Application and Outcomes of Minimal-Dose Versus Standard-Dose Radiation in Peripheral Endovascular Intervention (KAR Endovascular Study)
Abstract
1. Introduction
2. Material and Methods
Statistical Methods
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreassi, M.G.; Piccaluga, E.; Guagliumi, G.; Del Greco, M.; Gaita, F.; Picano, E. Occupational Health Risks in Cardiac Catheterization Laboratory Workers. Circ. Cardiovasc. Interv. 2016, 9, e003273. [Google Scholar] [CrossRef] [PubMed]
- Karatasakis, A.; Brilakis, H.S.; Danek, B.A.; Karacsonyi, J.; Martinez-Parachini, J.R.; Nguyen-Trong, P.J.; Alame, A.J.; Roesle, M.K.; Rangan, B.V.; Rosenfield, K.; et al. Radiation-associated lens changes in the cardiac catheterization laboratory: Results from the IC-CATARACT (CATaracts Attributed to RAdiation in the CaTh lab) study. Catheter. Cardiovasc. Interv. 2018, 91, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Lertsuwunseri, V.; Srimahachota, S.; Krisanachinda, A.; Tulvatana, W.; Khambhiphant, B.; Sudchai, W.; Rehani, M. Eye lens dosimetry and the study on radiation cataract in interventional cardiologists. Phys. Med. 2017, 44, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Elmaraezy, A.; Ebraheem Morra, M.; Tarek Mohammed, A.; Al-Habaa, A.; Elgebaly, A.; Abdelmotaleb Ghazy, A.; Khalil, A.M.; Tien Huy, N.; Hirayama, K. Risk of cataract among interventional cardiologists and catheterization lab staff: A systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2017, 90, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Roguin, A.; Goldstein, J.; Bar, O.; Goldstein, J.A. Brain and neck tumors among physicians performing interventional procedures. Am. J. Cardiol. 2013, 111, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.W.; Tra, Y.; Garratt, K.N.; Powell, W.; Lopez-Cruz, G.; Chambers, C.; Goldstein, J.A.; Society for Cardiovascular Angiography and Interventions. Occupational health hazards of interventional cardiologists in the current decade: Results of the 2014 SCAI membership survey. Catheter. Cardiovasc. Interv. 2015, 86, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Venneri, L.; Rossi, F.; Botto, N.; Andreassi, M.G.; Salcone, N.; Emad, A.; Lazzeri, M.; Gori, C.; Vano, E.; Picano, E. Cancer risk from professional exposure in staff working in cardiac catheterization laboratory: Insights from the National Research Council’s Biological Effects of Ionizing Radiation VII Report. Am. Heart J. 2009, 157, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Attigah, N.; Oikonomou, K.; Hinz, U.; Knoch, T.; Demirel, S.; Verhoeven, E.; Bockler, D. Radiation exposure to eye lens and operator hands during endovascular procedures in hybrid operating rooms. J. Vasc. Surg. 2016, 63, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Sigterman, T.A.; Bolt, L.J.; Snoeijs, M.G.; Krasznai, A.G.; Heijboer, R.; Schurink, G.W.; Bouwman, L.H. Radiation Exposure during Percutaneous Transluminal Angioplasty for Symptomatic Peripheral Arterial Disease. Ann. Vasc. Surg. 2016, 33, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Balter, S.; Hopewell, J.W.; Miller, D.L.; Wagner, L.K.; Zelefsky, M.J. Fluoroscopically guided interventional procedures: A review of radiation effects on patients’ skin and hair. Radiology 2010, 254, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, M.L.; Arbique, G.M.; Guild, J.B.; Timaran, C.; Valentine, R.J.; Anderson, J.A. Radiation-induced skin injury after complex endovascular procedures. J. Vasc. Surg. 2014, 60, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Teleb, M.; Albaghdadi, A.; Ibrahim, A.; Mukherjee, D. Efficacy of Low-Dose Compared with Standard-Dose Radiation for Cardiac Catheterization and Intervention (KAR RAD Study). J. Invasive Cardiol. 2019, 31, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Amlani, V.; Ludwigs, K.; Rawshani, A.; Thuresson, M.; Falkenberg, M.; Smidfelt, K.; Nordanstig, J. Editor’s Choice—Major Adverse Limb Events in Patients Undergoing Revascularisation for Lower Limb Peripheral Arterial Disease: A Nationwide Observational Study. Eur. J. Vasc. Endovasc. Surg. 2024, 68, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Bax, M.; Romanov, V.; Junday, K.; Giannoulatou, E.; Martinac, B.; Kovacic, J.C.; Liu, R.; Iismaa, S.E.; Graham, R.M. Arterial dissections: Common features and new perspectives. Front. Cardiovasc. Med. 2022, 9, 1055862. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Bhagwandeen, R.; Collins, N. Contemporary Management of Coronary Artery Perforation. Heart Lung Circ. 2019, 28, e121–e125. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.E.; Blaine, C.; Dawnay, A.; Devonald, M.A.; Ftouh, S.; Laing, C.; Latchem, S.; Lewington, A.; Milford, D.V.; Ostermann, M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015, 87, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.; Spiliopoulos, S.; Kitrou, P.; Krokidis, M.; Karnabatidis, D. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2018, 7, e011245. [Google Scholar] [CrossRef] [PubMed]
- Goldsweig, A.M.; Kennedy, K.F.; Abbott, J.D.; Jones, W.S.; Velagapudi, P.; Rutar, F.J.; Curtis, J.C.; Tsai, T.T.; Aronow, H.D. Patient Radiation Dosage During Lower Extremity Endovascular Intervention. JACC Cardiovasc. Interv. 2019, 12, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Weinberg, I.; Leichter, I.; Klimov, A.; Giri, J.; Bloom, A.I. Patient radiation exposure during percutaneous endovascular revascularization of the lower extremity. J. Vasc. Surg. 2013, 58, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Akkus, N.I.; Mina, G.S.; Abdulbaki, A.; Shafiei, F.; Tandon, N. Using 7.5 frames per second reduces radiation exposure in lower extremity peripheral vascular interventions. Vascular 2015, 23, 240–244. [Google Scholar] [CrossRef] [PubMed]
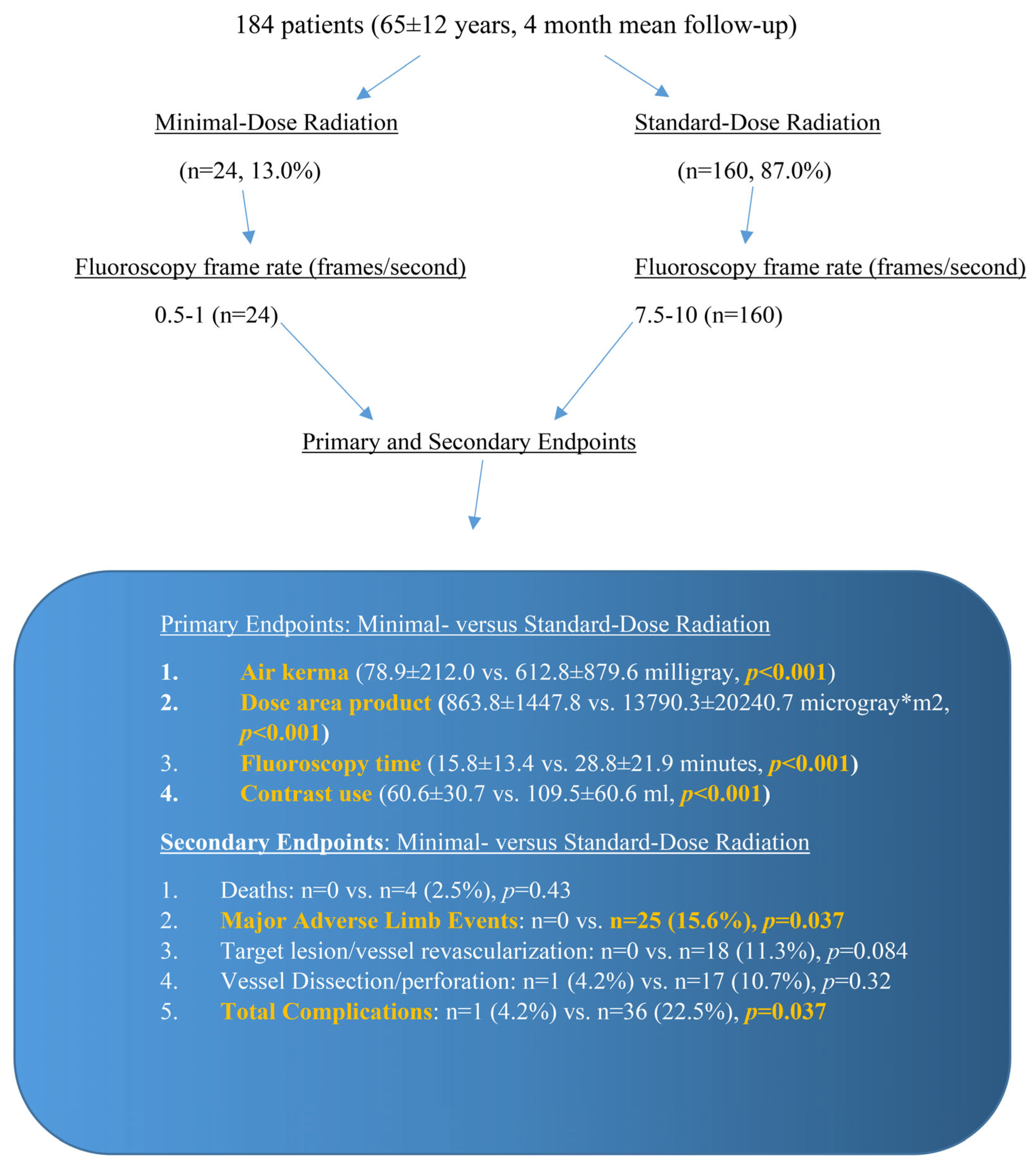

| Total | Minimal | Standard | p-Value | |
|---|---|---|---|---|
| n (%) | 184 | 24 (13.0%) | 160 (87.0%) | |
| Age, mean (SD*) | 65.0 (11.9) | 67.8 (9.6) | 64.6 (12.2) | 0.22 |
| Weight (kg), mean (SD*) | 76.4 (17.1) | 74.7 (14.7) | 76.7 (17.4) | 0.59 |
| Height (cm), mean (SD*) | 163.9 (12.4) | 163.4 (7.0) | 163.9 (13.0) | 0.84 |
| Body Mass Index (kg/m2), mean (SD*) | 28.3 (6.0) | 28.0 (5.0) | 28.3 (6.2) | 0.79 |
| Body Surface Area (m2), mean (SD*) | 3.7 (17.6) | 1.8 (0.2) | 4.0 (18.9) | 0.59 |
| Fluoroscopy Time (min), mean (SD*) | 27.1 (21.4) | 15.8 (13.4) | 28.8 (21.9) | <0.001 |
| DAP† (uGy×m2), mean (SD*) | 12,085.7 (19,361.4) | 863.8 (1447.8) | 13,790.3 (20,240.7) | <0.001 |
| Air Kerma (mGy), mean (SD*) | 542.4 (842.4) | 78.9 (212.0) | 612.8 (879.6) | <0.001 |
| Contrast (mL), mean (SD*) | 102.2 (59.7) | 60.6 (30.7) | 109.5 (60.6) | <0.001 |
| Follow-up (months), mean (SD*) | 3.9 (3.6) | 4.3 (5.2) | 3.8 (3.2) | 0.52 |
| Risk Factors Diabetes | 124 (67.4%) | 20 (83.3%) | 104 (65.0%) | 0.074 |
| Hypertension | 138 (75.0%) | 20 (83.3%) | 118 (73.8%) | 0.31 |
| Hyperlipidemia | 98 (53.3%) | 11 (45.8%) | 87 (54.4%) | 0.43 |
| Past Medical History of CAD‡ | 57 (31.0%) | 10 (41.7%) | 47 (29.4%) | 0.22 |
| Past History of MI/PCI§ | 49 (26.6%) | 9 (37.5%) | 40 (25.0%) | 0.20 |
| Atrial fibrillation/Atrial Flutter | 13 (7.1%) | 5 (20.8%) | 8 (5.0%) | 0.005 |
| History of CKD/ESRD||, On Hemodialysis | 6 (3.3%) | 1 (4.2%) | 5 (3.1%) | 0.79 |
| History of Peripheral Intervention | 46 (25.0%) | 9 (37.5%) | 37 (23.1%) | 0.13 |
| History of Vascular Surgery | 5 (2.7%) | 2 (8.3%) | 3 (1.9%) | 0.070 |
| History of Acute/Critical Limb Ischemia | 29 (15.8%) | 7 (29.2%) | 22 (13.8%) | 0.053 |
| Current/Former Smoker | 90 (48.9%) | 9 (37.5%) | 81 (50.6%) | 0.23 |
| History of Amputation | 28 (15.2%) | 6 (25.0%) | 22 (13.8%) | 0.15 |
| Procedural Indications | ||||
| Intermittent Claudication | 27 (14.7%) | 5 (20.8%) | 22 (13.8%) | 0.36 |
| Critical Limb Ischemia | 89 (48.4%) | 15 (62.5%) | 74 (46.3%) | 0.14 |
| Acute Limb Ischemia | 30 (16.3%) | 3 (12.5%) | 27 (16.9%) | 0.59 |
| Lower Extremity Edema/DVT{/May Thurner/CTO# Vein | 25 (13.6%) | 1 (4.2%) | 24 (15.0%) | 0.15 |
| Venous Stenosis | 2 (1.1%) | 0 (0.0%) | 2 (1.3%) | 0.58 |
| Left Heart Catheterization with Peripheral Angiogram | 6 (3.3%) | 0 (0.0%) | 6 (3.8%) | 0.33 |
| Peripheral Aneurysm | 4 (2.2%) | 0 (0.0%) | 4 (2.5%) | 0.43 |
| Interventional Location and Access Site Suprainguinal Intervention | 40 (21.7%) | 2 (8.3%) | 38 (23.8%) | 0.088 |
| Infrainguinal Intervention | 92 (50.0%) | 8 (33.3%) | 84 (52.5%) | 0.080 |
| Infrapopliteal Intervention | 66 (35.9%) | 10 (41.7%) | 56 (35.0%) | 0.53 |
| Femoral Access | 156 (84.8%) | 24 (100.0%) | 132 (82.5%) | 0.026 |
| Pedal Access | 18 (9.8%) | 0 (0.0%) | 18 (11.3%) | 0.084 |
| Brachial/Radial Access | 28 (15.2%) | 0 (0.0%) | 28 (17.5%) | 0.026 |
| Retrograde Approach | 177 (96.2%) | 23 (95.8%) | 154 (96.3%) | 0.92 |
| Antegrade Approach | 1 (0.5%) | 1 (4.2%) | 0 (0.0%) | 0.010 |
| Peripheral Intervention | 144 (78.3%) | 17 (70.8%) | 127 (79.4%) | 0.34 |
| CTO# Intervention | 54 (29.3%) | 9 (37.5%) | 45 (28.1%) | 0.35 |
| Venous Intervention | 19 (10.3%) | 1 (4.2%) | 18 (11.3%) | 0.29 |
| Staged Intervention | 17 (9.2%) | 3 (12.5%) | 14 (8.8%) | 0.55 |
| TASC** Classification A | 16 (8.7%) | 4 (16.7%) | 12 (7.5%) | 0.14 |
| TASC** Classification B | 54 (29.3%) | 9 (37.5%) | 45 (28.1%) | 0.35 |
| TASC** Classification C | 56 (30.4%) | 3 (12.5%) | 53 (33.1%) | 0.041 |
| TASC** Classification D | 13 (7.1%) | 5 (20.8%) | 8 (5.0%) | 0.005 |
| Rutherford Grade I | 27 (14.7%) | 5 (20.8%) | 22 (13.8%) | 0.36 |
| Rutherford Grade II | 55 (29.9%) | 10 (41.7%) | 45 (28.1%) | 0.18 |
| Rutherford Grade III | 51 (27.7%) | 5 (20.8%) | 46 (28.7%) | 0.42 |
| Ruth Grade IV | 1 (0.5%) | 1 (4.2%) | 0 (0.0%) | 0.01 |
| Fontaine Stage I | 0% | |||
| Fontaine Stage II | 33 (17.9%) | 5 (20.8%) | 28 (17.5%) | 0.69 |
| Fontaine Stage III | 37 (20.1%) | 7 (29.2%) | 30 (18.8%) | 0.24 |
| Fontaine Stage IV | 50 (27.2%) | 6 (25.0%) | 44 (27.5%) | 0.8 |
| Peripheral Diagnostic Angiogram | Minimal Dose | Standard Dose | p-Value |
|---|---|---|---|
| n (%) | 7 (29.2%) | 32 (20.0%) | |
| Age, mean (SD*) | 64.4 (6.5) | 64.7 (9.4) | 0.95 |
| Weight (kg), mean (SD*) | 74.4 (16.6) | 74.9 (18.1) | 0.95 |
| Height (cm), mean (SD*) | 164.7 (6.2) | 162.5 (10.6) | 0.59 |
| Body Mass Index (kg/m2), mean (SD*) | 27.3 (6.2) | 28.3 (6.4) | 0.71 |
| Body Surface Area (m2), mean (SD*) | 1.8 (0.2) | 7.1 (29.9) | 0.65 |
| Fluoroscopy Time (min), mean (SD*) | 7.9 (8.9) | 16.2 (18.9) | 0.007 |
| DAP† (uGy×m2), mean (SD*) | 1233.5 (2494.6) | 12,432.3 (8759.1) | <0.001 |
| Air Kerma (mGy), mean (SD*) | 67.0 (147.7) | 550.8 (431.2) | <0.001 |
| Contrast (mL), mean (SD*) | 42.6 (28.0) | 81.5 (44.9) | 0.013 |
| Follow-up (months), mean (SD*) | 3.8 (6.3) | 3.3 (2.8) | 0.43 |
| Peripheral Intervention | Minimal Dose | Standard Dose | p-Value |
| n (%) | 17 (70.8%) | 128 (80.0%) | |
| Age, mean (SD*) | 69.1 (10.5) | 64.5 (12.8) | 0.16 |
| Weight (kg), mean (SD*) | 74.8 (14.5) | 77.1 (17.3) | 0.59 |
| Height (cm), mean (SD*) | 162.8 (7.5) | 164.3 (13.6) | 0.67 |
| Body Mass Index (kg/m2), mean (SD*) | 28.3 (4.5) | 28.3 (6.2) | 0.96 |
| Body Surface Area (m2), mean (SD*) | 1.8 (0.2) | 3.2 (15.0) | 0.72 |
| Fluoroscopy Time (min), mean (SD*) | 19.0 (13.8) | 31.8 (21.5) | <0.001 |
| DAP† (uGy×m2), mean (SD*) | 711.6 (771.5) | 14,108.6 (22,099.9) | <0.001 |
| Air Kerma (mGy), mean (SD*) | 83.8 (237.3) | 627.3 (955.5) | <0.001 |
| Contrast (mL), mean (SD*) | 68.1 (29.4) | 116.3 (62.2) | 0.006 |
| Follow-up (months), mean (SD*) | 4.5 (4.9) | 3.9 (3.3) | 0.90 |
| Total | Minimal | Standard | p-Value | |
|---|---|---|---|---|
| Variables (n, %) | 184 | 24 (13.0%) | 160 (87.0%) | |
| Total Complications | 37 (20.1%) | 1 (4.2%) | 36 (22.5%) | 0.037 |
| Major Adverse Limb Event | 25 (13.6%) | 0 (0.0%) | 25 (15.6%) | 0.037 |
| Myocardial Infarction | 4 (2.2%) | 0 (0.0%) | 4 (2.5%) | 0.43 |
| Stroke | 1 (0.5%) | 0 (0.0%) | 1 (0.6%) | 0.70 |
| Dissection/Perforation | 18 (9.8%) | 1 (4.2%) | 17 (10.6%) | 0.32 |
| Acute Kidney Injury | 24 (13.0%) | 0 (0.0%) | 24 (15.0%) | 0.042 |
| Target Lesion/vessel Revascularization | 18 (9.8%) | 0 (0.0%) | 18 (11.3%) | 0.084 |
| Death | 4 (2.2%) | 0 (0.0%) | 4 (2.5%) | 0.43 |
| Peripheral Diagnostic Angiogram | Minimal Dose | Standard Dose | ||
| Total Complications | 0 (0%) | 2 (6%) | 0.50 | |
| Major Adverse Limb Event | 0% | 0% | ||
| Myocardial Infarction | 0 (0%) | 1 (3%) | 0.64 | |
| Stroke | 0 (0%) | 1 (3%) | 0.64 | |
| Dissection/Perforation | 0% | 0% | ||
| Acute Kidney Injury | 0 (0%) | 3 (9%) | 0.40 | |
| Target Lesion/Vessel Revascularization | 0% | 1 (3%) | 0.64 | |
| Death | 0% | 1 (3%) | 0.64 | |
| Peripheral Endovascular Intervention | Minimal Dose | Standard Dose | ||
| Total Complications | 1 (5.9%) | 34 (26.6%) | 0.061 | |
| Major Adverse Limb Event | 0 (0.0%) | 25 (19.5%) | 0.045 | |
| Myocardial Infarction | 0 (0.0%) | 3 (2.3%) | 0.52 | |
| Stroke | 0% | 0% | ||
| Dissection/Perforation | 1 (5.9%) | 17 (13.3%) | 0.38 | |
| Acute Kidney Injury | 0 (0.0%) | 21 (16.4%) | 0.071 | |
| Target Lesion/vessel Revascularization | 0 (0.0%) | 17 (13.3%) | 0.11 | |
| Death | 0 (0.0%) | 3 (2.3%) | 0.52 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kar, S.; Espinoza, C. Application and Outcomes of Minimal-Dose Versus Standard-Dose Radiation in Peripheral Endovascular Intervention (KAR Endovascular Study). J. Cardiovasc. Dev. Dis. 2025, 12, 284. https://doi.org/10.3390/jcdd12080284
Kar S, Espinoza C. Application and Outcomes of Minimal-Dose Versus Standard-Dose Radiation in Peripheral Endovascular Intervention (KAR Endovascular Study). Journal of Cardiovascular Development and Disease. 2025; 12(8):284. https://doi.org/10.3390/jcdd12080284
Chicago/Turabian StyleKar, Subrata, and Clifton Espinoza. 2025. "Application and Outcomes of Minimal-Dose Versus Standard-Dose Radiation in Peripheral Endovascular Intervention (KAR Endovascular Study)" Journal of Cardiovascular Development and Disease 12, no. 8: 284. https://doi.org/10.3390/jcdd12080284
APA StyleKar, S., & Espinoza, C. (2025). Application and Outcomes of Minimal-Dose Versus Standard-Dose Radiation in Peripheral Endovascular Intervention (KAR Endovascular Study). Journal of Cardiovascular Development and Disease, 12(8), 284. https://doi.org/10.3390/jcdd12080284






