosis or edema | 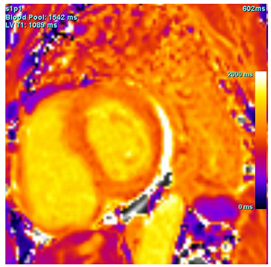 |
| T2 Time | T2 time may be focally increased in regions of edema | 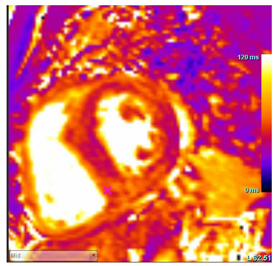 |
| LGE |
|  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussien, M.; Bermudez, F.; Bering, P.T.; Weissman, G.; Hays, A.G.; Sheikh, F.H. Cardiac Magnetic Resonance Imaging in the Evaluation and Prognosis of Infiltrative Cardiomyopathies. J. Cardiovasc. Dev. Dis. 2025, 12, 154. https://doi.org/10.3390/jcdd12040154
Hussien M, Bermudez F, Bering PT, Weissman G, Hays AG, Sheikh FH. Cardiac Magnetic Resonance Imaging in the Evaluation and Prognosis of Infiltrative Cardiomyopathies. Journal of Cardiovascular Development and Disease. 2025; 12(4):154. https://doi.org/10.3390/jcdd12040154
Chicago/Turabian StyleHussien, Merna, Francisca Bermudez, Patrick T. Bering, Gaby Weissman, Allison G. Hays, and Farooq H. Sheikh. 2025. "Cardiac Magnetic Resonance Imaging in the Evaluation and Prognosis of Infiltrative Cardiomyopathies" Journal of Cardiovascular Development and Disease 12, no. 4: 154. https://doi.org/10.3390/jcdd12040154
APA StyleHussien, M., Bermudez, F., Bering, P. T., Weissman, G., Hays, A. G., & Sheikh, F. H. (2025). Cardiac Magnetic Resonance Imaging in the Evaluation and Prognosis of Infiltrative Cardiomyopathies. Journal of Cardiovascular Development and Disease, 12(4), 154. https://doi.org/10.3390/jcdd12040154






