Multimodality Imaging in Eosinophilic Myocarditis: A Rare Cause of Heart Failure
Abstract
1. Introduction
2. Etiology
3. Diagnosis
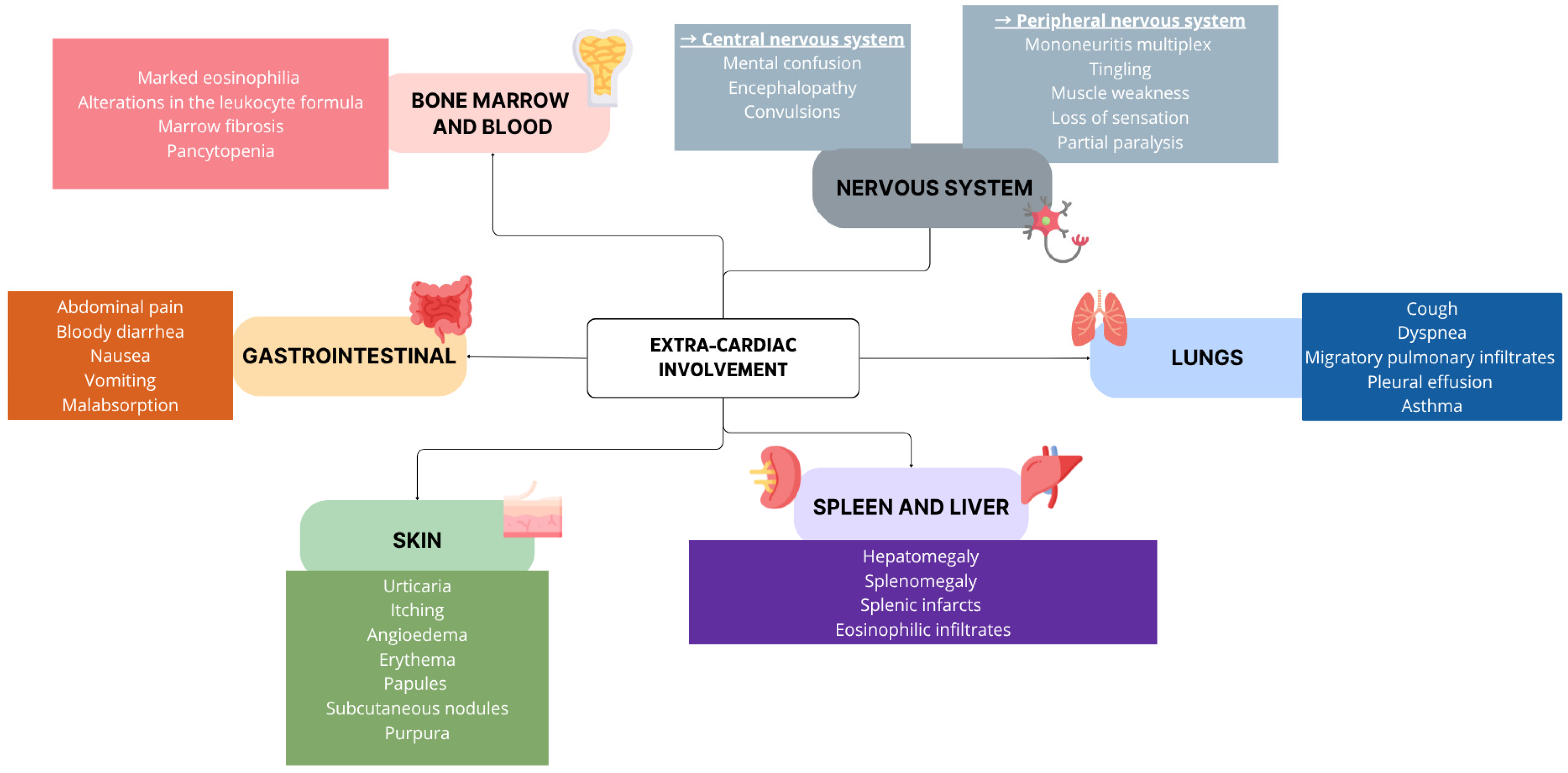
3.1. Clinical Features
3.2. Initial Work-Up
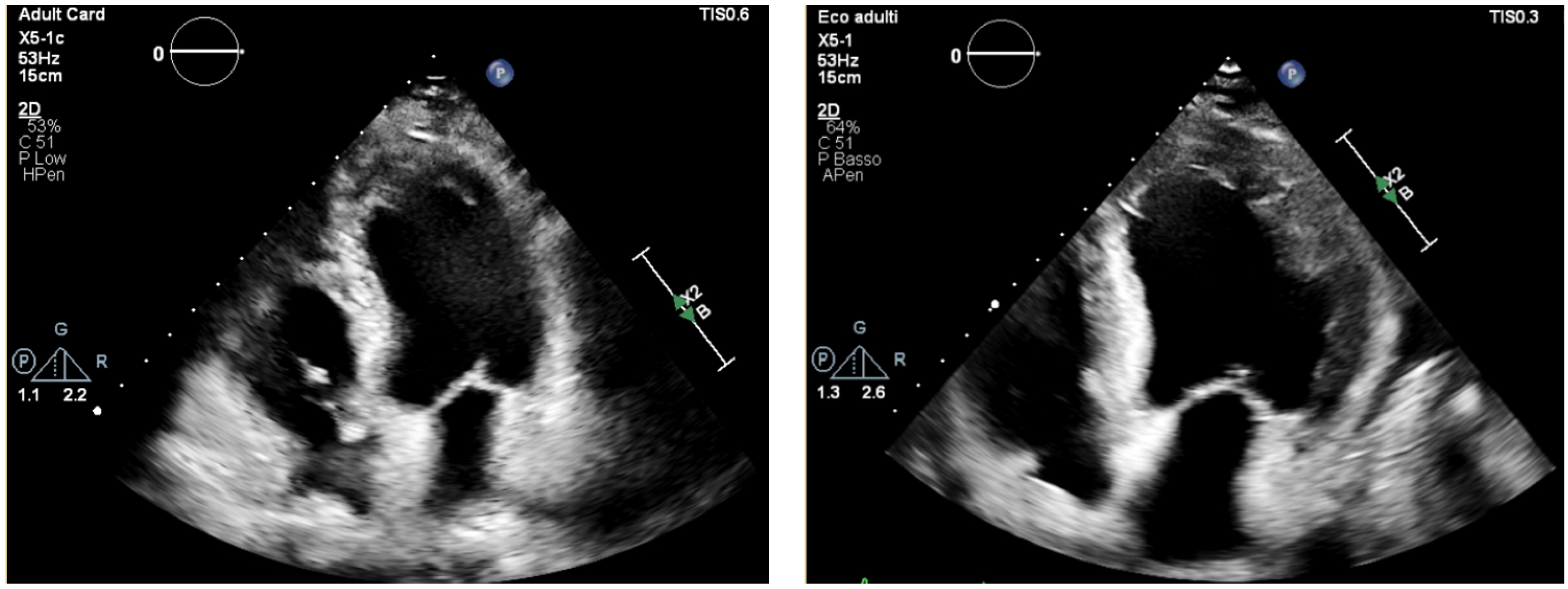
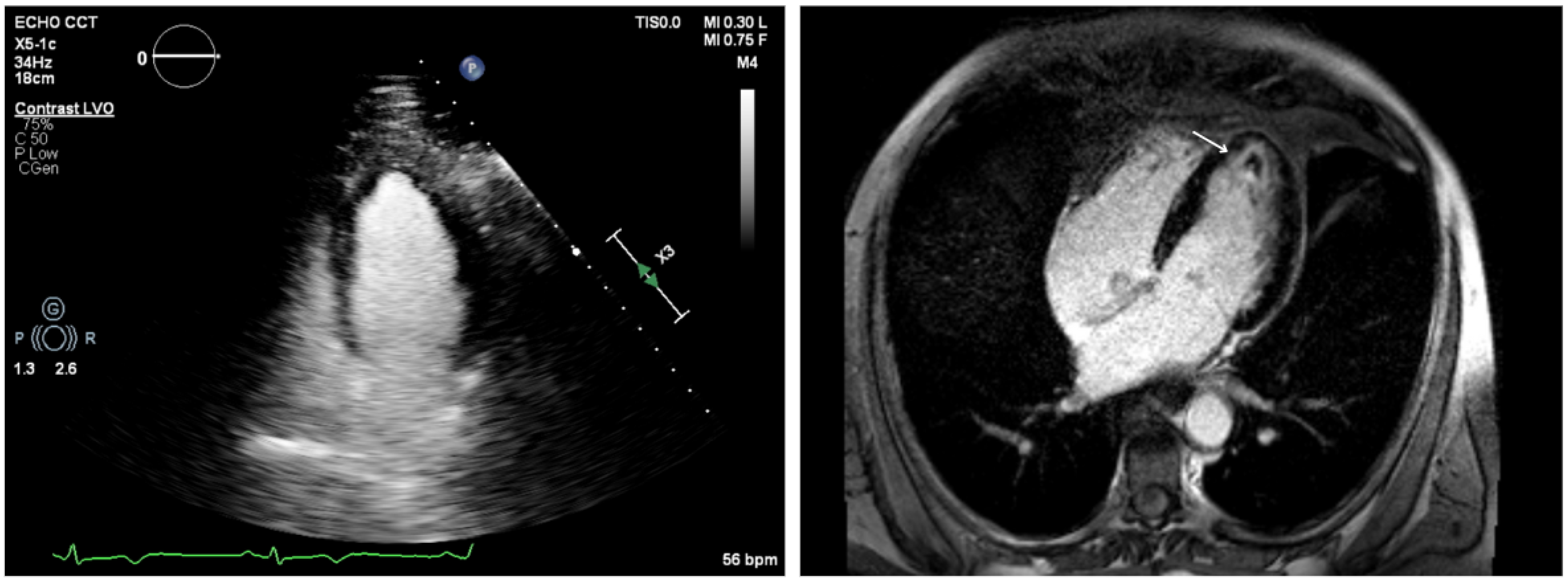
3.3. Echocardiography
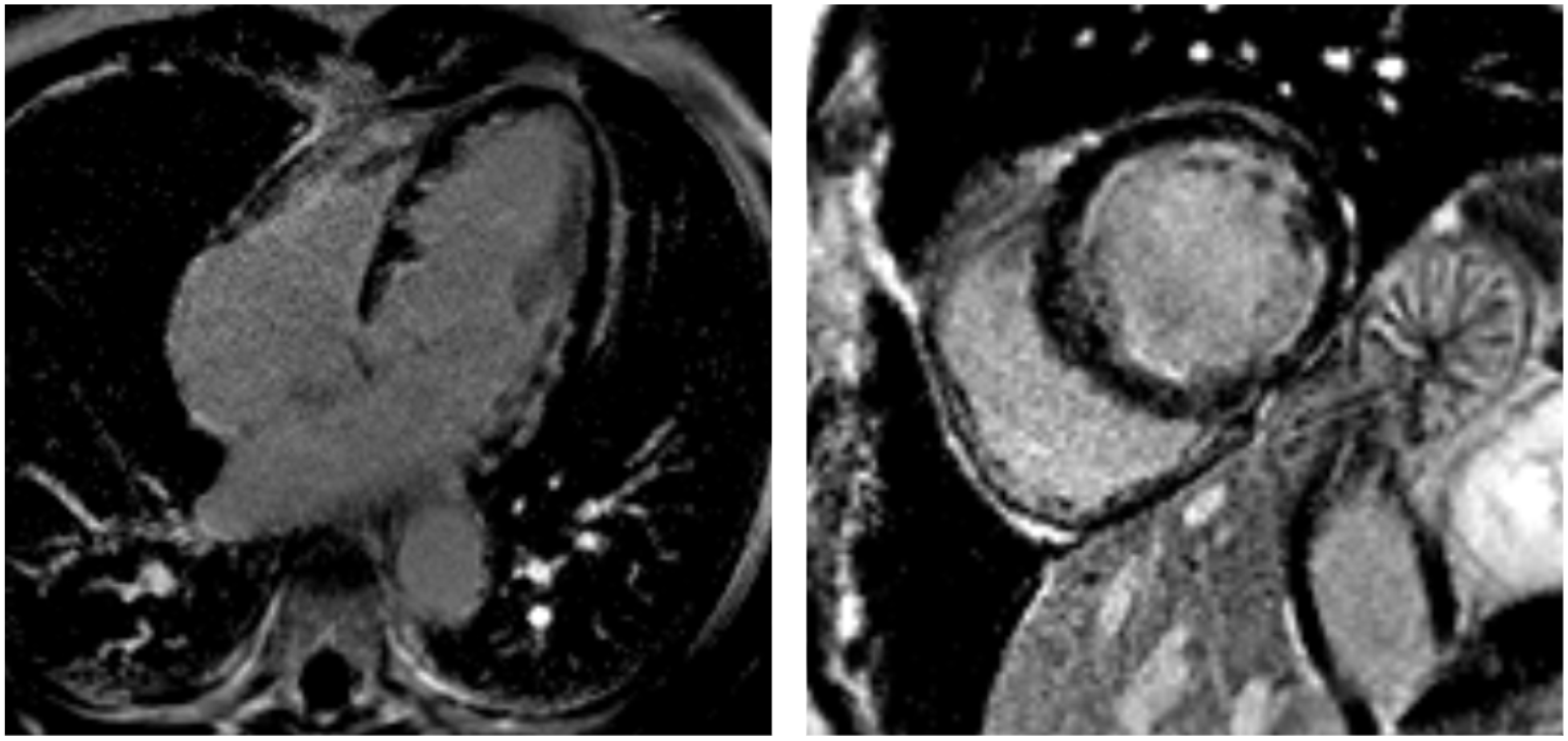
3.4. Cardiac Magnetic Resonance
3.5. Cardiac CT
3.6. [18F]FDG Positron Emission Tomography
3.7. Endomyocardial Biopsy
4. Treatment and Follow-Up
4.1. Fulminant and Acute Non-Fulminant Myocarditis
4.2. Thromboembolic Complications
4.3. Inflammatory Disease
4.4. Multimodality Imaging in the Follow-Up
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| [18F]FDG | [18F]fluorodeoxyglucose |
| AC | acute myocarditis |
| AHF | acute heart failure |
| ANA | antinuclear antibodies |
| ANCA | anti-neutrophil cytoplasmic antibodies |
| CAD | coronary artery disease |
| cine-SSFP | cine steady-state free precession |
| CMR | cardiac magnetic resonance |
| CRP | C-reactive protein |
| CS | cardiogenic shock |
| CT | computed tomography |
| CXR | chest X-ray |
| DOACs | direct oral anticoagulants |
| DRESS | drug reaction with eosinophilia and systemic symptoms |
| ECG | electrocardiography |
| ECV | extracellular volume |
| EF | ejection fraction |
| EGPA | eosinophilic granulomatosis with polyangiitis |
| EM | eosinophilic myocarditis |
| EMB | endomyocardial biopsy |
| ESR | erythrocyte sedimentation rate |
| FM | fulminant myocarditis |
| GLS | global longitudinal speckle |
| HES | hypereosinophilic syndrome |
| HFrEF | heart failure with reduced ejection fraction |
| HTx | heart transplantation |
| IL-5 | interleukin-5 |
| INR | international normalized ratio |
| IVIG | intravenous immunoglobulin |
| LGE | late gadolinium enhancement |
| LV | left ventricular |
| LVAD | left ventricular assist device |
| LVEF | left ventricular ejection fraction |
| LVMD | left ventricular mechanical dispersion |
| OAC | oral anticoagulant |
| PET | positron emission tomography |
| PET/CT | positron emission tomography/computed tomography |
| t-MCS | temporary mechanical circulatory support |
| T2w-STIR | T2-weighted short tau inversion recovery |
| TDI | tissue doppler imaging |
| TTE | transthoracic echocardiography |
| VKAs | vitamin K antagonists |
References
- Yamaguchi, Y.; Suda, T.; Ohta, S.; Tominaga, K.; Miura, Y.; Kasahara, T. Analysis of the survival of mature human eosinophils: Interleukin-5 prevents apoptosis in mature human eosinophils. Blood 1991, 78, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Shomali, W.; Gotlib, J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2019, 94, 1149–1167. [Google Scholar] [CrossRef] [PubMed]
- Klion, A.D.; Ackerman, S.J.; Bochner, B.S. Contributions of Eosinophils to Human Health and Disease. Annu. Rev. Pathol. 2020, 15, 179–209. [Google Scholar] [CrossRef] [PubMed]
- Brambatti, M.; Matassini, M.V.; Adler, E.D.; Klingel, K.; Camici, P.G.; Ammirati, E. Eosinophilic Myocarditis: Characteristics, Treatment, and Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2363–2375. [Google Scholar] [CrossRef]
- Fiszenson-Albala, F.; Auzerie, V.; Mahe, E.; Farinotti, R.; Durand-Stocco, C.; Crickx, B.; Descamps, V. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br. J. Dermatol. 2003, 149, 1018–1022. [Google Scholar] [CrossRef]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy. Circ. Heart Fail. 2020, 13, e007405. [Google Scholar] [CrossRef]
- Younis, A.; Matetzky, S.; Mulla, W.; Masalha, E.; Afel, Y.; Chernomordik, F.; Fardman, A.; Goitein, O.; Ben-Zekry, S.; Peled, Y.; et al. Epidemiology Characteristics and Outcome of Patients with Clinically Diagnosed Acute Myocarditis. Am. J. Med. 2020, 133, 492–499. [Google Scholar] [CrossRef]
- Kindermann, I.; Barth, C.; Mahfoud, F.; Ukena, C.; Lenski, M.; Yilmaz, A.; Klingel, K.; Kandolf, R.; Sechtem, U.; Cooper, L.T.; et al. Update on myocarditis. J. Am. Coll. Cardiol. 2012, 59, 779–792. [Google Scholar] [CrossRef]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients with Acute Myocarditis: Multicenter Lombardy Registry. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef]
- White, J.A.; Hansen, R.; Abdelhaleem, A.; Mikami, Y.; Peng, M.; Rivest, S.; Satriano, A.; Dykstra, S.; Flewitt, J.; Heydari, B.; et al. Natural History of Myocardial Injury and Chamber Remodeling in Acute Myocarditis. Circ. Cardiovasc. Imaging 2019, 12, e008614. [Google Scholar] [CrossRef]
- Khachatryan, A.; Harutyunyan, H.; Psotka, M.; Batikyan, A.; Cinar, T.; Khorsandi, M.; Alejandro, J.; Tamazyn, V.; Sargsyan, M. Hypereosinophilia and Left Ventricular Thrombus: A Case Report and Literature Review. Cureus 2024, 16, e61674. [Google Scholar] [CrossRef]
- Yoshizawa, H.; Ikeda, Y.; Hatakeyama, K.; Yoshida, K.I. Myocardial ischemic injury derived from multiple thromboemboli due to eosinophilic endomyocarditis (Löffler endocarditis) causing right ventricular rupture. Pathol. Int. 2021, 71, 722–724. [Google Scholar] [CrossRef]
- deMello, D.E.; Liapis, H.; Jureidini, S.; Nouri, S.; Kephart, G.M.; Gleich, G.J. Cardiac localization of eosinophil-granule major basic protein in acute necrotizing myocarditis. N. Engl. J. Med. 1990, 323, 1542–1545. [Google Scholar] [CrossRef] [PubMed]
- Sandarsh, S.; Bishnoi, R.J.; Shashank, R.B.; Miller, B.J.; Freudenreich, O.; McEvoy, J.P. Monitoring for myocarditis during treatment initiation with clozapine. Acta Psychiatr. Scand. 2021, 144, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Gilotra, N.A.; Minkove, N.; Bennett, M.K.; Tedford, R.J.; Steenbergen, C.; Judge, D.P.; Haluska, M.K.; Russel, S.D. Lack of Relationship Between Serum Cardiac Troponin I Level and Giant Cell Myocarditis Diagnosis and Outcomes. J. Card. Fail. 2016, 22, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Arima, M.; Kanoh, T. Eosinophilic myocarditis associated with dense deposits of eosinophil cationic protein (ECP) in endomyocardium with high serum ECP. Heart 1999, 81, 669–671. [Google Scholar] [CrossRef]
- Birnie, D.H.; Nery, P.B.; Ha, A.C.; Beanlands, R.S.B. Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 2016, 68, 411–421. [Google Scholar] [CrossRef]
- Perel-Winkler, A.; Bokhari, S.; Perez-Recio, T.; Zartoshti, A.; Askanase, A.; Geraldino-Pardilla, L. Myocarditis in systemic lupus erythematosus diagnosed by 18F-fluorodeoxyglucose positron emission tomography. Lupus Sci. Med. 2018, 5, e000265. [Google Scholar] [CrossRef]
- Bennett, M.K.; Gilotra, N.A.; Harrington, C.; Rao, S.; Dunn, J.M.; Freitag, T.B.; Halushka, M.K.; Russel, S.D. Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from 2000–2009. Circ. Heart Fail. 2013, 6, 676–684. [Google Scholar] [CrossRef]
- Asada, A.M.; Kahwash, R.; Trovato, V. Eosinophilic Myocarditis: A Concise Review. Curr. Cardiol. Rep. 2025, 27, 38. [Google Scholar] [CrossRef]
- Verstraeten, A.S.; De Weerdt, A.; van Den Eynden, G.; Van Marck, E.; Snoeckx, A.; Jorens, P.G. Excessive eosinophilia as paraneoplastic syndrome in a patient with non-small-cell lung carcinoma: A case report and review of the literature. Acta Clin. Belg. 2011, 66, 293–297. [Google Scholar] [PubMed]
- Piccirillo, F.; Mastroberardino, S.; Nafisio, V.; Fiorentino, M.; Segreti, A.; Nusca, A.; Ussia, G.P.; Grigioni, F. Eosinophilic Myocarditis: From Bench to Bedside. Biomedicines 2024, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Yang, Z.; Peng, Y.; Wang, L.; Yuan, X. Diagnosis and treatment of eosinophilic myocarditis. J. Transl. Autoimmun. 2021, 4, 100118. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, H.; Siddiqui, M.; Uddin, S.M.M.; Haq, A.; Yaqoob, U. The Clinicopathological Profile of Eosinophilic Myocarditis. Cureus 2018, 10, e3677. [Google Scholar] [CrossRef]
- Nguyen, Y.; Guillevin, L. Eosinophilic Granulomatosis with Polyangiitis (Churg-Strauss). Semin. Respir. Crit. Care Med. 2018, 39, 471–481. [Google Scholar] [CrossRef]
- Valent, P.; Klion, A.D.; Horny, H.P.; Roufosse, F.; Gotlib, J.; Weller, P.F.; Hellmann, A.; Matzgeroth, G.; Leiferman, K.M.; Arock, M.; et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J. Allergy Clin. Immunol. 2012, 130, 607–612.e9. [Google Scholar] [CrossRef]
- Hsiao, J.F.; Koshino, Y.; Bonnichsen, C.R.; Yu, Y.; Miller, F.A.; Pellikka, P.A.; Cooper, L.T.; Villaraga, H.R. Speckle tracking echocardiography in acute myocarditis. Int. J. Cardiovasc. Imaging 2013, 29, 275–284. [Google Scholar] [CrossRef]
- Lancellotti, P.; Price, S.; Edvardsen, T.; Cosyns, B.; Neskovic, A.N.; Dulgheru, R.; Flachskampf, F.A.; Hassager, C.; Pasquet, A.; Gargani, L.; et al. The use of echocardiography in acute cardiovascular care: Recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 119–146. [Google Scholar] [CrossRef]
- Frederiksen, C.A.; Stilling, C.; Kim, W.Y.; Poulsen, S.H. Two Different Clinical Presentations and Stages of Loeffler Endocarditis Diagnosed by Multimodality Investigations. CASE 2023, 7, 496–501. [Google Scholar] [CrossRef]
- Kawakami, H.; Nerlekar, N.; Haugaa, K.H.; Edvardsen, T.; Marwick, T.H. Prediction of Ventricular Arrhythmias With Left Ventricular Mechanical Dispersion: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2020, 13 Pt 2, 562–572. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Smedsrud, M.K.; Steen, T.; Kongsgaard, E.; Loennechen, J.P.; Skjaerpe, T.; Voigt, J.-U.; Willems, R.; Smith, G.; Smiseth, O.A.; et al. Mechanical dispersion assessed by myocardial strain in patients after myocardial infarction for risk prediction of ventricular arrhythmia. JACC Cardiovasc. Imaging 2010, 3, 247–256. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Goebel, B.; Dahlslett, T.; Meyer, K.; Jung, C.; Lauten, A.; Figulla, H.R.; Poerner, T.C.; Edvardsen, T. Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2012, 25, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Gibson, D.G.; Foale, R.; Heer, K.; Spry, C.J.; Oakley, C.M.; Goodwin, J.F. Echocardiographic features of eosinophilic endomyocardial disease. Heart 1982, 48, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Fakadej, T.; Hathaway, Q.A.; Balar, A.B.; Amin, M.S.; Lakhani, D.A.; Kim, C. Eosinophilic myocarditis: Case report and brief review of the literature. Radiol. Case Rep. 2023, 18, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Ananthasubramaniam, K. Evaluation of cardiac involvement in hypereosinophilic syndrome: Complementary roles of transthoracic, transesophageal, and contrast echocardiography. Echocardiography 2006, 23, 689–691. [Google Scholar] [CrossRef]
- Abdelmoneim, S.S.; Pellikka, P.A.; Mulvagh, S.L. Contrast echocardiography for assessment of left ventricular thrombi. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2014, 33, 1337–1344. [Google Scholar] [CrossRef]
- Russo, M.; Ismibayli, Z.; Antonaci, S.; Piccinni, G.C. Eosinophilic myocarditis: From etiology to diagnostics and therapy. Minerva Cardiol. Angiol. 2024, 72, 656–673. [Google Scholar] [CrossRef]
- Al-Kaisey, A.M.; Meher-Homji, Z.; Hayward, P.; Jones, E. Mitral and tricuspid valve repair in hypereosinophilic syndrome. BMJ Case Rep. 2019, 12, e228951. [Google Scholar] [CrossRef]
- Szczerba, E.; Kowalik, R.; Gorska, K.; Mierzejewski, M.; Slowikowska, A.; Bednarczyk, T.; Marchel, M.; Krenke, R.; Opolski, G. Severe mitral stenosis secondary to eosinophilic granulomatosis resolving after pharmacological treatment. Echocardiography 2018, 35, 2099–2103. [Google Scholar] [CrossRef]
- Ommen, S.R.; Seward, J.B.; Tajik, A.J. Clinical and echocardiographic features of hypereosinophilic syndromes. Am. J. Cardiol. 2000, 86, 110–113. [Google Scholar] [CrossRef]
- Marwick, T.H.; Shah, S.J.; Thomas, J.D. Myocardial Strain in the Assessment of Patients with Heart Failure: A Review. JAMA Cardiol. 2019, 4, 287–294. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Faron, A.; Isaak, A.; Dabir, D.; Kuetting, D.; Feisst, A.; Schmeel, F.C.; Sprinkart, A.M.; Thomas, D. Comparison of Original and 2018 Lake Louise Criteria for Diagnosis of Acute Myocarditis: Results of a Validation Cohort. Radiol. Cardiothorac. Imaging 2019, 1, e190010. [Google Scholar] [CrossRef] [PubMed]
- Luetkens, J.A.; Homsi, R.; Dabir, D.; Kuetting, D.L.; Marx, C.; Doerner, J.; Schlesinger-Irsch, U.; Andrié, R.; Sprinkart, A.M.; Schmeel, F.C.; et al. Comprehensive Cardiac Magnetic Resonance for Short-Term Follow-Up in Acute Myocarditis. J. Am. Heart Assoc. 2016, 5, e003603. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Verardo, R.; Galea, N.; Lavalle, C.; Bagnato, G.; Scialla, R.; Chimenti, C. Hypersensitivity Myocarditis after COVID-19 mRNA Vaccination. J. Clin. Med. 2022, 11, 1660. [Google Scholar] [CrossRef]
- Ohtani, K.; Takahama, S.; Kato, S.; Higo, T. Acute necrotizing eosinophilic myocarditis after COVID-19 vaccination. Eur. Heart J. 2022, 43, 2640. [Google Scholar] [CrossRef] [PubMed]
- Dinis, P.; Teixeira, R.; Puga, L.; Lourenço, C.; Cachulo, M.C.; Gonçalves, L. Eosinophilic Myocarditis: Clinical Case and Literature Review. Arq. Bras. Cardiol. 2018, 110, 597–599. [Google Scholar] [CrossRef]
- Kindermann, M.; Sood, N.; Ehrlich, P.; Klingel, K. Fast spontaneous recovery from acute necrotizing eosinophilic myopericarditis without need for immunosuppressive therapy: A case report of a 27-year-old male. Eur. Heart J. Case Rep. 2020, 4, 1–5. [Google Scholar] [CrossRef]
- Björkenstam, M.; Bobbio, E.; Mellberg, T.; Polte, C.L.; Bergh, N.; Giallauria, F.; Bollano, E. Case report of eosinophilic granulomatosis with polyangitis presenting as acute myocarditis. Clin. Case Rep. 2022, 10, e6446. [Google Scholar] [CrossRef]
- Syed, I.S.; Martinez, M.W.; Feng, D.L.; Glockner, J.F. Cardiac magnetic resonance imaging of eosinophilic endomyocardial disease. Int. J. Cardiol. 2008, 126, e50–e52. [Google Scholar] [CrossRef]
- Debl, K.; Djavidani, B.; Seitz, J.; Nitz, W.; Schmid, F.X.; Muders, F.; Buchner, S.; Feuerbach, S.; Riegger, G.; Luchner, A. Planimetry of aortic valve area in aortic stenosis by magnetic resonance imaging. Investig. Radiol. 2005, 40, 631–636. [Google Scholar] [CrossRef]
- Looi, J.L.; Ruygrok, P.; Royle, G.; Raos, Z.; Hood, C.; Kerr, A.J. Acute eosinophilic endomyocarditis: Early diagnosis and localisation of the lesion by cardiac magnetic resonance imaging. Int. J. Cardiovasc. Imaging 2010, 26 (Suppl. S1), 151–154. [Google Scholar] [CrossRef]
- Mengesha, B.; Meir, K.; Gural, A.; Asleh, R. A case series of eosinophilic myocarditis: Different faces of the same coin. Eur. Heart J. Case Rep. 2022, 6, ytac388. [Google Scholar] [CrossRef]
- EACVI CMR Pocket Guides. Available online: https://www.escardio.org/Sub-specialty-communities/European-Association-of-Cardiovascular-Imaging-(EACVI)/Research-and-Publications/CMR-Pocket-Guides (accessed on 13 May 2025).
- Pöyhönen, P.; Rågback, J.; Mäyränpää, M.I.; Nordenswan, H.K.; Lehtonen, J.; Shenoy, C.; Kupari, M. Cardiac magnetic resonance in histologically proven eosinophilic myocarditis. J. Cardiovasc. Magn. Reson. 2023, 25, 79. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648, 2648a–2648d. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Lee, K.K.; Bularga, A.; O’Brien, R.; Ferry, A.V.; Doudesis, D.; Fujisawa, T.; Kelly, S.; Stewart, S.; Wereski, R.; Cranley, D.; et al. Troponin-Guided Coronary Computed Tomographic Angiography After Exclusion of Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 78, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Le Polain de Waroux, J.B.; Pouleur, A.C.; Goffinet, C.; Pasquet, A.; Vanoverschelde, J.L.; Gerber, B.L. Combined coronary and late-enhanced multidetector-computed tomography for delineation of the etiology of left ventricular dysfunction: Comparison with coronary angiography and contrast-enhanced cardiac magnetic resonance imaging. Eur. Heart J. 2008, 29, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Estornell-Erill, J.; Igual-Muñoz, B.; Monmeneu-Menadas, J.V.; Soriano-Navarro, C.; Valle-Muñoz, A.; Vilar-Herrero, J.V.; Perez-Bosca, L.; Paya-Serrano, R.; Martinez-Alzamora, N.; Ridocci-Soriano, F. Etiological diagnosis of left ventricular dysfunction: Computed tomography compared with coronary angiography and cardiac magnetic resonance. Rev. Esp. Cardiol. Engl. Ed. 2012, 65, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.V.; Shah, M.; McCarthy, B.; Garcia, A.; Ferketich, A.K. Multi-detector row cardiac computed tomography accurately quantifies right and left ventricular size and function compared with cardiac magnetic resonance. Am. Heart J. 2006, 151, 736–744. [Google Scholar] [CrossRef]
- Maffei, E.; Messalli, G.; Martini, C.; Nieman, K.; Catalano, O.; Rossi, A.; Seitun, S.; Guaricci, A.I.; Tedeschi, C.; Mollet, N.R.; et al. Left and right ventricle assessment with Cardiac CT: Validation study vs. Cardiac MR. Eur. Radiol. 2012, 22, 1041–1049. [Google Scholar] [CrossRef]
- Asferg, C.; Usinger, L.; Kristensen, T.S.; Abdulla, J. Accuracy of multi-slice computed tomography for measurement of left ventricular ejection fraction compared with cardiac magnetic resonance imaging and two-dimensional transthoracic echocardiography: A systematic review and meta-analysis. Eur. J. Radiol. 2012, 81, e757–e762. [Google Scholar] [CrossRef]
- Sharma, A.; Einstein, A.J.; Vallakati, A.; Arbab-Zadeh, A.; Mukherjee, D.; Lichstein, E. Meta-analysis of global left ventricular function comparing multidetector computed tomography with cardiac magnetic resonance imaging. Am. J. Cardiol. 2014, 113, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, M.S.; Achenbach, S.; Marwan, M.; Seltmann, M.; Muschiol, G.; Ropers, D.; Daniel, W.G.; Pflederer, T. Left ventricular thrombus attenuation characterization in cardiac computed tomography angiography. J. Cardiovasc. Comput. Tomogr. 2012, 6, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Cruz Rodriguez, J.B.; Okajima, K.; Greenberg, B.H. Management of left ventricular thrombus: A narrative review. Ann. Transl. Med. 2021, 9, 520. [Google Scholar] [CrossRef]
- Nakahara, T.; Fujimoto, S.; Jinzaki, M. Molecular imaging of cardiovascular disease: Current status and future perspective. J. Cardiol. 2025, 85, 386–398. [Google Scholar] [CrossRef]
- Yokoyama, R.; Miyagawa, M.; Okayama, H.; Inoue, T.; Miki, H.; Ogimoto, A.; Higaki, J.; Mochizuki, T. Quantitative analysis of myocardial 18F-fluorodeoxyglucose uptake by PET/CT for detection of cardiac sarcoidosis. Int. J. Cardiol. 2015, 195, 180–187. [Google Scholar] [CrossRef]
- Martineau, P.; Grégoire, J.; Harel, F.; Pelletier-Galarneau, M. Assessing cardiovascular infection and inflammation with FDG-PET. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 46–58. [Google Scholar]
- Cho, S.G. Can FDG PET Serve as a Clinically Relevant Tool for Detecting Active Non-sarcoidotic Myocarditis? Nucl. Med. Mol. Imaging 2024, 58, 406–417. [Google Scholar] [CrossRef]
- Caobelli, F.; Cabrero, J.B.; Galea, N.; Haaf, P.; Loewe, C.; Luetkens, J.A.; Muscogiuri, G.; Francone, M. Cardiovascular magnetic resonance (CMR) and positron emission tomography (PET) imaging in the diagnosis and follow-up of patients with acute myocarditis and chronic inflammatory cardiomyopathy: A review paper with practical recommendations on behalf of the European Society of Cardiovascular Radiology (ESCR). Int. J. Cardiovasc. Imaging 2023, 39, 2221–2235. [Google Scholar]
- Lucinian, Y.A.; Martineau, P.; Abikhzer, G.; Harel, F.; Pelletier-Galarneau, M. Novel tracers to assess myocardial inflammation with radionuclide imaging. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 2024, 42, 102012. [Google Scholar] [CrossRef]
- Peslier, H.; Metrard, G.; Malmare, B.A.; Bailly, M. A Rare Case of Eosinophilic Myocarditis Described with 18F-FDG PET/CT. Clin. Nucl. Med. 2025, 50, 172–173. [Google Scholar] [CrossRef]
- Si, J.; Zhang, X.; Chen, N.; Sun, F.; Du, P.; Li, Z.; Tian, D.; Sun, X.; Sun, G.; Cong, T.; et al. Case Report: Multimodal Imaging Guides the Management of an Eosinophilic Leukemia Patient with Eosinophilic Myocarditis and Intracardiac Thrombus. Front. Cardiovasc. Med. 2022, 9, 903323. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, K.; Dohi, K.; Omori, T.; Tanimura, M.; Sugiura, E.; Nakamori, S.; Sawai, T.; Imanaka-Yoshida, K.; Yamada, N.; Ito, M. A Survival Case of Fulminant Right-Side Dominant Eosinophilic Myocarditis. Int. Heart J. 2017, 58, 459–462. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hagiwara, H.; Fukushima, A.; Iwano, H.; Anzai, T. Refractory cardiac myocarditis associated with drug rash with eosinophilia and systemic symptoms syndrome due to anti-bipolar disorder drugs: A case report. Eur. Heart J.-Case Rep. 2018, 2, yty100. [Google Scholar] [CrossRef] [PubMed]
- Thomas Aretz, H. Myocarditis: The Dallas criteria. Hum. Pathol. 1987, 18, 619–624. [Google Scholar] [CrossRef]
- Bermpeis, K.; Esposito, G.; Gallinoro, E.; Paolisso, P.; Bertolone, D.T.; Fabbricatore, D.; Mileva, N.; Munhoz, D.; Buckley, J.; Wyffels, E.; et al. Safety of Right and Left Ventricular Endomyocardial Biopsy in Heart Transplantation and Cardiomyopathy Patients. JACC Heart Fail. 2022, 10, 963–973. [Google Scholar] [CrossRef]
- Ammirati, E.; Buono, A.; Moroni, F.; Gigli, L.; Power, J.R.; Ciabatti, M.; Garascia, A.; Adler, E.D.; Pieroni, M. State-of-the-Art of Endomyocardial Biopsy on Acute Myocarditis and Chronic Inflammatory Cardiomyopathy. Curr. Cardiol. Rep. 2022, 24, 597–609. [Google Scholar] [CrossRef]
- Parrillo, J.E. Transvenous Endomyocardial Biopsy: Clinical Indications, Potential Complications, and Future Applications. Chest 1986, 90, 155–157. [Google Scholar] [CrossRef]
- Chow, L.H.; Radio, S.J.; Sears, T.D.; Mcmanus, B.M. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J. Am. Coll. Cardiol. 1989, 14, 915–920. [Google Scholar] [CrossRef]
- Kühl, U.; Noutsias, M.; Seeberg, B.; Schultheiss, H.P. Immunohistological evidence for a chronic intramyocardial inflammatory process in dilated cardiomyopathy. Heart 1996, 75, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Maisch, B.; Richter, A.; Sandmöller, A.; Portig, I.; Pankuweit, S. Members of Project 9a in the BMBF-Heart Failure Network. Inflammatory Dilated Cardiomyopathy (DCMI). Herz-Kreislauf-Erkrank. 2005, 30, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Veronese, G.; Brambatti, M.; Merlo, M.; Cipriani, M.; Potena, L.; Sormani, P.; Aoki, T.; Sugimura, K.; Sawamura, A.; et al. Fulminant Versus Acute Nonfulminant Myocarditis in Patients With Left Ventricular Systolic Dysfunction. J. Am. Coll. Cardiol. 2019, 74, 299–311. [Google Scholar] [CrossRef]
- Sinha, S.S.; Morrow, D.A.; Kapur, N.K.; Kataria, R.; Roswell, R.O. 2025 Concise Clinical Guidance: An ACC Expert Consensus Statement on the Evaluation and Management of Cardiogenic Shock. JACC 2025, 85, 1618–1641. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Cionchel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Levine, G.N.; McEvoy, J.W.; Fang, J.C.; Ibeh, C.; McCarthy, C.P.; Misra, A.; Shah, Z.I.; Shenoy, C.; Spinler, S.A.; Vallurupalli, S.; et al. Management of Patients at Risk for and With Left Ventricular Thrombus: A Scientific Statement from the American Heart Association. Circulation 2022, 146, e205–23. [Google Scholar] [CrossRef]
- Chen, H.S.; Wang, W.; Wu, S.N.; Liu, J.P. Corticosteroids for viral myocarditis. Cochrane Database Syst. Rev. 2013, 2013, CD004471. [Google Scholar] [CrossRef]
- Kishimoto, C.; Shioji, K.; Hashimoto, T.; Nonogi, H.; Lee, J.D.; Kato, S.; Hiramitsu, S.; Morimoto, S.-i. Therapy with immunoglobulin in patients with acute myocarditis and cardiomyopathy: Analysis of leukocyte balance. Heart Vessel. 2014, 29, 336–342. [Google Scholar] [CrossRef]
- Techasatian, W.; Gozun, M.; Vo, K.; Yokoyama, J.; Nagamine, T.; Shah, P.; Vu, K.; Zhang, J.; Nishimura, Y. Eosinophilic myocarditis: Systematic review. Heart Br. Card. Soc. 2024, 110, 687–693. [Google Scholar] [CrossRef]
- Mason, J.W.; Billingham, M.E.; Ricci, D.R. Treatment of acute inflammatory myocarditis assisted by endomyocardial biopsy. Am. J. Cardiol. 1980, 45, 1037–1044. [Google Scholar] [CrossRef]
- O’Connell, J.B.; Mason, J.W. Diagnosing and treating active myocarditis. West. J. Med. 1989, 150, 431–435. [Google Scholar]
- Farhat, N.; Bouhabib, M.; Joye, R.; Vallée, J.P.; Beghetti, M. Contribution of imaging modalities to eosinophilic myocarditis diagnosis: A case report. Eur. Heart J.-Case Rep. 2022, 6, ytac058. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Cheng, T.O.; Fei, H.; Ren, P.; He, Y.; Wang, X.; Lu, Q.; Han, W.; Li, K.; Li, L.; et al. The diagnostic value of transthoracic echocardiography for eosinophilic myocarditis: A single center experience from China. Int. J. Cardiol. 2015, 201, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Eppenberger, M.; Hack, D.; Ammann, P.; Rickli, H.; Maeder, M.T. Acute Eosinophilic Myocarditis with Dramatic Response to Steroid Therapy. Tex. Heart Inst. J. 2013, 40, 326–330. [Google Scholar] [PubMed]
- Kim, E.Y.; Chang, S.A.; Lee, Y.K.; Choi, J.O.; Choe, Y.H. Early Non-Invasive Diagnosis and Treatment of Acute Eosinophlic Myopericarditis by Cardiac Magnetic Resonance. J. Korean Med. Sci. 2011, 26, 1522–1526. [Google Scholar] [CrossRef]
- Sharifkazemi, M.; Rezaian, G.; Lotfi, M. Evaluation of myocardial performance by serial speckle tracking echocardiography in diagnosis and follow-up of a patient with eosinophilic myocarditis. Echo Res. Pract. 2023, 10, 1. [Google Scholar] [CrossRef]
- Debl, K.; Djavidani, B.; Buchner, S.; Poschenrieder, F.; Heinicke, N.; Feuerbach, S.; Riegger, G.; Luchner, A. Time course of eosinophilic myocarditis visualized by CMR. J. Cardiovasc. Magn. Reson. 2008, 10, 21. [Google Scholar] [CrossRef]

| CLINICAL SCENARIO | EMB RATIONALE | IMAGING MODALITY | IMAGING ROLE IN BIOPSY |
|---|---|---|---|
| Fulminant myocarditis with shock | Rapid diagnosi sto guide immunosoppressive/antiviral therapy. Note: imaging may be not always feasible in unstable patient. | CMR, [18F]FDG, PET/CT | Identify areas with highest oedema or tracer uptake if patients’ conditions allow imaging |
| Unclear diagnosis after imaging | Histological confirmation (e.g., EM vs. other) | CMR | Locate subendocardial LGE or diffuse edema to target biopsy site |
| No reponse to therapy | Explore alternative or coexisting causes | PET | Confirm persistent inflammation and guide sampling |
| Isolated LV involvement | Consider LV biopsy (with caution) | CMR | Confirm lack of RV involvement and focus on LV lesions for biopsy guidance |
| Focal myocarditis | Increase diagnostic yield | CMR PET | Avoid inaffected areas, reduce false negatives |
| Systemic disease with cardiac involvement (EGPA, HES) | Confism eosinophilic infiltration | PET/TC CMR | Define cardiac and extracardiac disease pattern, guide EMB to active sites |
| LINE | THERAPY | DOSE | DURATION |
|---|---|---|---|
| FIRST LINE | Methylprednisone (IV pulse therapy) Prednisone (oral) | 500 mg to 1 g/day 1 mg/kg/day Max: 60–80 mg/day | 3–5 days Several weeks, with a slow taper over 3–6 months |
| ADJUNCTIVE OR RESCUE THERAPY | Intravenous immunoglobulin (IVIG) | 2 g/kg total dose, typically given as: 400 mg/kg/day 1 g/kg/day | For 5 days For 2 days |
| IF STEROID-REFRACTORY | Azathioprine Mycophenolate mofetii Cyclophosphamide | 1–2 mg/kg 1–2 g 1–2 mg/kg orally Or IV pulse (500–1000 mg/m2) | Daily Daily Daily monthly |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viccaro, V.; Valotta, A.; Checcoli, E.; Landi, S.; Cattaneo, F.; Milzi, A.; Duchini, M.; Viani, G.M.; Caretta, A.; Schlossbauer, S.; et al. Multimodality Imaging in Eosinophilic Myocarditis: A Rare Cause of Heart Failure. J. Cardiovasc. Dev. Dis. 2025, 12, 320. https://doi.org/10.3390/jcdd12080320
Viccaro V, Valotta A, Checcoli E, Landi S, Cattaneo F, Milzi A, Duchini M, Viani GM, Caretta A, Schlossbauer S, et al. Multimodality Imaging in Eosinophilic Myocarditis: A Rare Cause of Heart Failure. Journal of Cardiovascular Development and Disease. 2025; 12(8):320. https://doi.org/10.3390/jcdd12080320
Chicago/Turabian StyleViccaro, Vincenzo, Amabile Valotta, Elena Checcoli, Susanna Landi, Fabio Cattaneo, Andrea Milzi, Mattia Duchini, Giacomo Maria Viani, Alessandro Caretta, Susanne Schlossbauer, and et al. 2025. "Multimodality Imaging in Eosinophilic Myocarditis: A Rare Cause of Heart Failure" Journal of Cardiovascular Development and Disease 12, no. 8: 320. https://doi.org/10.3390/jcdd12080320
APA StyleViccaro, V., Valotta, A., Checcoli, E., Landi, S., Cattaneo, F., Milzi, A., Duchini, M., Viani, G. M., Caretta, A., Schlossbauer, S., Landi, A., Leo, L. A., Treglia, G., Pedrazzini, G., Valgimigli, M., & Pavon, A. G. (2025). Multimodality Imaging in Eosinophilic Myocarditis: A Rare Cause of Heart Failure. Journal of Cardiovascular Development and Disease, 12(8), 320. https://doi.org/10.3390/jcdd12080320







