Fibroblast Growth Factor 23 Is a Strong Predictor of Adverse Events After Left Ventricular Assist Device Implantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory Parameters and Measurements
2.3. Collection of Clinical Data
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Postoperative Complications
3.3. Preoperative Plasma FGF23 Levels Predict Postoperative RHF, Post-HMII/3 Mortality and Post-HMII/3 Dialysis
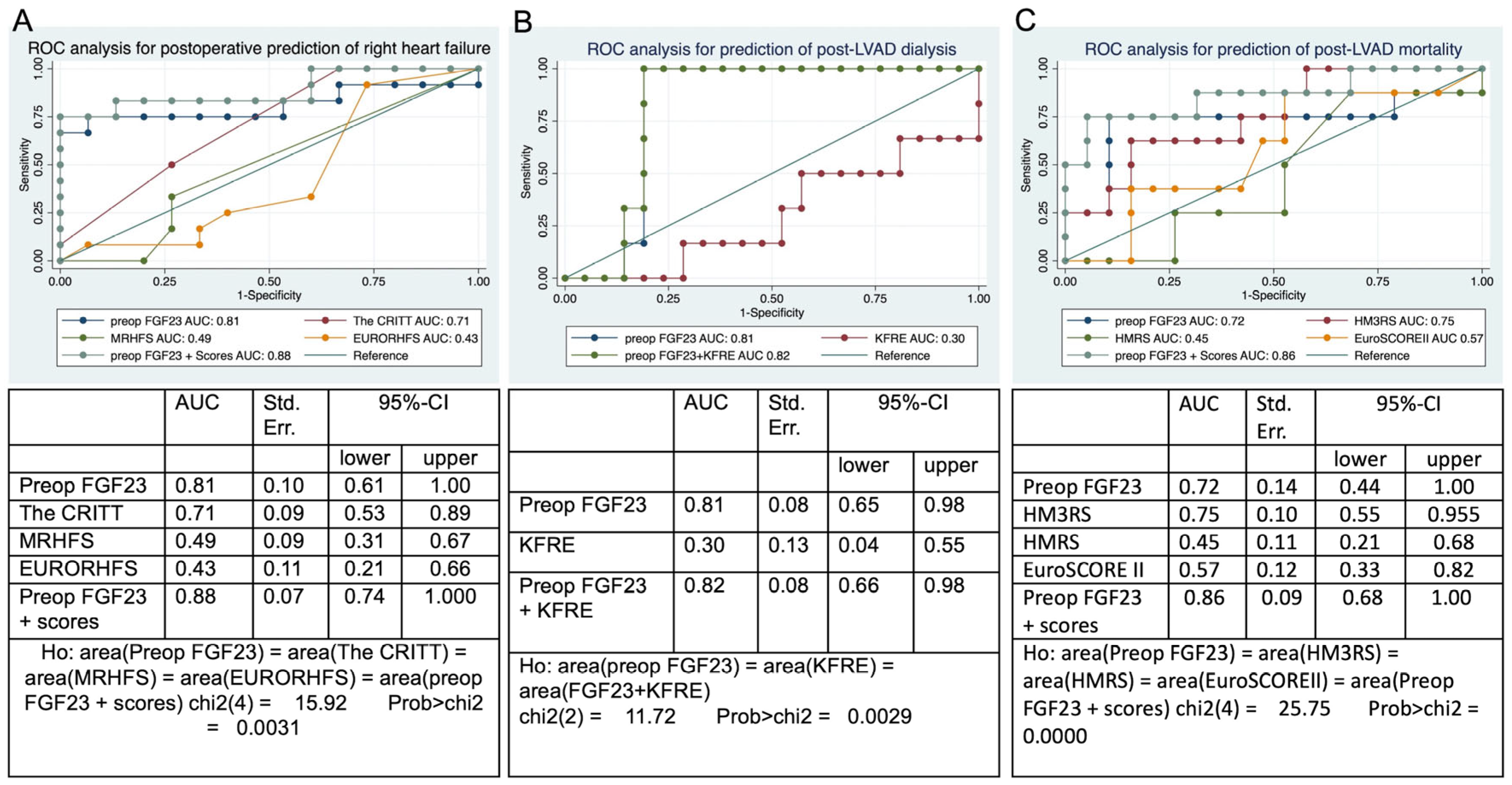
3.4. Receiver Operating Characteristic Analysis
4. Discussion
Strength and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 95%-CI | 95% confidence interval |
| AHT | Arterial hypertension |
| AST | Aspartate aminotransferase |
| AKI | Acute kidney injury |
| AUC | Area under the curve |
| BMI | Body mass index Kg/m2 |
| BSA | Body surface area m2 |
| BUN | Blood urea nitrogen |
| Bio-ADM | Bioactive adrenomedullin |
| CPB | Cardiopulmonary bypass |
| CKD | Chronic kidney disease |
| COPD | Chronic obstructive pulmonary disease |
| CRITT score | CVP greater than 15 mm Hg (C); severe RV dysfunction (R); preoperative mechanical ventilation/intubation (I); severe tricuspid regurgitation (T); and tachycardia (T) |
| CVD | Cerebrovascular disease |
| CVP | Central venous pressure |
| DCM | Dilative cardiomyopathy |
| EDTA | Ethylenediaminetetraacetic acid blood sample |
| EuroSCORE II | European system for cardiac operative risk evaluation II |
| EURORHFS | The European Registry for Patients with Mechanical Circulatory Support right heart failure score |
| FGF-23 | Fibroblast growth factor 23 |
| IDDM | Insulin-dependent diabetes mellitus |
| HLP | Hyperlipoproteinemia |
| HMII | HeartMate II |
| HM3 | HeartMate 3 |
| HMRS | The HeartMate II risk score |
| HM3RS | The HeartMate 3 risk score |
| HF | Heart failure |
| ICU | Intensive care unit |
| iNO | Inhaled nitroxide |
| INTERMACS | the Interagency Registry for Mechanically Assisted Circulatory Support |
| INR | International normalized ratio |
| ICM | Ischemic cardiomyopathy |
| KFR | kidney failure risk score |
| LVADs | Left ventricular assist devices |
| LOS | Length of stay |
| MRHFS | Michigan-right-heart-failure risk score |
| mPAP | Mean pulmonary artery pressure |
| OR | Odds ratio |
| PAD | Peripheral arterial disease |
| Penkid | Proenkephalin A |
| PHT | Pulmonary hypertension |
| RAP/PCWP | Right atrium pressure/postcapillary wedge pressure ratio |
| ROC | Receiver operating characteristic analysis |
| RHF | Right heart failure |
| RVAD | Right ventricular assist device |
| RVFAC | RV fractional area change |
| sPAP | Systolic pulmonary pressure |
| TASV | Tricuspid annular systolic velocity |
| TAPSE | Tricuspid annular plane systolic excursion |
| TIA | Transitory ischemic attack |
References
- Kanwar, M.K.; Lohmueller, L.C.; Teuteberg, J.; Kormos, R.L.; Rogers, J.G.; Benza, R.L.; Lindenfeld, J.; McIlvennan, C.; Bailey, S.H.; Murali, S.; et al. Risk Assessment in Patients with a Left Ventricular Assist Device Across INTERMACS Profiles Using Bayesian Analysis. Asaio J. 2019, 65, 436–441. [Google Scholar] [CrossRef]
- van der Horst, S.F.B.; de Jong, Y.; van Rein, N.; Jukema, J.W.; Palmen, M.; Janssen, E.; Bonneville, E.F.; Klok, F.A.; Huisman, M.V.; Tops, L.F.; et al. Performance of risk scores in predicting major bleeding in left ventricular assist device recipients: A comparative external validation. Res. Pract. Thromb. Haemost. 2024, 8, 102437. [Google Scholar] [CrossRef] [PubMed]
- Shahian, D.M.; Grover, F.L. Biomarkers and risk models in cardiac surgery. Circulation 2014, 130, 932–935. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, J.R.; Jacobs, J.P.; Alam, S.S.; Thiessen-Philbrook, H.; Everett, A.; Likosky, D.S.; Lobdell, K.; Wyler von Ballmoos, M.C.; Parker, D.M.; Garg, A.X.; et al. Utility of Biomarkers to Improve Prediction of Readmission or Mortality After Cardiac Surgery. Ann. Thorac. Surg. 2018, 106, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Speer, T.; Groesdonk, H.V.; Zapf, B.; Buescher, V.; Beyse, M.; Duerr, L.; Gewert, S.; Krauss, P.; Poppleton, A.; Wagenpfeil, S.; et al. A single preoperative FGF23 measurement is a strong predictor of outcome in patients undergoing elective cardiac surgery: A prospective observational study. Crit. Care 2015, 19, 190. [Google Scholar] [CrossRef]
- Hofer, F.; Hammer, A.; Pailer, U.; Koller, L.; Kazem, N.; Steinacher, E.; Steinlechner, B.; Andreas, M.; Laufer, G.; Wojta, J.; et al. Relationship of Fibroblast Growth Factor 23 with Hospitalization for Heart Failure and Cardiovascular Outcomes in Patients Undergoing Cardiac Surgery. J. Am. Heart Assoc. 2023, 12, e027875. [Google Scholar] [CrossRef]
- Hu, M.C.; Shiizaki, K.; Kuro-o, M.; Moe, O.W. Fibroblast growth factor 23 and Klotho: Physiology and pathophysiology of an endocrine network of mineral metabolism. Annu. Rev. Physiol. 2013, 75, 503–533. [Google Scholar] [CrossRef]
- Kurosu, H.; Kuro, O.M. The Klotho gene family as a regulator of endocrine fibroblast growth factors. Mol. Cell Endocrinol. 2009, 299, 72–78. [Google Scholar] [CrossRef]
- Navarro-García, J.A.; Fernández-Velasco, M.; Delgado, C.; Delgado, J.F.; Kuro, O.M.; Ruilope, L.M.; Ruiz-Hurtado, G. PTH, vitamin D, and the FGF-23-klotho axis and heart: Going beyond the confines of nephrology. Eur. J. Clin. Investig. 2018, 48, e12902. [Google Scholar] [CrossRef]
- Zittermann, A.; Morshuis, M.; Kuhn, J.; Pilz, S.; Ernst, J.B.; Oezpeker, C.; Dreier, J.; Knabbe, C.; Gummert, J.F.; Milting, H. Vitamin D metabolites and fibroblast growth factor-23 in patients with left ventricular assist device implants: Association with stroke and mortality risk. Eur. J. Nutr. 2016, 55, 305–313. [Google Scholar] [CrossRef]
- Vazquez-Sanchez, S.; Poveda, J.; Navarro-Garcia, J.A.; Gonzalez-Lafuente, L.; Rodriguez-Sanchez, E.; Ruilope, L.M.; Ruiz-Hurtado, G. An Overview of FGF-23 as a Novel Candidate Biomarker of Cardiovascular Risk. Front. Physiol. 2021, 12, 632260. [Google Scholar] [CrossRef]
- Bergmark, B.A.; Udell, J.A.; Morrow, D.A.; Cannon, C.P.; Steen, D.L.; Jarolim, P.; Budaj, A.; Hamm, C.; Guo, J.; Im, K.; et al. Association of Fibroblast Growth Factor 23 with Recurrent Cardiovascular Events in Patients After an Acute Coronary Syndrome: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Negishi, K.; Kobayashi, M.; Ochiai, I.; Yamazaki, Y.; Hasegawa, H.; Yamashita, T.; Shimizu, T.; Kasama, S.; Kurabayashi, M. Association between fibroblast growth factor 23 and left ventricular hypertrophy in maintenance hemodialysis patients. Comparison with B-type natriuretic peptide and cardiac troponin T. Circ. J. 2010, 74, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, O.M.; Januzzi, J.L.; Isakova, T.; Laliberte, K.; Smith, K.; Collerone, G.; Sarwar, A.; Hoffmann, U.; Coglianese, E.; Christenson, R.; et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009, 119, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Widmann, L.; Keranov, S.; Jafari, L.; Liebetrau, C.; Keller, T.; Troidl, C.; Kriechbaum, S.; Voss, S.; Arsalan, M.; Richter, M.J.; et al. Fibroblast growth factor 23 as a biomarker of right ventricular dysfunction in pulmonary hypertension. Clin. Res. Cardiol. 2023, 112, 1382–1393. [Google Scholar] [CrossRef]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.C.; Sloan, A.; Isakova, T.; Gutiérrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef]
- Akhabue, E.; Vu, T.T.; Vaidya, A.; Michos, E.D.; de Boer, I.H.; Kestenbaum, B.; Allison, M.; Szklo, M.; Ouyang, P.; Yancy, C.W.; et al. Fibroblast Growth Factor-23, Heart Failure Risk, and Renin-Angiotensin-Aldosterone-System Blockade in Hypertension: The MESA Study. Am. J. Hypertens. 2019, 32, 18–25. [Google Scholar] [CrossRef]
- Shah, P.; Yuzefpolskaya, M.; Hickey, G.W.; Breathett, K.; Wever-Pinzon, O.; Ton, V.K.; Hiesinger, W.; Koehl, D.; Kirklin, J.K.; Cantor, R.S.; et al. Twelfth Interagency Registry for Mechanically Assisted Circulatory Support Report: Readmissions After Left Ventricular Assist Device. Ann. Thorac. Surg. 2022, 113, 722–737. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Delgado, C.; Baweja, M.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; Roberts, G.V.; et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. J. Am. Soc. Nephrol. 2021, 32, 2994–3015. [Google Scholar] [CrossRef]
- Tangri, N.; Grams, M.E.; Levey, A.S.; Coresh, J.; Appel, L.J.; Astor, B.C.; Chodick, G.; Collins, A.J.; Djurdjev, O.; Elley, C.R.; et al. Multinational Assessment of Accuracy of Equations for Predicting Risk of Kidney Failure: A Meta-analysis. JAMA 2016, 315, 164–174. [Google Scholar] [CrossRef]
- Cowger, J.; Sundareswaran, K.; Rogers, J.G.; Park, S.J.; Pagani, F.D.; Bhat, G.; Jaski, B.; Farrar, D.J.; Slaughter, M.S. Predicting survival in patients receiving continuous flow left ventricular assist devices: The HeartMate II risk score. J. Am. Coll. Cardiol. 2013, 61, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Nayak, A.; Morris, A.A.; Lanfear, D.E.; Nemeh, H.; Desai, S.; Bansal, A.; Guerrero-Miranda, C.; Hall, S.; Cleveland JC, J.r.; et al. Prediction of Survival After Implantation of a Fully Magnetically Levitated Left Ventricular Assist Device. JACC Heart Fail. 2022, 10, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–744; discussion 735–744. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.C.; Koelling, T.M.; Pagani, F.D.; Aaronson, K.D. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J. Am. Coll. Cardiol. 2008, 51, 2163–2172. [Google Scholar] [CrossRef]
- Atluri, P.; Goldstone, A.B.; Fairman, A.S.; MacArthur, J.W.; Shudo, Y.; Cohen, J.E.; Acker, A.L.; Hiesinger, W.; Howard, J.L.; Acker, M.A.; et al. Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann. Thorac. Surg. 2013, 96, 857–863; discussion 854–863. [Google Scholar] [CrossRef]
- Soliman, O.I.I.; Akin, S.; Muslem, R.; Boersma, E.; Manintveld, O.C.; Krabatsch, T.; Gummert, J.F.; de By, T.; Bogers, A.; Zijlstra, F.; et al. Derivation and Validation of a Novel Right-Sided Heart Failure Model After Implantation of Continuous Flow Left Ventricular Assist Devices: The EUROMACS (European Registry for Patients with Mechanical Circulatory Support) Right-Sided Heart Failure Risk Score. Circulation 2018, 137, 891–906. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. Fibroblast growth factors. Genome Biol. 2001, 2, Reviews3005.1. [Google Scholar] [CrossRef]
- Rodelo-Haad, C.; Santamaria, R.; Muñoz-Castañeda, J.R.; Pendón-Ruiz de Mier, M.V.; Martin-Malo, A.; Rodriguez, M. FGF23, Biomarker or Target? Toxins 2019, 11, 175. [Google Scholar] [CrossRef]
- Mattinzoli, D.; Molinari, P.; Romero-Gonzalez, G.; Bover, J.; Cicero, E.; Pesce, F.; Abinti, M.; Conti, C.; Castellano, G.; Alfieri, C. Is there a role in acute kidney injury for FGF23 and Klotho? Clin. Kidney J. 2023, 16, 1555–1562. [Google Scholar] [CrossRef]
- Koller, L.; Kleber, M.E.; Brandenburg, V.M.; Goliasch, G.; Richter, B.; Sulzgruber, P.; Scharnagl, H.; Silbernagel, G.; Grammer, T.B.; Delgado, G.; et al. Fibroblast Growth Factor 23 Is an Independent and Specific Predictor of Mortality in Patients With Heart Failure and Reduced Ejection Fraction. Circ. Heart Fail. 2015, 8, 1059–1067. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Voors, A.A.; Damman, K.; van der Meer, P.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; van der Harst, P.; Hillege, H.L.; et al. Fibroblast growth factor 23 is related to profiles indicating volume overload, poor therapy optimization and prognosis in patients with new-onset and worsening heart failure. Int. J. Cardiol. 2018, 253, 84–90. [Google Scholar] [CrossRef]
- Navarro-García, J.A.; Delgado, C.; Fernández-Velasco, M.; Val-Blasco, A.; Rodríguez-Sánchez, E.; Aceves-Ripoll, J.; Gómez-Hurtado, N.; Bada-Bosch, T.; Mérida-Herrero, E.; Hernández, E.; et al. Fibroblast growth factor-23 promotes rhythm alterations and contractile dysfunction in adult ventricular cardiomyocytes. Nephrol. Dial. Transplant. 2019, 34, 1864–1875. [Google Scholar] [CrossRef]
- Bouzina, H.; Hesselstrand, R.; Rådegran, G. Higher plasma fibroblast growth factor 23 levels are associated with a higher risk profile in pulmonary arterial hypertension. Pulm. Circ. 2019, 9, 2045894019895446. [Google Scholar] [CrossRef]

| Characteristic | N = 27 |
|---|---|
| Age years | 70 (60, 73) |
| Female n (%) | 4 (15%) |
| BMI kg/m2 | 29.7 (25.2, 33.6) |
| BSA m2 | 2.09 (1.99, 2.34) |
| IDDM n (%) | 7 (26%) |
| PAD n (%) | 3 (11%) |
| CVD n (%) | 5 (19%) |
| aHT n (%) | 26 (96%) |
| Nicotine n (%) | 15 (56%) |
| EF (%) | 17.9 ± 2.9% |
| Heartmate 3 n (%) | 25 (92.6%) |
| Heartmate II n (%) | 2 (7.4%) |
| TIA n (%) | 2 (7.4%) |
| HLP | 10 (37%) |
| CKD stages | |
| 0 n (%) | 14 (52%) |
| 1 n (%) | 4 (14.8%) |
| 3 n (%) | 9 (33%) |
| Preoperative dialysis n (%) | 1 (3.7%) |
| COPD | |
| 0 n (%) | 24 (89%) |
| 2 n (%) | 1 (3.7%) |
| 3 n (%) | 2 (7.4%) |
| PHT n (%) | 17 (63%) |
| Prior cardiac surgery n (%) | 3 (11%) |
| Risk scores | |
| INTERMCS 2 n (%) | 3 (11%) |
| INTERMCS 3 n (%) | 9 (33%) |
| INTERMCS 4 n (%) | 15 (56%) |
| CRITT | 1.22 ± 0.8 |
| MRHFS | 0.74 ± 1.35 |
| EURORHFS | 3.17 ± 2.53 |
| HM3RS | 3.75 ± 0.64 |
| KFRE (%) | 4.60 ± 5.77% |
| HMRS | 1.43 ± 0.69 |
| EuroSCOREII (%) | 11.73 (7.51, 17.65) |
| Echocardiography | |
| LVEDD cm | 6.24 ± 0.55 |
| TAPSE mm | 13.89 ± 2.24 |
| TASV cm/s | 9.44 ± 1.19 |
| Preop laboratory | |
| Creatinine mg/dL | 1.43 (1.08, 1.72) |
| INR | 1.02 (0.97, 1.07) |
| Hemoglobin mg/dL | 11.17 ± 2.38 |
| Thrombocyte/nL | 227 (194, 321) |
| Albumin g/dL | 3.90 (3.60, 4.20) |
| AST U/L | 23 (21, 33) |
| Hematocrit (%) | 37.6 (34.4, 41.5) |
| BUN mg/dL | 56 (41, 62) |
| Bilirubin mg/dL | 0.44 (0.39, 0.60) |
| FGF23 plasma levels | |
| Preoperative FGF23 RU/mL | 243 (105, 1211) |
| ICU FGF23 RU/mL | 1474 (391, 6790) |
| FGF23 24 h RU/mL | 1791 (800, 11,900) |
| Preoperative right heart catheterization | |
| CO L/m | 3.79 ± 1.10 |
| CVP mmHg | 13.96 ± 5.72 |
| mPAP mmHg | 31.78 ± 15.82 |
| SPAP mHg | 45.48 ± 15.07 |
| PCWP mmHg | 23.74 ± 10.63 |
| Perioperative data | |
| OP-Zeit minutes | 239.16 ± 72.66 |
| Bypass-Zeit minutes | 110.96 ± 45.09 |
| Cross-Clamp Zeit minutes | 9.12 ± 9.88 |
| ICU stay days | 6 (4, 22) |
| ICU readmission n (%) | 6 (22%) |
| Hospital LOS days | 21 (13, 55) |
| mechanical ventilation duration h | 168 (16, 912) |
| 30-day mortality n (%) | 5 (19%) |
| >30-day mortality n (%) | 3 (11%) |
| Postoperative pneumonia n (%) | 14 (52%) |
| Post-sepsis n (%) | 7 (26%) |
| Delirium n (%) | 7 (26%) |
| AKI with dialysis n (%) | 7 (26%) |
| AKI without dialysis n (%) | 5 (19%) |
| Re-thoracotomy n (%) | 11 (41%) |
| RHF n (%) | 12 (44.4%) |
| RVAD implantation n (%) | 2 (7.4%) |
| iNO inhalation h | 14 (0, 53) |
| Device thrombus n (%) | 0 |
| Ischemic stroke n (%) | 2 (8.0%) |
| Hemorrhagic stroke | 1 (4.0%) |
| Laboratory parameters before hospital discharge | |
| fHb mg/dL | 40 (27, 60) |
| Hb g/dL | 10.20 (8.90, 10.90) |
| Platelet count/nL | 274 (170, 330) |
| INR | 2.50 (2.10, 2.90) |
| LDH U/L | 294 (254, 349) |
| AST U/L | 48 (20, 52) |
| ALT U/L | 40 (15, 60) |
| Bilirubin mg/dL | 0.34 (0.33, 0.44) |
| Creatinine mg/dL | 1.19 (0.88, 1.82) |
| BUN mg/dL | 56 (50, 66) |
| Albumin g/dL | 2.80 (2.20, 2.90) |
| Postoperative Right Heart Failure as Dependent Variable | 95% Confidence Interval | |||||
| Predictor | Estimate | SE | p | Odds Ratio | Lower | Upper |
| Intercept | 2.57 | 1.53 | 0.092 | 13.11 | 0.66 | 261.69 |
| Preop FGF23 | 0.31 | 0.28 | 0.031 | 1.37 | 0.78 | 2.38 |
| The CRITT | −0.89 | 1.09 | 0.412 | 0.41 | 0.05 | 3.45 |
| EURORHFS | −0.00 | 0.00 | 0.270 | 1.00 | 0.99 | 1.00 |
| MRHFS | −0.08 | 0.54 | 0.882 | 0.92 | 0.32 | 2.67 |
| Post-HMII/3 mortality as dependent variable | 95% Confidence Interval | |||||
| Predictor | Estimate | SE | p | Odds ratio | Lower | Upper |
| Intercept | −10.21 | 5.23 | 0.051 | 0.00 | 0.00 | 1.04 |
| Preop FGF23 | 0.00 | 0.00 | 0.025 | 1.10 | 0.90 | 1.60 |
| HM3RS | 1.82 | 1.07 | 0.088 | 6.20 | 0.76 | 50.49 |
| HMRS | 0.65 | 1.14 | 0.568 | 1.92 | 0.20 | 18.06 |
| EuroSCOREII | −0.01 | 0.04 | 0.735 | 0.99 | 0.92 | 1.06 |
| Post-HMII/3 dialysis as dependent variable | 95% Confidence Interval | |||||
| Predictor | Estimate | SE | p | Odds ratio | Lower | Upper |
| Intercept | −2.48 | 1.16 | 0.032 | 0.08 | 0.01 | 0.80 |
| Preop FGF23 | 0.00 | 0.00 | 0.032 | 1.09 | 0.91 | 1.44 |
| KFRE | −0.11 | 0.16 | 0.487 | 0.90 | 0.66 | 1.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yared, W.; Dogan, L.; Fassli, A.M.; Moza, A.; Goetzenich, A.; Stoppe, C.; Mohammed, A.F.A.; Kraemer, S.; Tewarie, L.; Abugameh, A.; et al. Fibroblast Growth Factor 23 Is a Strong Predictor of Adverse Events After Left Ventricular Assist Device Implantation. J. Cardiovasc. Dev. Dis. 2025, 12, 290. https://doi.org/10.3390/jcdd12080290
Yared W, Dogan L, Fassli AM, Moza A, Goetzenich A, Stoppe C, Mohammed AFA, Kraemer S, Tewarie L, Abugameh A, et al. Fibroblast Growth Factor 23 Is a Strong Predictor of Adverse Events After Left Ventricular Assist Device Implantation. Journal of Cardiovascular Development and Disease. 2025; 12(8):290. https://doi.org/10.3390/jcdd12080290
Chicago/Turabian StyleYared, Wissam, Leyla Dogan, Ahsannullah Madad Fassli, Ajay Moza, Andreas Goetzenich, Christian Stoppe, Ahmed F. A. Mohammed, Sandra Kraemer, Lachmandath Tewarie, Ahmad Abugameh, and et al. 2025. "Fibroblast Growth Factor 23 Is a Strong Predictor of Adverse Events After Left Ventricular Assist Device Implantation" Journal of Cardiovascular Development and Disease 12, no. 8: 290. https://doi.org/10.3390/jcdd12080290
APA StyleYared, W., Dogan, L., Fassli, A. M., Moza, A., Goetzenich, A., Stoppe, C., Mohammed, A. F. A., Kraemer, S., Tewarie, L., Abugameh, A., & Zayat, R. (2025). Fibroblast Growth Factor 23 Is a Strong Predictor of Adverse Events After Left Ventricular Assist Device Implantation. Journal of Cardiovascular Development and Disease, 12(8), 290. https://doi.org/10.3390/jcdd12080290






