Nonclinical Human Cardiac New Approach Methodologies (NAMs) Predict Vanoxerine-Induced Proarrhythmic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Complex Cardiac MPS Cell Source
2.2. Cardiac Differentiation
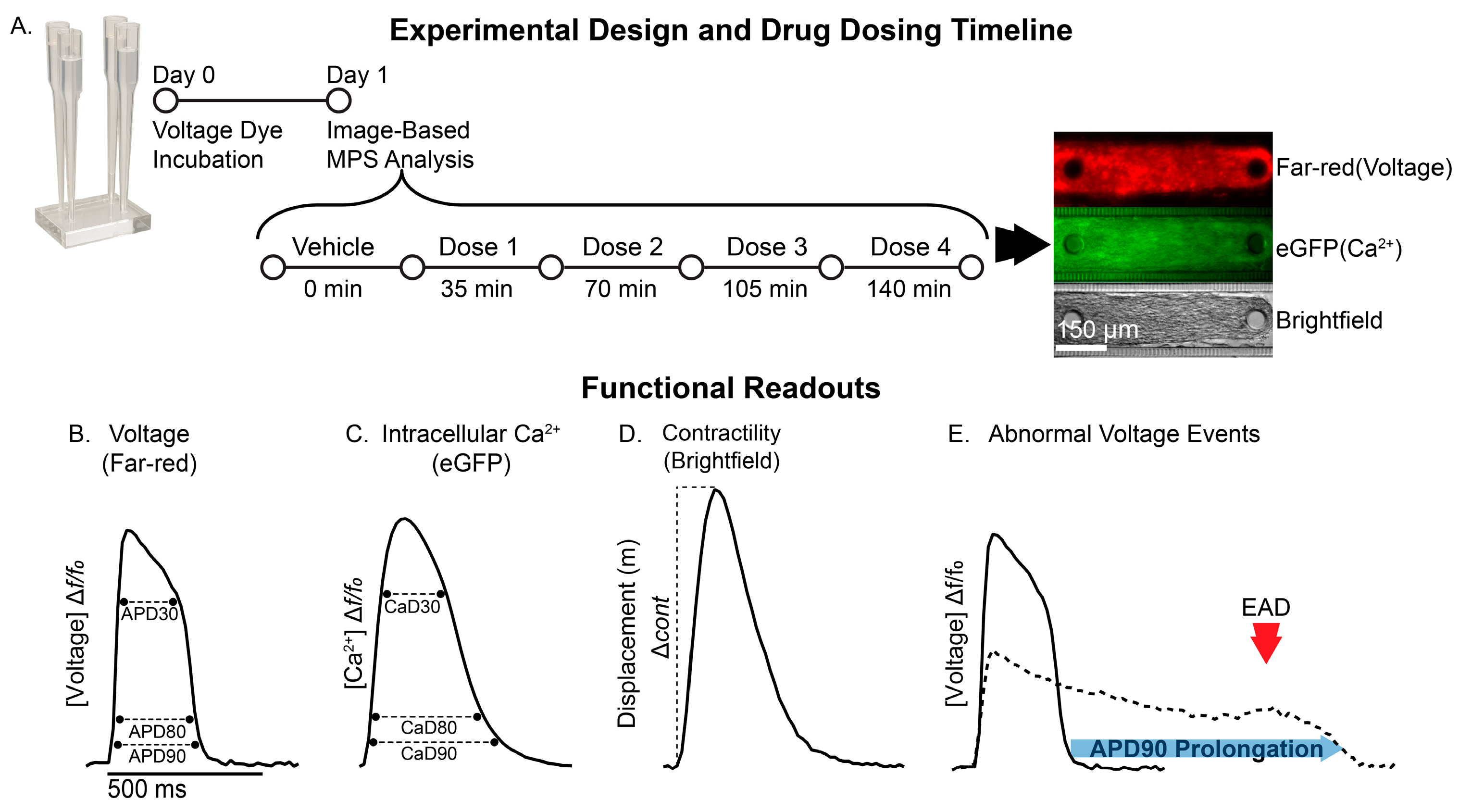
2.3. Loading of the Complex Cardiac Microphysiological System
2.4. Pharmacological Studies
2.5. Image Acquisition
2.6. Data Analysis
2.7. MEA Recordings
2.8. LC-MS/MS Analysis
2.9. Statistical Analysis
3. Results
3.1. Vanoxerine Delays Repolarization and Induces EADs in the Complex Cardiac MPS
3.2. Vanoxerine Effects Display Frequency Dependence in Cardiac MPS
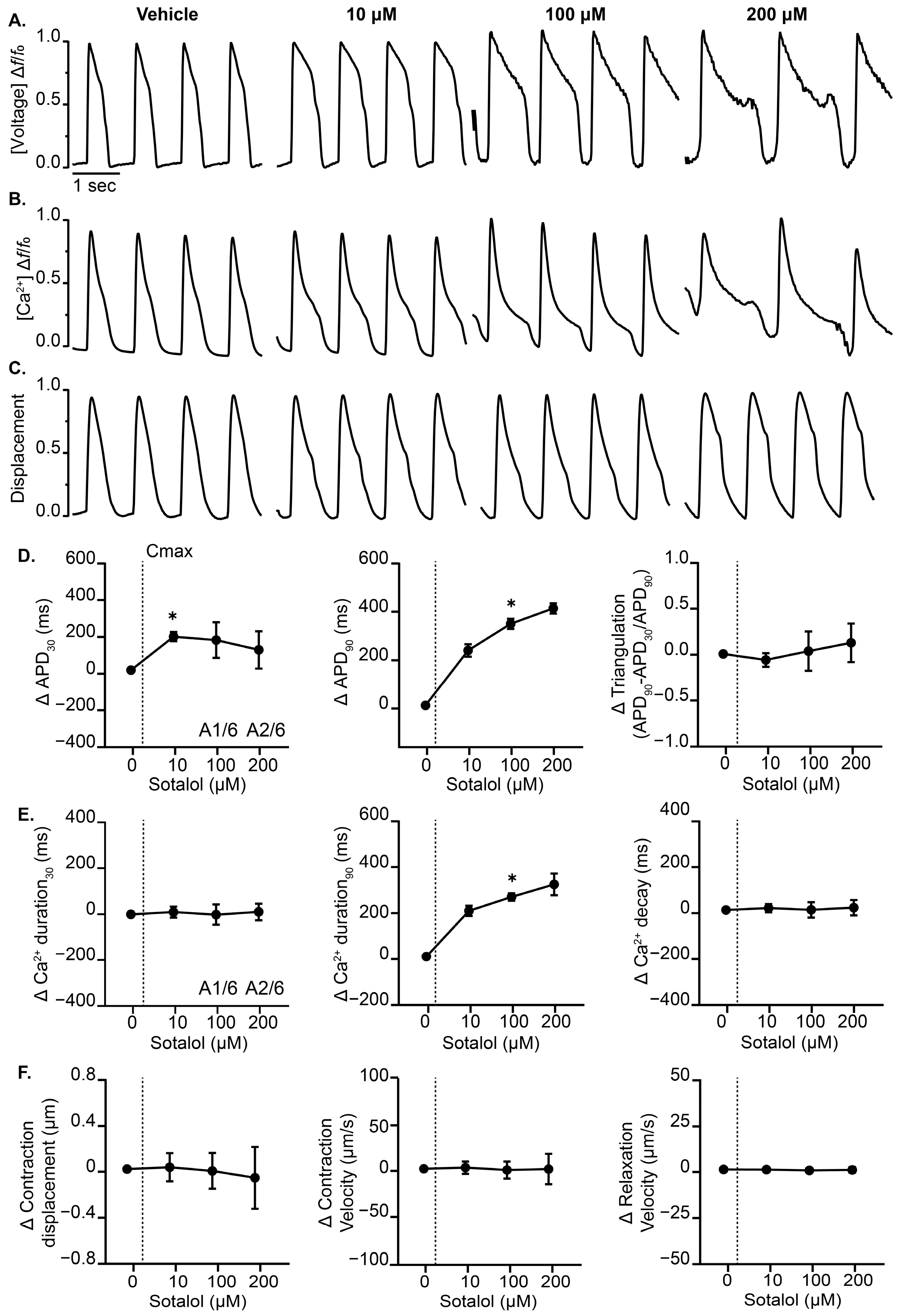
3.3. Effects of Cardiotoxic Drugs with Known Mechanism on the Complex Cardiac MPS
3.4. Complex Cardiac MPS Exposure Analysis
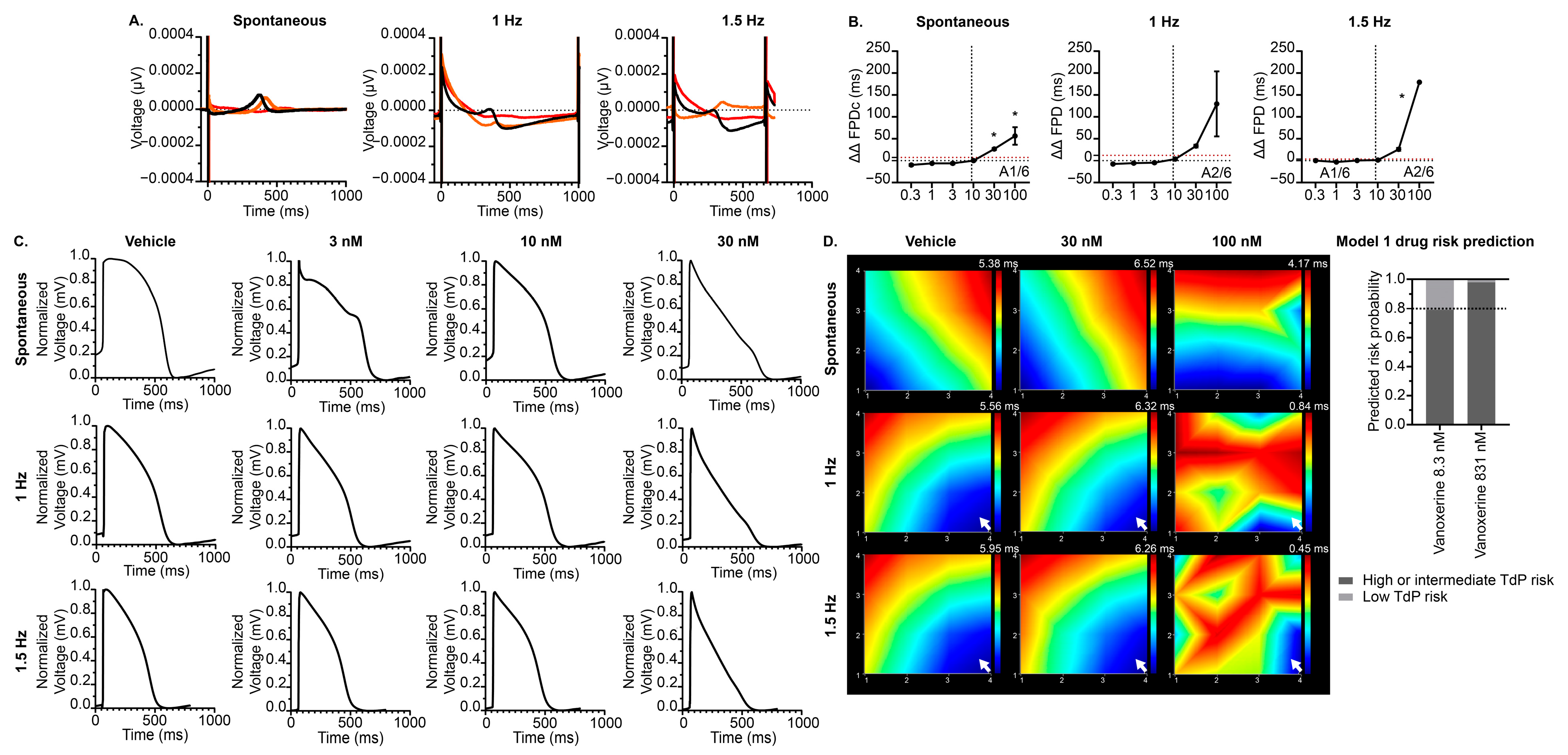
3.5. Effects of Vanoxerine on the hiPSC-CM MEA CiPA Model
4. Discussion
4.1. Nonclinical Prediction of Vanoxerine-Induced Proarrhythmia Risk
4.2. Comparison of Nonclinical and Clinical Vanoxerine Studies
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NAMs | New approach methodologies |
| MPS | Microphysiological system |
| TdP | Torsade de points |
| VSO | Voltage-sensing optical |
| CiPA | Comprehensive in vitro proarrhythmia assay |
| MEA | Multielectrode array |
| EC | Excitation–contraction |
| hiPSC-CM | Human-induced pluripotent stem cell-derived cardiomyocyte |
| AF | Atrial fibrillation |
| MICEs | Multiple ion channel effects |
| AFL | Atrial flutter |
| MM | Maturation media |
| BeRST | Berkeley rhodamine-based sensor of transmembrane potential |
| hERG | Human ether-a-go-go-related gene |
| Cav1.2 | L-type calcium channel |
| hNav1.5 | Human Nav1.5 sodium channel |
| LAA | L-ascorbic acid |
| cTnT | Cardiac troponin T |
| APD | Action potential duration |
| CaTD | Calcium transient duration |
| BPM | Beats per minute |
| FPD | Field potential duration |
| EAD | Early after depolarization |
| LEAP | Local extracellular action potential |
| Cmax | Maximum serum concentration |
References
- Dame, K.; Ribeiro, A.J.S. Microengineered systems with iPSC-derived cardiac and hepatic cells to evaluate drug adverse effects. Exp. Biol. Med. 2021, 246, 317–331. [Google Scholar] [CrossRef]
- Ferri, N.; Siegl, P.; Corsini, A.; Herrmann, J.; Lerman, A.; Benghozi, R. Drug attrition during pre-clinical and clinical development: Understanding and managing drug-induced cardiotoxicity. Pharmacol. Ther. 2013, 138, 470–484. [Google Scholar] [CrossRef]
- Garcia, M.I.; Dame, K.; Charwat, V.; Siemons, B.A.; Finsberg, H.; Bhardwaj, B.; Yokosawa, R.; Goswami, I.; Bruckner, D.; Wall, S.T.; et al. Human induced pluripotent stem cell-derived cardiomyocytes and their use in a cardiac organ-on-a-chip to assay electrophysiology, calcium and contractility. Nat. Protoc. 2025. [Google Scholar] [CrossRef]
- Charrez, B.; Charwat, V.; Siemons, B.; Finsberg, H.; Miller, E.W.; Edwards, A.G.; Healy, K.E. In vitro safety “clinical trial” of the cardiac liability of drug polytherapy. Clin. Transl. Sci. 2021, 14, 1155–1165. [Google Scholar] [CrossRef]
- Mathur, A.; Loskill, P.; Shao, K.; Huebsch, N.; Hong, S.; Marcus, S.G.; Marks, N.; Mandegar, M.; Conklin, B.R.; Lee, L.P.; et al. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci. Rep. 2015, 5, 8883. [Google Scholar] [CrossRef]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef]
- Arefin, A.; Mendoza, M.; Dame, K.; Garcia, M.I.; Strauss, D.G.; Ribeiro, A.J.S. Reproducibility of drug-induced effects on the contractility of an engineered heart tissue derived from human pluripotent stem cells. Front. Pharmacol. 2023, 14, 1212092. [Google Scholar] [CrossRef]
- Charrez, B.; Charwat, V.; Siemons, B.A.; Goswami, I.; Sakolish, C.; Luo, Y.S.; Finsberg, H.; Edwards, A.G.; Miller, E.W.; Rusyn, I.; et al. Heart Muscle Microphysiological System for Cardiac Liability Prediction of Repurposed COVID-19 Therapeutics. Front. Pharmacol. 2021, 12, 684252. [Google Scholar] [CrossRef]
- Charwat, V.; Charrez, B.; Siemons, B.A.; Finsberg, H.; Jaeger, K.H.; Edwards, A.G.; Huebsch, N.; Wall, S.; Miller, E.; Tveito, A.; et al. Validating the Arrhythmogenic Potential of High-, Intermediate-, and Low-Risk Drugs in a Human-Induced Pluripotent Stem Cell-Derived Cardiac Microphysiological System. ACS Pharmacol. Transl. Sci. 2022, 5, 652–667. [Google Scholar] [CrossRef]
- Choi, S.W.; Elmaleh, D.R.; Hanson, R.N.; Fischman, A.J. Design, synthesis, and biological evaluation of novel non-piperazine analogues of 1-[2-(diphenylmethoxy)ethyl]- and 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazines as dopamine transporter inhibitors. J. Med. Chem. 1999, 42, 3647–3656. [Google Scholar] [CrossRef]
- Lacerda, A.E.; Kuryshev, Y.A.; Yan, G.X.; Waldo, A.L.; Brown, A.M. Vanoxerine: Cellular mechanism of a new antiarrhythmic. J. Cardiovasc. Electrophysiol. 2010, 21, 301–310. [Google Scholar] [CrossRef]
- Dittrich, H.C.; Feld, G.K.; Bahnson, T.D.; Camm, A.J.; Golitsyn, S.; Katz, A.; Koontz, J.I.; Kowey, P.R.; Waldo, A.L.; Brown, A.M. COR-ART: A multicenter, randomized, double-blind, placebo-controlled dose-ranging study to evaluate single oral doses of vanoxerine for conversion of recent-onset atrial fibrillation or flutter to normal sinus rhythm. Heart Rhythm. 2015, 12, 1105–1112. [Google Scholar] [CrossRef]
- Piccini, J.P.; Pritchett, E.L.; Davison, B.A.; Cotter, G.; Wiener, L.E.; Koch, G.; Feld, G.; Waldo, A.; van Gelder, I.C.; Camm, A.J.; et al. Randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of a single oral dose of vanoxerine for the conversion of subjects with recent onset atrial fibrillation or flutter to normal sinus rhythm: RESTORE SR. Heart Rhythm. 2016, 13, 1777–1783. [Google Scholar] [CrossRef]
- Obejero-Paz, C.A.; Bruening-Wright, A.; Kramer, J.; Hawryluk, P.; Tatalovic, M.; Dittrich, H.C.; Brown, A.M. Quantitative Profiling of the Effects of Vanoxerine on Human Cardiac Ion Channels and its Application to Cardiac Risk. Sci. Rep. 2015, 5, 17623. [Google Scholar] [CrossRef]
- Cherstniakova, S.A.; Bi, D.; Fuller, D.R.; Mojsiak, J.Z.; Collins, J.M.; Cantilena, L.R. Metabolism of vanoxerine, 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine, by human cytochrome P450 enzymes. Drug Metab. Dispos. 2001, 29, 1216–1220. [Google Scholar]
- Matsumoto, N.; Khrestian, C.M.; Ryu, K.; Lacerda, A.E.; Brown, A.M.; Waldo, A.L. Vanoxerine, a new drug for terminating atrial fibrillation and flutter. J. Cardiovasc. Electrophysiol. 2010, 21, 311–319. [Google Scholar] [CrossRef]
- Maddah, M.; Heidmann, J.D.; Mandegar, M.A.; Walker, C.D.; Bolouki, S.; Conklin, B.R.; Loewke, K.E. A non-invasive platform for functional characterization of stem-cell-derived cardiomyocytes with applications in cardiotoxicity testing. Stem Cell Rep. 2015, 4, 621–631. [Google Scholar] [CrossRef]
- Huebsch, N.; Loskill, P.; Mandegar, M.A.; Marks, N.C.; Sheehan, A.S.; Ma, Z.; Mathur, A.; Nguyen, T.N.; Yoo, J.C.; Judge, L.M.; et al. Automated Video-Based Analysis of Contractility and Calcium Flux in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Cultured over Different Spatial Scales. Tissue Eng. Part. C Methods 2015, 21, 467–479. [Google Scholar] [CrossRef]
- Huebsch, N.; Charrez, B.; Neiman, G.; Siemons, B.; Boggess, S.C.; Wall, S.; Charwat, V.; Jaeger, K.H.; Cleres, D.; Telle, A.; et al. Metabolically driven maturation of human-induced-pluripotent-stem-cell-derived cardiac microtissues on microfluidic chips. Nat. Biomed. Eng. 2022, 6, 372–388. [Google Scholar] [CrossRef]
- Huang, Y.L.; Walker, A.S.; Miller, E.W. A Photostable Silicon Rhodamine Platform for Optical Voltage Sensing. J. Am. Chem. Soc. 2015, 137, 10767–10776. [Google Scholar] [CrossRef]
- Ribeiro, A.J.S.; Schwab, O.; Mandegar, M.A.; Ang, Y.S.; Conklin, B.R.; Srivastava, D.; Pruitt, B.L. Multi-Imaging Method to Assay the Contractile Mechanical Output of Micropatterned Human iPSC-Derived Cardiac Myocytes. Circ. Res. 2017, 120, 1572–1583. [Google Scholar] [CrossRef]
- Blinova, K.; Dang, Q.; Millard, D.; Smith, G.; Pierson, J.; Guo, L.; Brock, M.; Lu, H.R.; Kraushaar, U.; Zeng, H.; et al. International Multisite Study of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Drug Proarrhythmic Potential Assessment. Cell Rep. 2018, 24, 3582–3592. [Google Scholar] [CrossRef]
- Ma, J.; Guo, L.; Fiene, S.J.; Anson, B.D.; Thomson, J.A.; Kamp, T.J.; Kolaja, K.L.; Swanson, B.J.; January, C.T. High purity human-induced pluripotent stem cell-derived cardiomyocytes: Electrophysiological properties of action potentials and ionic currents. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2006–H2017. [Google Scholar] [CrossRef]
- Feaster, T.K.; Casciola, M.; Narkar, A.; Blinova, K. Acute effects of cardiac contractility modulation on human induced pluripotent stem cell-derived cardiomyocytes. Physiol. Rep. 2021, 9, e15085. [Google Scholar] [CrossRef]
- Tran, P.N.; Sheng, J.; Randolph, A.L.; Baron, C.A.; Thiebaud, N.; Ren, M.; Wu, M.; Johannesen, L.; Volpe, D.A.; Patel, D.; et al. Mechanisms of QT prolongation by buprenorphine cannot be explained by direct hERG channel block. PLoS ONE 2020, 15, e0241362. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- George, S.A.; Lin, Z.; Efimov, I.R. Simultaneous triple-parametric optical mapping of transmembrane potential, intracellular calcium and NADH for cardiac physiology assessment. Commun. Biol. 2022, 5, 319. [Google Scholar] [CrossRef]
- Kerr, C.M.; Richards, D.; Menick, D.R.; Deleon-Pennell, K.Y.; Mei, Y. Multicellular Human Cardiac Organoids Transcriptomically Model Distinct Tissue-Level Features of Adult Myocardium. Int. J. Mol. Sci. 2021, 22, 8482. [Google Scholar] [CrossRef]
- Richards, D.J.; Coyle, R.C.; Tan, Y.; Jia, J.; Wong, K.; Toomer, K.; Menick, D.R.; Mei, Y. Inspiration from heart development: Biomimetic development of functional human cardiac organoids. Biomaterials 2017, 142, 112–123. [Google Scholar] [CrossRef]
- Richards, D.J.; Li, Y.; Kerr, C.M.; Yao, J.; Beeson, G.C.; Coyle, R.C.; Chen, X.; Jia, J.; Damon, B.; Wilson, R.; et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 2020, 4, 446–462. [Google Scholar] [CrossRef]
- Tan, Y.; Richards, D.; Xu, R.; Stewart-Clark, S.; Mani, S.K.; Borg, T.K.; Menick, D.R.; Tian, B.; Mei, Y. Silicon nanowire-induced maturation of cardiomyocytes derived from human induced pluripotent stem cells. Nano Lett. 2015, 15, 2765–2772. [Google Scholar] [CrossRef]
- Feaster, T.K.; Casciola, M.; Narkar, A.; Blinova, K. Evaluation of Cardiac Contractility Modulation Therapy in 2D Human Stem Cell-Derived Cardiomyocytes. J. Vis. Exp. 2022, 190, e64848. [Google Scholar] [CrossRef]
- Feric, N.T.; Pallotta, I.; Singh, R.; Bogdanowicz, D.R.; Gustilo, M.; Chaudhary, K.; Willette, R.N.; Chendrimada, T.; Xu, X.; Graziano, M.P.; et al. Engineered Cardiac Tissues Generated in the Biowire II: A Platform for Human-Based Drug Discovery. Toxicol. Sci. 2019, 172, 89–97. [Google Scholar] [CrossRef]
- Heinson, Y.W.; Han, J.L.; Entcheva, E. OptoDyCE-plate as an affordable high throughput imager for all optical cardiac electrophysiology. J. Mol. Cell Cardiol. Plus 2023, 6, 100054. [Google Scholar] [CrossRef]
- Strock, C.J. Evaluating the Arrhythmic Potential of Vanoxerine in Human iPSC Derived Cardiomyocytes on a Multiwell MEA; Society of Toxicology Poster Session: 2017. Available online: https://www.axionbiosystems.com/resources/poster/evaluating-arrhythmic-potential-vanoxerine-human-ipsc-derived-cardiomyocytes (accessed on 16 May 2025).
- Hagiwara-Nagasawa, M.; Kambayashi, R.; Goto, A.; Nunoi, Y.; Izumi-Nakaseko, H.; Takei, Y.; Matsumoto, A.; Sugiyama, A. Cardiohemodynamic and Arrhythmogenic Effects of the Anti-Atrial Fibrillatory Compound Vanoxerine in Halothane-Anesthetized Dogs. Cardiovasc. Toxicol. 2021, 21, 206–215. [Google Scholar] [CrossRef]
- FDA Announces Plan to Phase Out Animal Testing Requirement for Monoclonal Antibodies and Other Drugs. Available online: https://www.fda.gov/news-events/press-announcements/fda-announces-plan-phase-out-animal-testing-requirement-monoclonal-antibodies-and-other-drugs (accessed on 10 April 2025).
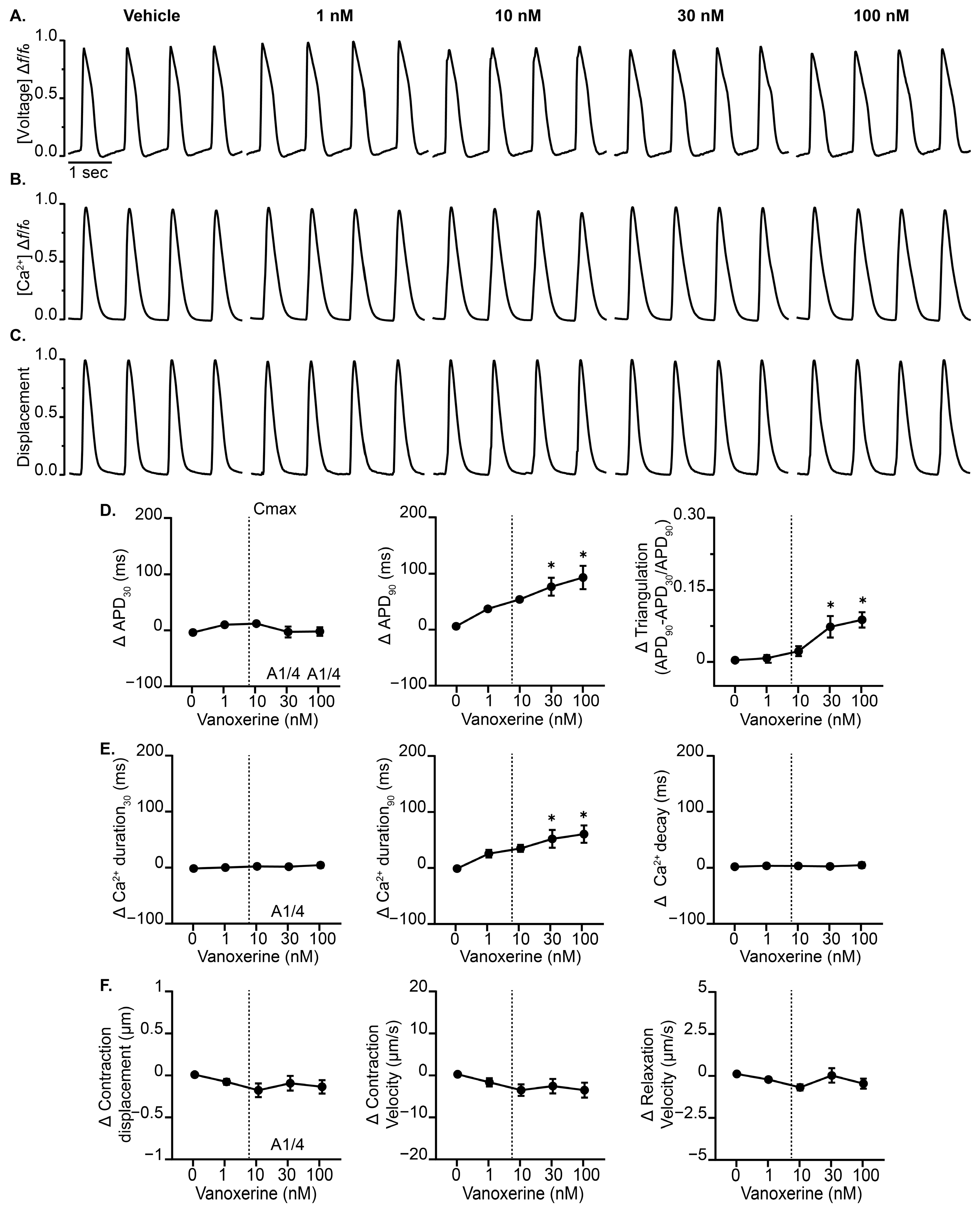

| Parameter | 1 nM | 10 nM | 30 nM | 100 nM |
|---|---|---|---|---|
| Voltage | ||||
| Δ APD30cF (ms) | 30.1 ± 2.9 | 54.7 ± 14.7 * | 57.8 ± 21.7 * | 60.92 ± 17.9 * |
| Δ APD80cF (ms) | 117.0 ± 17 | 360.4 ± 101.4 * | 425.5 ± 93.8 * | 344.8 ± 66 * |
| Δ Triangulation | 0.04 ± 0.01 | 0.1 ± 0.05 | 0.2 ± 0.05 * | 0.1 ± 0.04 |
| Observed EADs | 0/4 | 2/4 | 2/4 | 1/4 |
| ΔBPM | −2.9 ± 2.3 | −10.6 ± 3.7 * | −7.8 ± 2.4 | −11.7 ± 3.5 * |
| Intracellular Calcium | ||||
| Δ CaTD30cF (ms) | 16.1 ± 5.8 | 32.3 ± 14.6 | 50.2 ± 32.9 * | 41.2 ± 21.5 * |
| Δ CaTD80cF (ms) | 92.5 ± 14.2 | 286.0 ± 106.5 * | 332.5 ± 93.8 * | 281.8 ± 78.9 * |
| Δ Ca decay (ms) | 4.8 ± 1.8 | 1.51 ± 4.8 | 5.9 ± 6.9 | 7.9 ± 7 |
| Observed EADs | 0/4 | 2/4 | 2/4 | 2/4 |
| ΔBPM | −5.1 ± 1.7 | −9.5 ± 3.2 | −12.1 ± 4.8 * | −8.5 ± 3.4 |
| Contractility | ||||
| Δ Contraction displacement (µm) | −03 ± 0.03 | 0.06 ± 0.04 | −0.09 ± 0.06 | −0.1 ± 0.09 |
| Δ Contraction velocity (µm/s) | −1.37 ± 0.7 | −2.06 ± 1.1 | −2.81 ± 1.4 | −3.57 ± 1.9 |
| Δ Relaxation velocity (µm/s) | −0.4 ± 0.1 | −0.65 ± 0.2 | −0.73 ± 0.2 | −0.71 ± 0.3 |
| Observed EADs | 0/4 | 1/4 | 2/4 | 1/4 |
| ΔBPM | −5.8 ± 2.2 | −9.9 ± 4.1 | −12.3 ± 3.9 | −12.6 ± 3.9 * |
| N | 4 | 4 | 4 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, M.I.; Bhardwaj, B.; Dame, K.; Charwat, V.; Siemons, B.A.; Goswami, I.; Ismaiel, O.A.; Mistry, S.; Feaster, T.K.; Healy, K.E.; et al. Nonclinical Human Cardiac New Approach Methodologies (NAMs) Predict Vanoxerine-Induced Proarrhythmic Potential. J. Cardiovasc. Dev. Dis. 2025, 12, 285. https://doi.org/10.3390/jcdd12080285
Garcia MI, Bhardwaj B, Dame K, Charwat V, Siemons BA, Goswami I, Ismaiel OA, Mistry S, Feaster TK, Healy KE, et al. Nonclinical Human Cardiac New Approach Methodologies (NAMs) Predict Vanoxerine-Induced Proarrhythmic Potential. Journal of Cardiovascular Development and Disease. 2025; 12(8):285. https://doi.org/10.3390/jcdd12080285
Chicago/Turabian StyleGarcia, M. Iveth, Bhavya Bhardwaj, Keri Dame, Verena Charwat, Brian A. Siemons, Ishan Goswami, Omnia A. Ismaiel, Sabyasachy Mistry, Tromondae K. Feaster, Kevin E. Healy, and et al. 2025. "Nonclinical Human Cardiac New Approach Methodologies (NAMs) Predict Vanoxerine-Induced Proarrhythmic Potential" Journal of Cardiovascular Development and Disease 12, no. 8: 285. https://doi.org/10.3390/jcdd12080285
APA StyleGarcia, M. I., Bhardwaj, B., Dame, K., Charwat, V., Siemons, B. A., Goswami, I., Ismaiel, O. A., Mistry, S., Feaster, T. K., Healy, K. E., Ribeiro, A. J. S., & Blinova, K. (2025). Nonclinical Human Cardiac New Approach Methodologies (NAMs) Predict Vanoxerine-Induced Proarrhythmic Potential. Journal of Cardiovascular Development and Disease, 12(8), 285. https://doi.org/10.3390/jcdd12080285







