Plasma Soluble ST2 as a Prognostic Biomarker for Cardiovascular Events and Mortality in COVID-19 Patients
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection and Laboratory Assessments
2.3. Measurement of Plasma sST2 Levels
2.4. Study Endpoints and Outcome Definitions
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of COVID-19 Patients
3.2. Correlation Between Plasma sST2 Levels with Clinical Parameters
3.3. Plasma sST2 as an Independent Predictor of Cardiovascular Events and Mortality
3.4. Diagnostic Performance of sST2 in Predicting Cardiovascular Events and Mortality
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVD-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, C.; Bonow, R.O.; Trivedi, V.; Abbott, J.D.; Messerli, F.H.; Bhatt, D.L. Special Article—Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog. Cardiovasc. Dis. 2020, 63, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Altara, R.; Ghali, R.; Mallat, Z.; Cataliotti, A.; Booz, G.W.; Zouein, F.A. Conflicting vascular and metabolic impact of the IL-33/sST2 axis. Cardiovasc. Res. 2018, 114, 1578–1594. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; Bayes-Genis, A.; Mebazaa, A.; Bauersachs, J.; Cleland, J.G.F.; Coats, A.J.S.; Januzzi, J.L.; Maisel, A.S.; McDonald, K.; Mueller, T.; et al. Circulating heart failure biomarkers beyond natriuretic peptides: Review from the Biomarker Study Group of the Heart Failure Association (HFA), European Society of Cardiology (ESC). Eur. J. Heart Fail. 2021, 23, 1610–1632. [Google Scholar] [CrossRef] [PubMed]
- Emdin, M.; Aimo, A.; Vergaro, G.; Bayes-Genis, A.; Lupon, J.; Latini, R.; Meessen, J.; Anand, I.S.; Cohn, J.N.; Gravning, J.; et al. sST2 Predicts Outcome in Chronic Heart Failure Beyond NT-proBNP and High-Sensitivity Troponin T. J. Am. Coll. Cardiol. 2018, 72, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Sciatti, E.; Merlo, A.; Scangiuzzi, C.; Limonta, R.; Gori, M.; D’Elia, E.; Aimo, A.; Vergaro, G.; Emdin, M.; Senni, M. Prognostic Value of sST2 in Heart Failure. J. Clin. Med. 2023, 12, 3970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.; Ma, M.; He, Y. Soluble ST2 in coronary artery disease: Clinical biomarkers and treatment guidance. Front. Cardiovasc. Med. 2022, 9, 924461. [Google Scholar] [CrossRef] [PubMed]
- Weir, R.A.; Miller, A.M.; Murphy, G.E.; Clements, S.; Steedman, T.; Connell, J.M.; McInnes, I.B.; Dargie, H.J.; McMurray, J.J. Serum soluble ST2: A potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J. Am. Coll. Cardiol. 2010, 55, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, M.; Li, H.; Chen, Y.; Nie, X.; Cai, Y.; Xie, R.; Li, L.; Chen, P.; Sun, Y.; et al. Soluble ST2 Is a Sensitive and Specific Biomarker for Fulminant Myocarditis. J. Am. Heart Assoc. 2022, 11, e024417. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, K.; Ijaz, A.; Salahuddin, M.; Alam, A. Determination of high sensitive cardiac troponin I 99th percentile upper reference limits in a healthy Pakistani population. Pak. J. Med. Sci. 2020, 36, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, T.H.; Hur, M.; Kim, H.J.; Yang, H.S.; Lee, K.R.; Somma, S.D. Performance Evaluation of AFIAS ST2 and Ichroma ST2 Assays in Comparison with Presage ST2 Assay. Rev. Cardiovasc. Med. 2023, 24, 100. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Lavie, C.J.; Sanchis-Gomar, F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog. Cardiovasc. Dis. 2020, 63, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Sanada, S.; Kudinova, A.Y.; Steinhauser, M.L.; Handa, V.; Gannon, J.; Lee, R.T. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ. Heart Fail. 2009, 2, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L.; Mebazaa, A.; Di Somma, S. ST2 and prognosis in acutely decompensated heart failure: The International ST2 Consensus Panel. Am. J. Cardiol. 2015, 115, 26B–31B. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Kanneganti, T.D. Innate immunity, cytokine storm, and inflammatory cell death in COVID-19. J. Transl. Med. 2022, 20, 542. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Subbian, S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin. Microbiol. Rev. 2021, 34, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Marino, L.; Concistre, A.; Suppa, M.; Galardo, G.; Rosa, A.; Bertazzoni, G.; Pugliese, F.; Letizia, C.; Petramala, L. Prognostic Role of sST2 in Acute Heart Failure and COVID-19 Infection-A Narrative Review on Pathophysiology and Clinical Prospective. Int. J. Mol. Sci. 2022, 23, 8230. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Caro-Codon, J.; Rey, J.R.; Buno, A.; Iniesta, A.M.; Rosillo, S.O.; Castrejon-Castrejon, S.; Rodriguez-Sotelo, L.; Martinez, L.A.; Marco, I.; Merino, C.; et al. Characterization of NT-proBNP in a large cohort of COVID-19 patients. Eur. J. Heart Fail. 2021, 23, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.W.; Claggett, B.L.; Jering, K.S.; Vaduganathan, M.; Bhatt, A.S.; Rosenthal, N.; Solomon, S.D. Prognostic Value of Natriuretic Peptides and Cardiac Troponins in COVID-19. Circulation 2021, 144, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Kaluzna-Oleksy, M.; Migaj, J.; Sawczak, F.; Krysztofiak, H.; Lesiak, M.; Straburzynska-Migaj, E. sST2 and Heart Failure-Clinical Utility and Prognosis. J. Clin. Med. 2023, 12, 3136. [Google Scholar] [CrossRef] [PubMed]
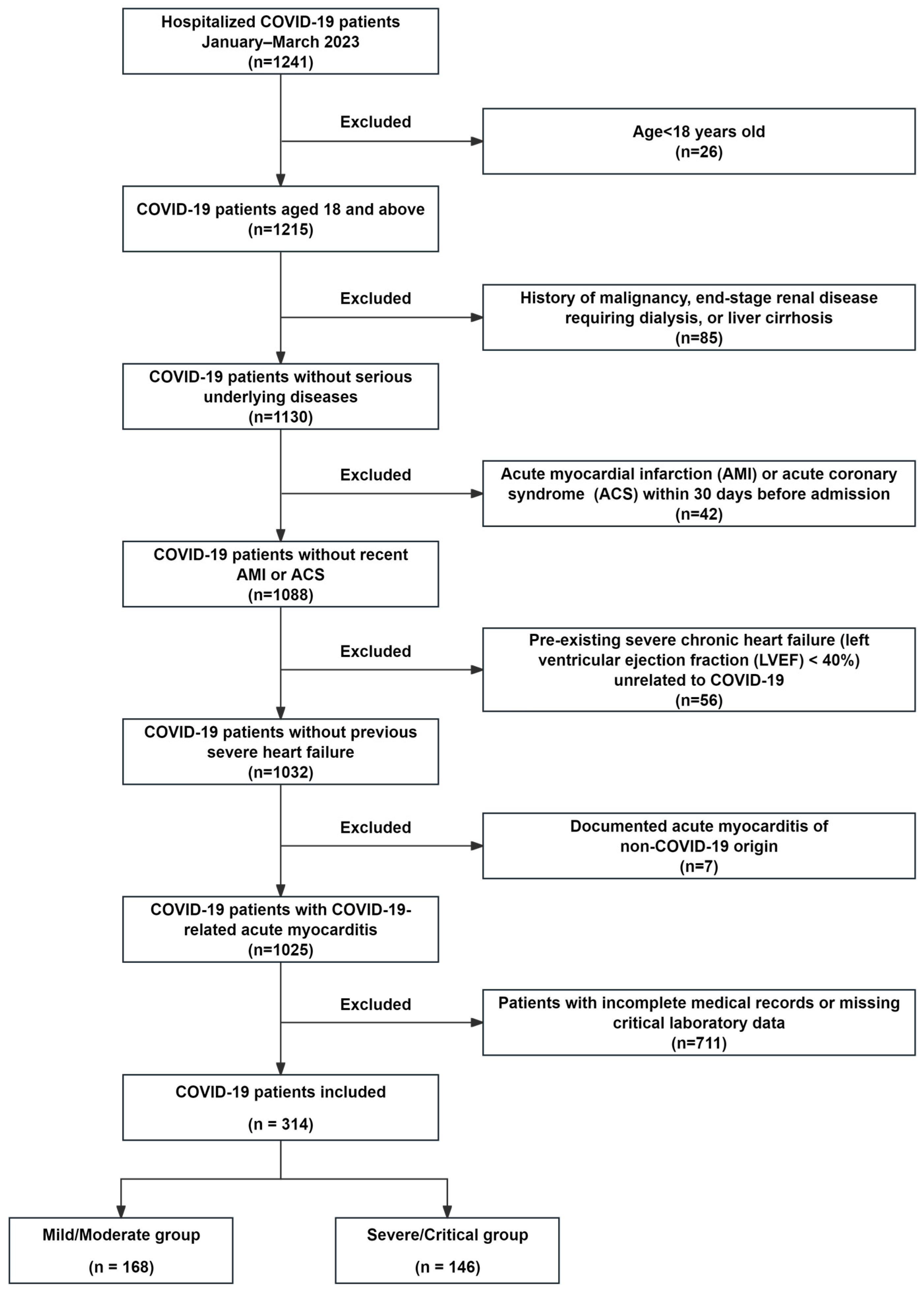

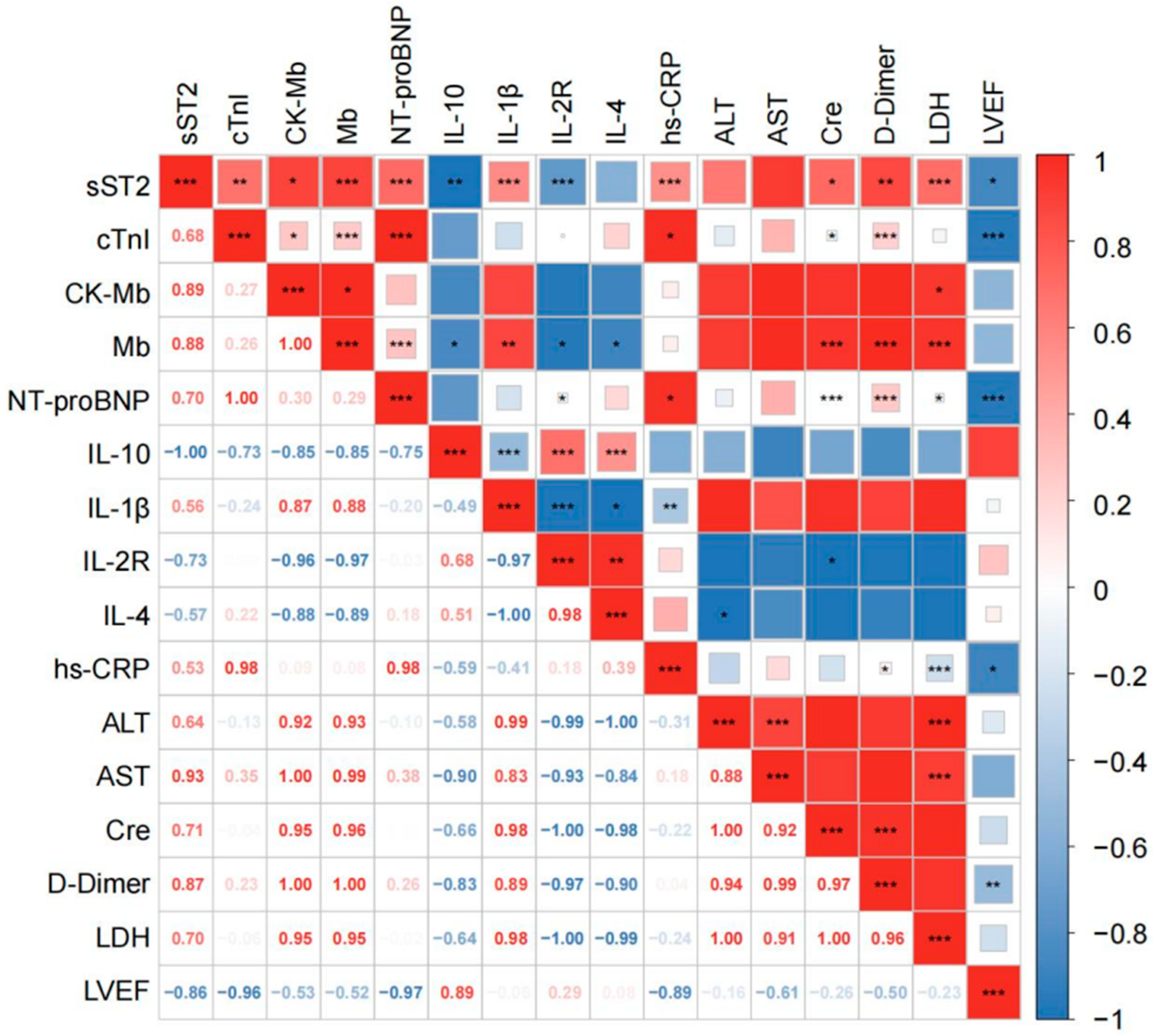
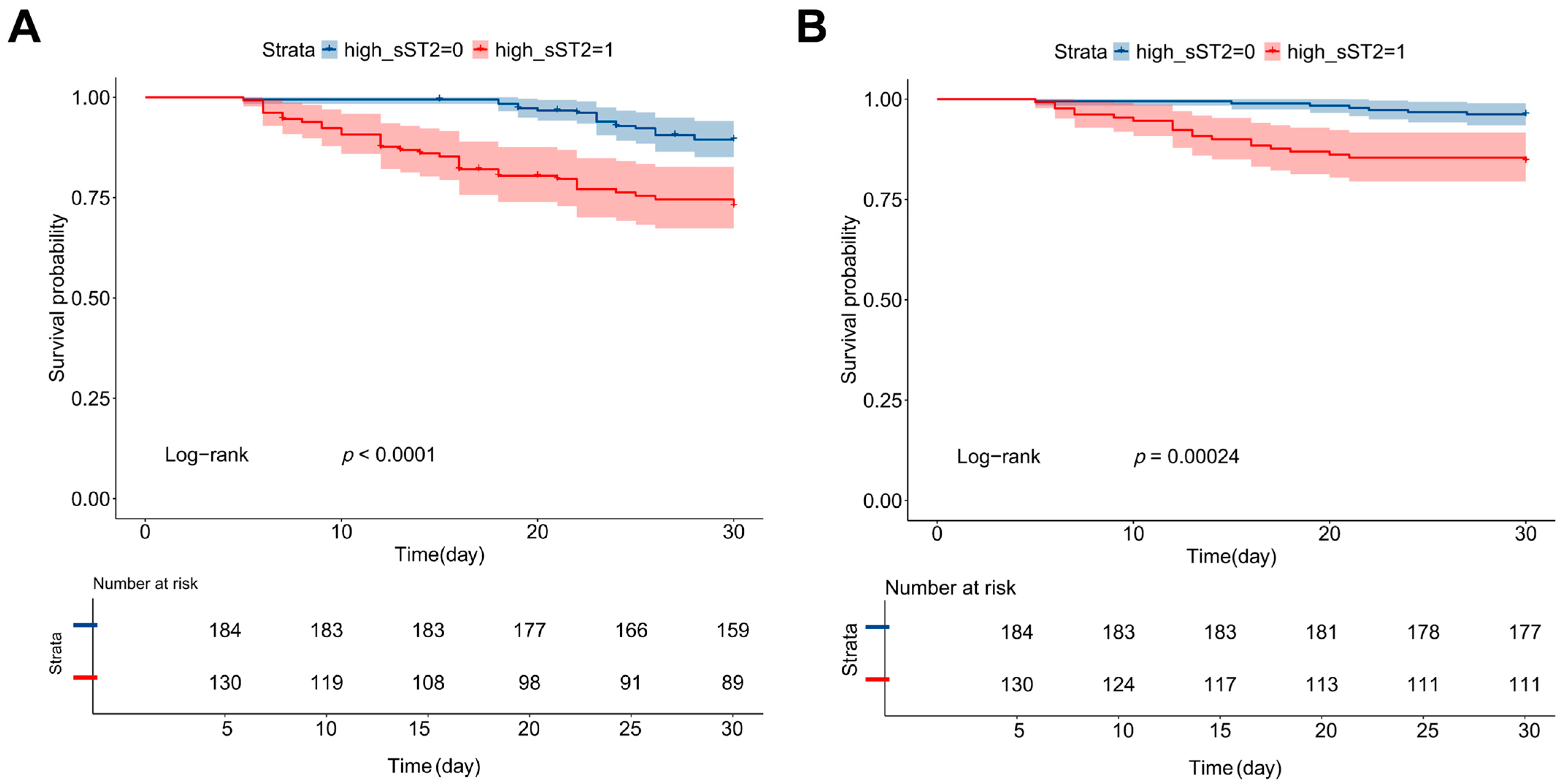
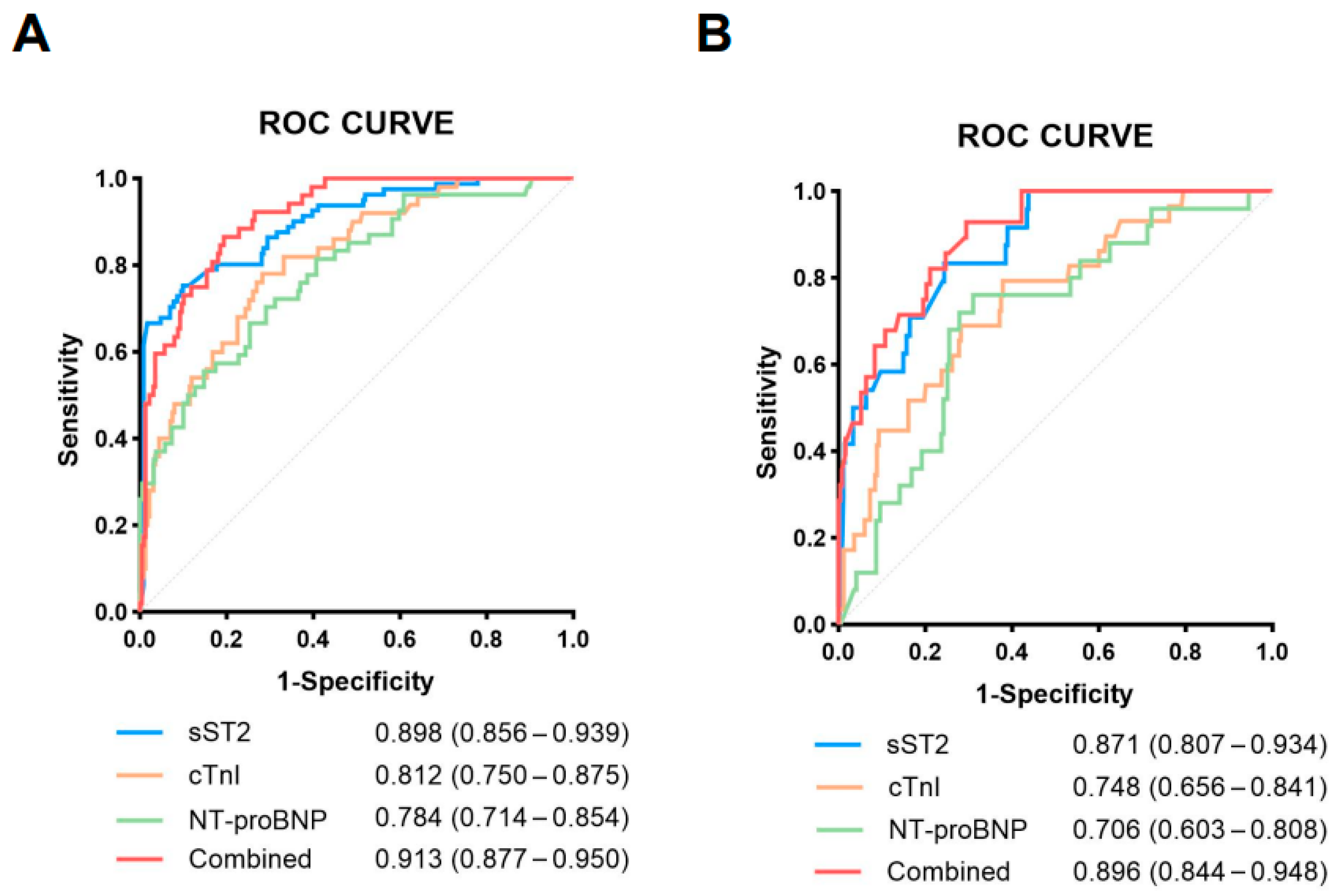
| Variables M (Q1, Q3)/n (%) | Total (n = 314) | Mild/Moderate (n = 168) | Severe/Critical (n = 146) | Statistic | p |
|---|---|---|---|---|---|
| Age | 64.000 (53.000, 75.000) | 59.000 (48.000, 72.000) | 70.000 (57.250, 79.000) | Z = −3.914 | <0.001 |
| Sex, male | 202 (64.331) | 99 (58.929) | 103 (70.548) | χ2 = 4.596 | 0.032 |
| cTnI, ng/L | 15.400 (5.150, 100.500) | 7.800 (2.800, 20.875) | 45.400 (10.400, 349.700) | Z = −7.013 | <0.001 |
| CK-Mb, μg/L | 1.200 (0.600, 2.775) | 0.700 (0.400, 1.375) | 1.750 (0.900, 4.475) | Z = −5.748 | <0.001 |
| Mb, μg/L | 87.450 (43.825, 214.125) | 55.100 (33.800, 106.850) | 146.400 (60.850, 636.750) | Z = −4.986 | <0.001 |
| NT-proBNP, ng/L | 1266.500 (366.250, 5416.250) | 836.000 (191.250, 2350.250) | 2355.000 (662.000, 8987.000) | Z = −4.607 | <0.001 |
| IL-10, ng/L | 7.060 (5.000, 19.300) | 5.500 (5.000, 11.200) | 9.100 (5.000, 25.150) | Z = −1.889 | 0.059 |
| IL-1β, ng/L | 6.300 (5.000, 16.250) | 5.000 (5.000, 9.600) | 7.100 (5.000, 19.975) | Z = −2.800 | 0.005 |
| IL-2, ng/L | 3.640 (3.087, 5.350) | 4.135 (3.473, 5.350) | 3.420 (2.990, 5.350) | Z = −0.798 | 0.425 |
| IL-2R, kIU/L | 1092.000 (794.000, 1760.000) | 937.000 (703.000, 1595.500) | 1212.000 (865.750, 1819.000) | Z = −2.301 | 0.021 |
| IL-4, ng/L | 3.000 (2.605, 3.232) | 3.000 (2.678, 3.550) | 2.925 (2.480, 3.130) | Z = −0.772 | 0.440 |
| WBC, ×109/L | 7.480 (4.680, 10.560) | 6.220 (3.860, 8.130) | 9.410 (6.380, 13.200) | Z = -6.338 | <0.001 |
| Lymphocytes, ×109/L | 0.730 (0.450, 1.310) | 0.940 (0.570, 1.580) | 0.600 (0.380, 0.990) | Z = −4.575 | <0.001 |
| Neutrophil, ×109/L | 5.360 (2.760, 8.350) | 3.830 (2.320, 6.260) | 7.290 (4.695, 10.970) | Z = −6.706 | <0.001 |
| Platelet, ×109/L | 193.000 (127.000, 259.000) | 195.000 (116.000, 249.000) | 191.500 (141.750, 270.250) | Z = −1.506 | 0.132 |
| Hb, g/L | 108.000 (87.000, 128.000) | 107.000 (83.000, 127.000) | 108.500 (92.000, 128.000) | Z = −0.661 | 0.509 |
| hsCRP, mg/L | 38.300 (9.975, 96.150) | 27.550 (4.775, 66.275) | 50.100 (26.725, 119.600) | Z = −4.565 | <0.001 |
| LDL, mmol/L | 1.875 (1.400, 2.522) | 1.860 (1.510, 2.650) | 1.890 (1.325, 2.460) | Z = −0.386 | 0.700 |
| HDL, mmol/L | 0.840 (0.640, 1.020) | 0.905 (0.758, 1.042) | 0.770 (0.620, 1.018) | Z = −1.927 | 0.054 |
| ALT, IU/L | 20.000 (13.000, 36.000) | 19.000 (13.000, 34.500) | 21.000 (12.750, 38.000) | Z = −0.638 | 0.524 |
| AST, IU/L | 24.000 (18.000, 36.750) | 23.000 (16.000, 32.750) | 27.000 (19.000, 41.250) | Z = −3.123 | 0.002 |
| Cre, µmol/L | 79.000 (61.000, 113.000) | 75.000 (60.250, 97.500) | 83.000 (62.500, 126.500) | Z = −1.787 | 0.074 |
| LDH, U/L | 270.500 (205.000, 398.000) | 219.500 (182.000, 301.500) | 351.500 (251.250, 475.000) | Z = −6.843 | <0.001 |
| LVEF% | 60.000 (58.000, 61.000) | 60.000 (60.000, 62.000) | 60.000 (58.000, 60.000) | Z = −2.274 | 0.023 |
| PaCO2 | 37.800 (35.300, 44.200) | 39.200 (36.300, 43.000) | 37.200 (32.850, 44.275) | Z = −1.245 | 0.213 |
| PaO2 | 79.500 (63.800, 105.350) | 91.200 (78.000, 114.000) | 66.650 (57.675, 94.525) | Z = −3.727 | <0.001 |
| Fever | 203 (64.650) | 101 (60.119) | 102 (69.863) | χ2 = 3.245 | 0.072 |
| Cough | 207 (65.924) | 99 (58.929) | 108 (73.973) | χ2 = 7.870 | 0.005 |
| Sputum | 131 (41.720) | 60 (35.714) | 71 (48.630) | χ2 = 5.359 | 0.021 |
| Shortness of breath | 78 (24.841) | 18 (10.714) | 60 (41.096) | χ2 = 38.619 | <0.001 |
| Dyspnea | 54 (17.252) | 14 (8.333) | 40 (27.586) | χ2 = 20.208 | <0.001 |
| Chest pain | 14 (4.459) | 9 (5.357) | 5 (3.425) | χ2 = 0.685 | 0.408 |
| Chest distress | 88 (28.025) | 36 (21.429) | 52 (35.616) | χ2 = 7.795 | 0.005 |
| Fatigue | 115 (36.741) | 60 (35.714) | 55 (37.931) | χ2 = 0.165 | 0.685 |
| Gastrointestinal Symptoms | 115 (36.624) | 47 (27.976) | 68 (46.575) | χ2 = 11.642 | <0.001 |
| Hypertension | 150 (47.771) | 71 (42.262) | 79 (54.110) | χ2 = 4.395 | 0.036 |
| Diabetes | 84 (26.752) | 37 (22.024) | 47 (32.192) | χ2 = 4.121 | 0.042 |
| Hyperlipidemia | 13 (4.140) | 7 (4.167) | 6 (4.110) | χ2 = 0.001 | 0.980 |
| Chronic renal failure | 58 (18.530) | 18 (10.778) | 40 (27.397) | χ2 = 14.251 | <0.001 |
| Chronic liver disease | 60 (19.108) | 29 (17.262) | 31 (21.233) | χ2 = 0.797 | 0.372 |
| Chronic lung disease | 18 (5.732) | 6 (3.571) | 12 (8.219) | χ2 = 3.123 | 0.077 |
| Solid organ transplantation | 24 (7.643) | 10 (5.952) | 14 (9.589) | χ2 = 1.463 | 0.226 |
| Obesity (BMI ≥ 30) | 15 (4.81) | 4 (2.41) | 11 (7.53) | χ2 = 4.460 | 0.035 |
| Imaging findings | 252 (86.598) | 133 (81.098) | 119 (93.701) | χ2 = 9.796 | 0.002 |
| Respiratory failure | 104 (33.121) | 0 (0.00) | 104 (71.233) | χ2 = 178.937 | <0.001 |
| Shock | 21 (7.420) | 0 (0.00) | 21 (16.667) | χ2 = 28.264 | <0.001 |
| Organ failure | 26 (9.253) | 0 (0.00) | 26 (20.968) | χ2 = 36.276 | <0.001 |
| Cardiovascular events | 53 (16.879) | 13 (7.738) | 40 (27.397) | χ2 = 21.518 | <0.001 |
| Death | 27 (8.599) | 0 (0.00) | 27 (18.493) | χ2 = 33.991 | <0.001 |
| Variables | Outcome for Cardiovascular Events | Outcome for All-Cause Mortality | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||||||||||||
| β | S.E | Z | p | HR (95%CI) | β | S.E | Z | p | HR (95%CI) | β | S.E | Z | p | HR (95%CI) | β | S.E | Z | p | HR (95%CI) | |
| sST2 | 1.263 | 0.232 | 5.434 | <0.001 | 3.537 (2.243–5.578) | 1.089 | 0.474 | 2.299 | 0.022 | 2.972 (1.174–7.521) | 1.349 | 0.341 | 3.96 | <0.001 | 3.852 (1.976–7.508) | 1.543 | 0.636 | 2.427 | 0.015 | 4.681 (1.346–16.280) |
| cTnI | 0.001 | 0.000 | 3.328 | <0.001 | 1.001 (1.001–1.001) | 0 | 0 | 1.481 | 0.139 | 1.000 (1.000–1.001) | 0.000 | 0.000 | 1.004 | 0.316 | 1.000 (1.000–1.000) | |||||
| CK-Mb | 0.004 | 0.010 | 0.429 | 0.668 | 1.004 (0.985–1.025) | 0.015 | 0.007 | 2.027 | 0.043 | 1.015 (1.001–1.029) | 0.023 | 0.018 | 1.246 | 0.213 | 1.023 (0.987–1.060) | |||||
| Mb | 0.001 | 0.000 | 3.593 | <0.001 | 1.001 (1.001–1.002) | 0 | 0.001 | 0.142 | 0.887 | 1.000 (0.999–1.001) | 0.002 | 0.000 | 3.897 | <0.001 | 1.002 (1.001–1.002) | 0.001 | 0.001 | 1.671 | 0.095 | 1.001 (1.000–1.002) |
| NT-proBNP | 0.001 | 0.000 | 3.743 | <0.001 | 1.001 (1.001–1.001) | 0 | 0 | −1.27 | 0.204 | 1.000 (1.000–1.000) | 0.001 | 0.000 | 2.484 | 0.013 | 1.001 (1.001–1.001) | 0 | 0 | −0.302 | 0.763 | 1.000 (1.000–1.000) |
| IL-1β | 0.023 | 0.008 | 3.034 | 0.002 | 1.024 (1.008–1.039) | 0.006 | 0.014 | 0.459 | 0.646 | 1.006 (0.979–1.034) | 0.028 | 0.008 | 3.574 | <0.001 | 1.028 (1.013–1.044) | 0.013 | 0.012 | 1.063 | 0.288 | 1.013 (0.989–1.038) |
| IL-2R | −0.000 | 0.000 | −0.328 | 0.743 | 1.000 (1.000–1.000) | 0.000 | 0.000 | 0.189 | 0.850 | 1.000 (1.000–1.000) | ||||||||||
| hsCRP | 0.005 | 0.002 | 2.930 | 0.003 | 1.005 (1.002–1.008) | 0 | 0.003 | −0.061 | 0.951 | 1.000 (0.993–1.006) | 0.008 | 0.002 | 3.790 | <0.001 | 1.008 (1.004–1.012) | 0 | 0.004 | −0.061 | 0.951 | 1.000 (0.992–1.007) |
| LDH | 0.001 | 0.000 | 2.179 | 0.029 | 1.001 (1.001–1.002) | −0.002 | 0.002 | −1.014 | 0.311 | 0.998 (0.995–1.001) | 0.002 | 0.001 | 2.821 | 0.005 | 1.002 (1.001–1.003) | −0.002 | 0.001 | −1.068 | 0.286 | 0.998 (0.995–1.001) |
| LVEF | −0.061 | 0.011 | −5.764 | <0.001 | 0.941 (0.921–0.961) | −0.078 | 0.033 | −2.391 | 0.017 | 0.925 (0.868–0.986) | −0.023 | 0.024 | −0.926 | 0.354 | 0.978 (0.932–1.026) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Zhuang, Y.; Li, H.; Wang, D.W. Plasma Soluble ST2 as a Prognostic Biomarker for Cardiovascular Events and Mortality in COVID-19 Patients. J. Cardiovasc. Dev. Dis. 2025, 12, 273. https://doi.org/10.3390/jcdd12070273
Yan Y, Zhuang Y, Li H, Wang DW. Plasma Soluble ST2 as a Prognostic Biomarker for Cardiovascular Events and Mortality in COVID-19 Patients. Journal of Cardiovascular Development and Disease. 2025; 12(7):273. https://doi.org/10.3390/jcdd12070273
Chicago/Turabian StyleYan, Yongcui, Yan Zhuang, Huihui Li, and Dao Wen Wang. 2025. "Plasma Soluble ST2 as a Prognostic Biomarker for Cardiovascular Events and Mortality in COVID-19 Patients" Journal of Cardiovascular Development and Disease 12, no. 7: 273. https://doi.org/10.3390/jcdd12070273
APA StyleYan, Y., Zhuang, Y., Li, H., & Wang, D. W. (2025). Plasma Soluble ST2 as a Prognostic Biomarker for Cardiovascular Events and Mortality in COVID-19 Patients. Journal of Cardiovascular Development and Disease, 12(7), 273. https://doi.org/10.3390/jcdd12070273







