Double QRS Transition Due to Anodal Capture During Left Bundle Branch Area Pacing: A Case Report
Abstract
1. Introduction
- -
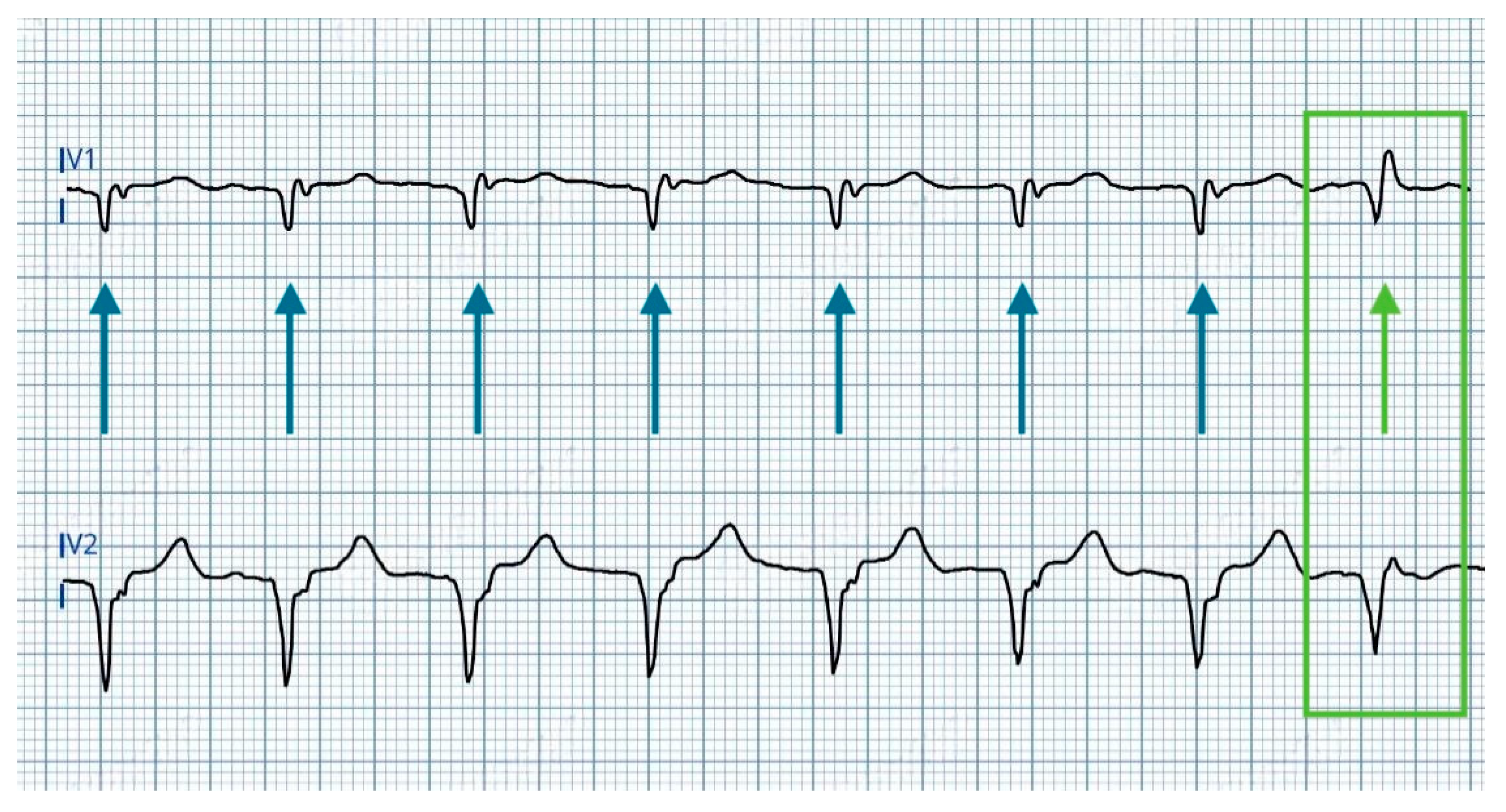
- A rounded R’ wave in lead V1, accompanied by prolongation of the V1 R-wave peak time (V1RWPT) exceeding 10 ms.
- -
- The development of a deeper S wave in leads V6 and I, while the V6RWPT remains unchanged.
- -
- Prolongation of the V6RWPT, indicating delayed activation of the left ventricular lateral wall.
- -
- Reduced R’ wave amplitude in V1, reflecting diminished direct LBB activation.
- -
- Disappearance of S wave in lead I and V6, consistent with altered ventricular depolarization patterns [10].
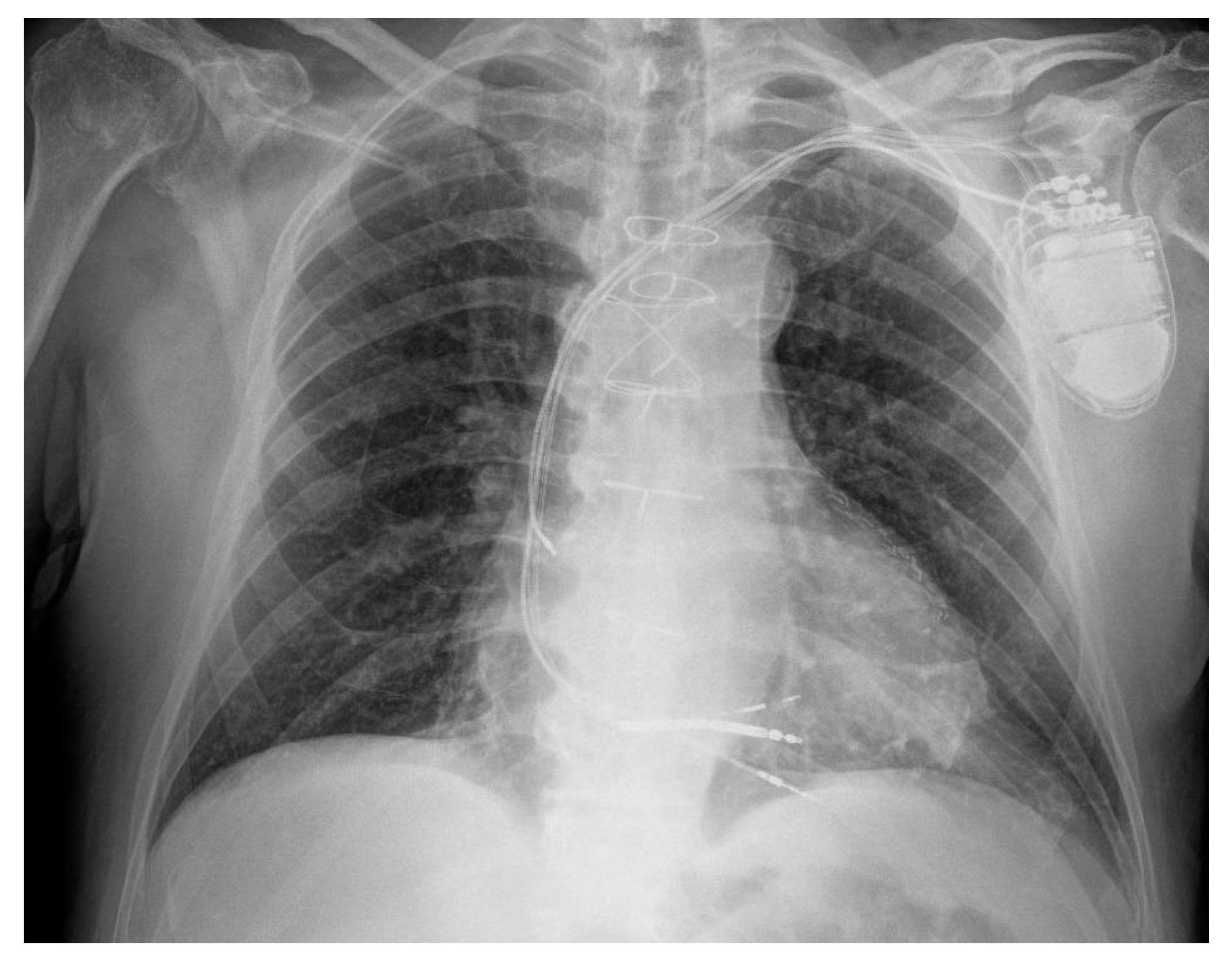
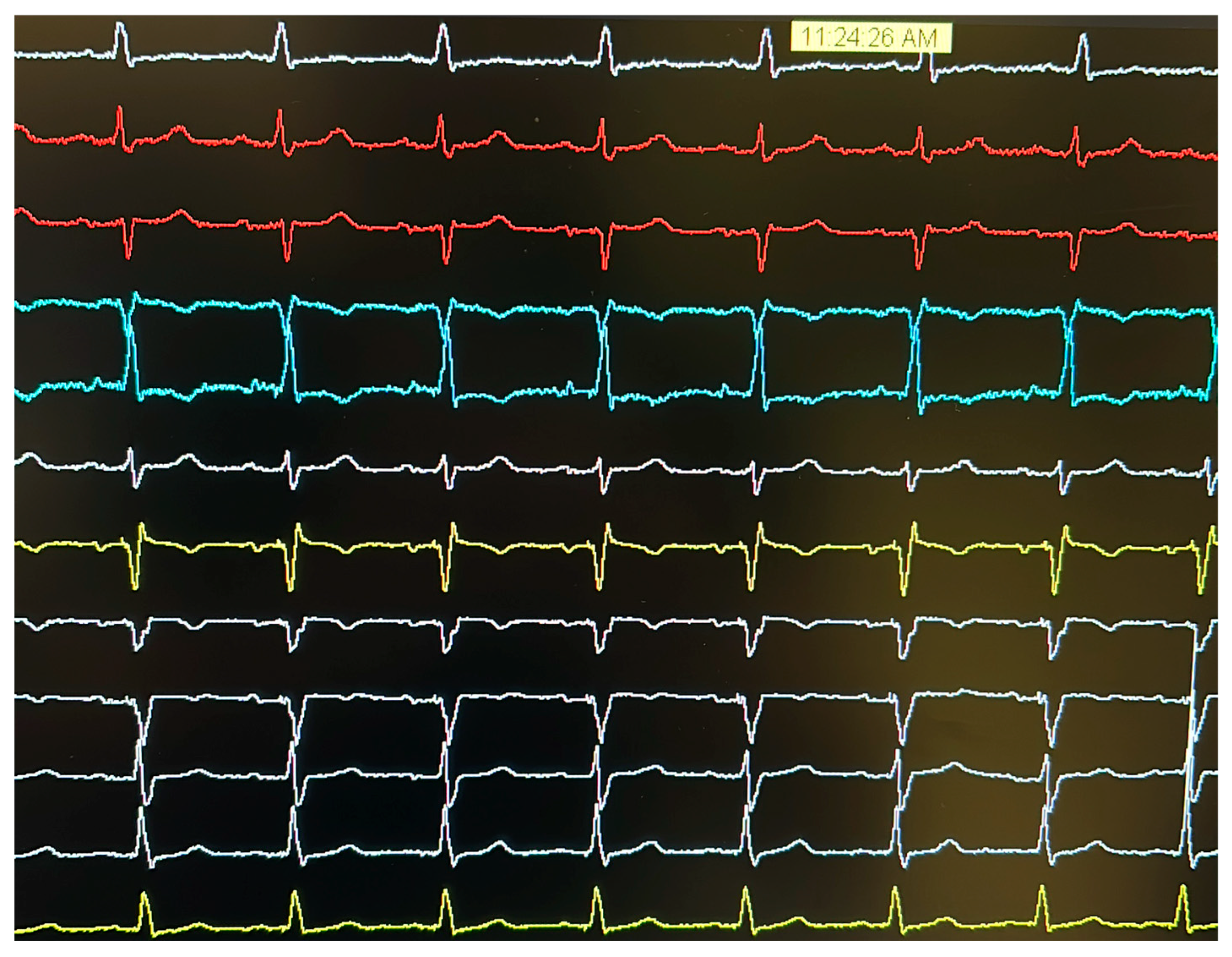
2. Case Presentation
- At >4.5 V@0.4 ms: dominance of anodal capture (RV apical pacing) due to high charge density at the anode.
- At 4.5 V–1.25 V@0.4 ms: ns-LBBAP due to progressive reduction in anodal interference.
- At ≤1.0 V@0.4 ms: s-LBBAP, with singular capture of the LBB.
3. Discussion
- -
- Confirm that anodal capture occurs only at voltages below the programmed pacing output, ensuring an appropriate safety margin.
- -
- Select a different anodal pole for bipolar pacing. In devices with a defibrillation lead, choosing a larger-area anode (e.g., the defibrillation coil) can reduce charge density.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRT | Cardiac resynchronization therapy |
| LBBAP | Left bundle branch area pacing |
| ICD | Implantable cardioverter defibrillator |
| CRT-D | Cardiac resynchronization therapy with defibrillator |
| RV | Right ventricular |
| LV | Left ventricular |
| CSP | Conduction system pacing |
| ns-LBBP | Non-selective left bundle branch pacing |
| s-LBBP | Selective left bundle branch pacing |
| LVSP | Left ventricular septal pacing |
| V6RWPT | V6 R-wave peak time |
| RBBB | Right bundle branch block |
| LBBB | Left bundle branch block |
| IVCD | Interventricular conduction delay |
| LBB | Left bundle branch |
| V1RWPT | V1 R-wave peak time |
| CABG | Coronary artery bypass grafting |
| LVEF | Left ventricular ejection fraction |
| COI | Current of injury |
References
- Kaszala, K.; Ellenbogen, K.A. Cardiac Pacing and ICDs, 7th ed; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020. [Google Scholar]
- Dekker, E. Direct current make and break thresholds for pacemaker electrodes on the canine ventricle. Circ. Res. 1970, 27, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Cranefield, P.F.; Hoffman, B.F.; Siebens, A.A. Anodal excitation of cardiac muscle. Am. J. Physiol. 1957, 190, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Tamborero, D.; Mont, L.; Alanis, R.; Berruezo, A.; Tolosana, J.M.; Sitges, M.; Vidal, B.; Brugada, J. Anodal capture in cardiac resynchronization therapy implications for device programming. Pacing Clin. Electrophysiol. 2006, 29, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Dendy, K.F.; Powell, B.D.; Cha, Y.M.; Espinosa, R.E.; Friedman, P.A.; Rea, R.F.; Hayes, D.L.; Redfield, M.M.; Asirvatham, S.J. Anodal stimulation: An underrecognized cause of nonresponders to cardiac resynchronization therapy. Indian Pacing Electrophysiol. J. 2011, 11, 64–72. [Google Scholar] [PubMed] [PubMed Central]
- Thibault, B.; Roy, D.; Guerra, P.G.; Macle, L.; Dubuc, M.; Gagné, P.; Greiss, I.; Novak, P.; Furlani, A.; Talajic, M. Anodal right ventricular capture during left ventricular stimulation in CRT-implantable cardioverter defibrillators. Pacing Clin. Electrophysiol. 2005, 28, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, X.; Gold, M.R. Left Bundle Branch Pacing: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.S.J.; Soh, R.; Boey, E.; Lee, J.Y.; de Leon, J.; Chan, S.P.; Gan, H.H.; Seow, S.C.; Kojodjojo, P. Comparison of Pacing Performance and Clinical Outcomes Between Left Bundle Branch and His Bundle Pacing. JACC Clin. Electrophysiol. 2023, 9, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Sharma, P.S.; Cano, Ó.; Ponnusamy, S.S.; Herweg, B.; Zanon, F.; Jastrzebski, M.; Zou, J.; Chelu, M.G.; Vernooy, K.; et al. Comparison of Left Bundle Branch Area Pacing and Biventricular Pacing in Candidates for Resynchronization Therapy. J. Am. Coll. Cardiol. 2023, 82, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Burri, H.; Jastrzebski, M.; Cano, Ó.; Čurila, K.; de Pooter, J.; Huang, W.; Israel, C.; Joza, J.; Vernooy, K.; Vijayaraman, P.; et al. EHRA clinical consensus statement on conduction system pacing implantation: Endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS). EP Eur. 2023, 25, 1208–1236. [Google Scholar] [CrossRef]
- Glikson, M.; Burri, H.; Abdin, A.; Cano, O.; Curila, K.; de Pooter, J.; Diaz, J.C.; Huang, W.; Israel, C.W.; Jastrzębski, M.; et al. European Society of Cardiology (ESC) clinical consensus statement on indications for conduction system pacing, with special contribution of the European Heart Rhythm Association of the ESC and endorsed by the Asia Pacific Heart Rhythm Society, the Canadian Heart Rhythm Society, the Heart Rhythm Society, and the Latin American Heart Rhythm Society. EP Eur. 2025, 27, euaf050. [Google Scholar] [CrossRef]
- Whinnett, Z.I.; Waxman, R.; Cornelussen, R.N.; Foley, P.; Chandrasekaran, B.; Vijayaraman, P.; Upadhyay, G.A.; Schaller, R.D.; Gardas, R.; Richardson, T.D.; et al. PO-02-062 Anodal Capture Decreases Left Ventricular Contractility During Left Bundle Branch Area Pacing. Heart Rhythm 2024, 21, S284–S285. [Google Scholar] [CrossRef]
- Ali, N.; Saqi, K.; Arnold, A.D.; Miyazawa, A.A.; Keene, D.; Chow, J.J.; Little, I.; Peters, N.S.; Kanagaratnam, P.; Qureshi, N.; et al. Left bundle branch pacing with and without anodal capture: Impact on ventricular activation pattern and acute haemodynamics. Europace 2023, 25, euad264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Criterion | Description | Notes |
|---|---|---|
| QRS transition | - During threshold testing: Transition from non-selective LBBP (ns-LBBP) to selective LBBP (s-LBBP) or left ventricular septal pacing (LVSP) - During programmed stimulation: Transition to s-LBBP with pacing output adjustments | Observable with pacing output changes |
| V6 R-wave peak time (V6RWPT) | Time from pacing stimulus to peak of R-wave in lead V6 | <75 ms if arrow native QRS or isolated RBBB <80 ms in case of LBBB, IVCD, RBBB + fascicular block, wide escape rhythm, or asystole |
| V6-V1 interpeak interval | Time difference between peak of R-wave in V6 and peak in V1 | >44 ms |
| Stimulus-to-potential alignment | Concordance between: 1. Intrinsic potential-V6RWPT; 2. Stimulus-V6RWPT. | Difference ≤ ±10 ms |
| RV Anodal Capture | ns-LBBAP | s-LBBAP | |
|---|---|---|---|
| Stim to peak interval (V6RWPT) | 88 ms | 74 ms | 74 ms |
| V6-V1 interval | - | 126 ms | 142 ms |
| V1RWPT | - | 52 ms | 68 ms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melpignano, A.; Vitali, F.; Canovi, L.; Bonini, J.; Vocale, L.R.; Bertini, M. Double QRS Transition Due to Anodal Capture During Left Bundle Branch Area Pacing: A Case Report. J. Cardiovasc. Dev. Dis. 2025, 12, 299. https://doi.org/10.3390/jcdd12080299
Melpignano A, Vitali F, Canovi L, Bonini J, Vocale LR, Bertini M. Double QRS Transition Due to Anodal Capture During Left Bundle Branch Area Pacing: A Case Report. Journal of Cardiovascular Development and Disease. 2025; 12(8):299. https://doi.org/10.3390/jcdd12080299
Chicago/Turabian StyleMelpignano, Angelo, Francesco Vitali, Luca Canovi, Jacopo Bonini, Ludovica Rita Vocale, and Matteo Bertini. 2025. "Double QRS Transition Due to Anodal Capture During Left Bundle Branch Area Pacing: A Case Report" Journal of Cardiovascular Development and Disease 12, no. 8: 299. https://doi.org/10.3390/jcdd12080299
APA StyleMelpignano, A., Vitali, F., Canovi, L., Bonini, J., Vocale, L. R., & Bertini, M. (2025). Double QRS Transition Due to Anodal Capture During Left Bundle Branch Area Pacing: A Case Report. Journal of Cardiovascular Development and Disease, 12(8), 299. https://doi.org/10.3390/jcdd12080299







