Activated Clotting Time and Haemostatic Complications in Patients Receiving ECMO Support: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Data Synthesis and Extraction
2.3. Quality Assessment of Studies
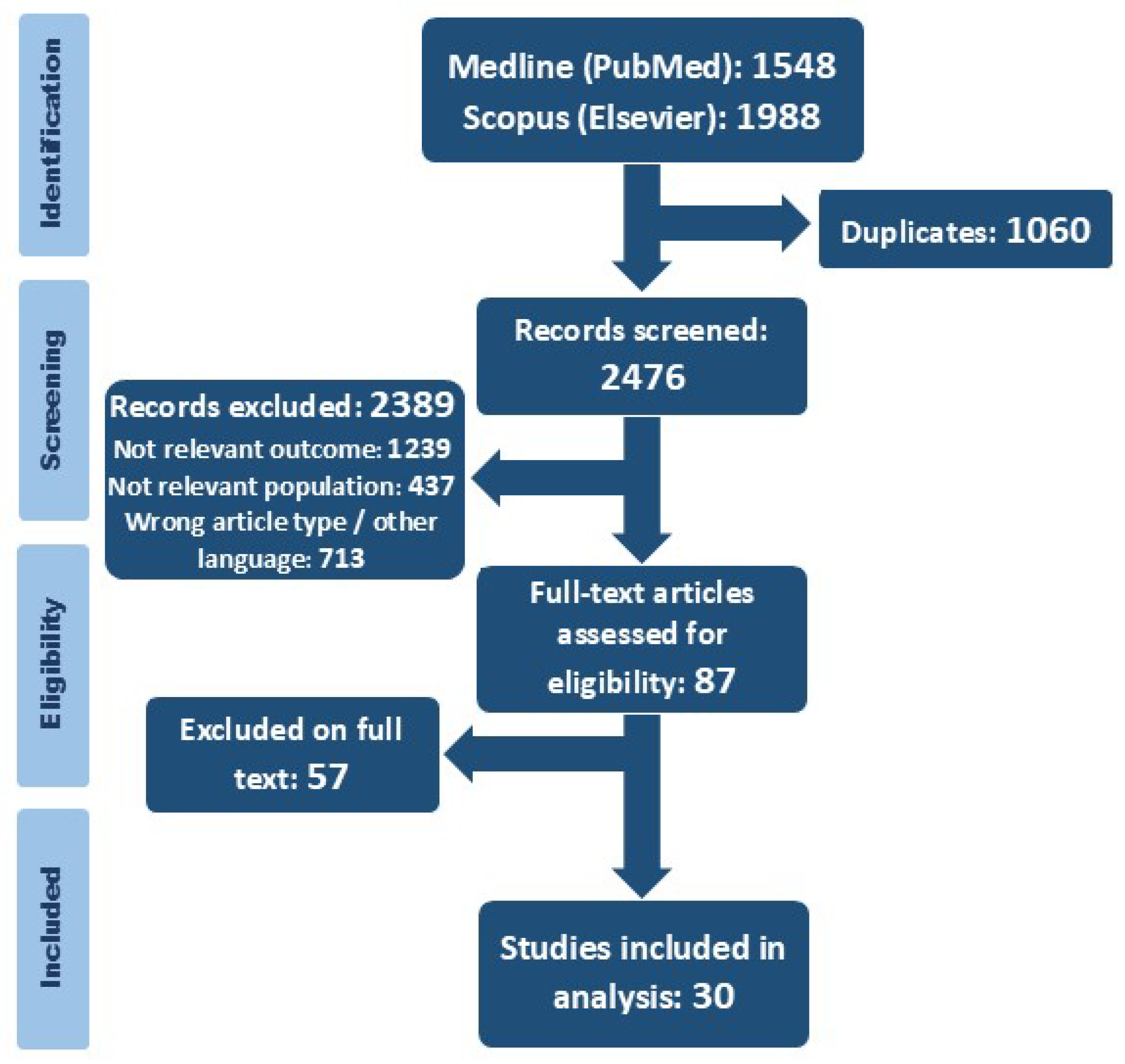
3. Results
3.1. Search Results and Description of Studies
3.2. Patient Population and Outcomes
3.3. Haemorrhagic Events and ACT
3.4. Thromboembolic Events and ACT
3.5. Adverse Events
4. Discussion
4.1. Monitoring of Anticoagulation During ECMO
4.2. Activated Clotting Time and Haemorrhagic Events
4.3. Activated Clotting Time and Thromboembolic Events
4.4. Adverse Events and Mortality
4.5. Future Development
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACT | Activated clotting time |
| aPTT | Activated partial thromboplastin time |
| ARDS | Acute respiratory distress syndrome |
| CDH | Congenital diaphragmatic hernia |
| ECMO | Extracorporeal membrane oxygenation |
| eCPR | Extracorporeal cardiopulmonary resuscitation |
| ELSO | Extracorporeal Life Support Organization |
| ICH | Intracerebral/cranial haemorrhage |
| ICU | Intensive care unit |
| NOS | Newcastle–Ottawa Scale |
| PR | Prospective |
| PT | Prothrombin time |
| ROTEM | Rotational thromboelastometry |
| RSV | Respiratory syncytial virus |
| RT | Retrospective |
| TEG | Thromboelastography |
| UFH | Unfractionated heparin |
| VA | Venoarterial |
| VV | Venovenous |
References
- Rajsic, S.; Breitkopf, R.; Oezpeker, U.C.; Bukumirić, Z.; Dobesberger, M.; Treml, B. The Role of Excessive Anticoagulation and Missing Hyperinflammation in ECMO-Associated Bleeding. J. Clin. Med. 2022, 11, 2314. [Google Scholar] [CrossRef] [PubMed]
- Rajsic, S.; Breitkopf, R.; Jadzic, D.; Krneta, M.P.; Tauber, H.; Treml, B. Anticoagulation Strategies during Extracorporeal Membrane Oxygenation: A Narrative Review. J. Clin. Med. 2022, 11, 5147. [Google Scholar] [CrossRef]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The Inflammatory Response to Extracorporeal Membrane Oxygenation (ECMO): A Review of the Pathophysiology. Crit. Care 2016, 20, 387. [Google Scholar] [CrossRef]
- Levy, J.H.; Staudinger, T.; Steiner, M.E. How to Manage Anticoagulation during Extracorporeal Membrane Oxygenation. Intensive Care Med. 2022, 48, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.A.; Hockings, L.E.; Andrews, R.K.; Aubron, C.; Gardiner, E.E.; Pellegrino, V.A.; Davis, A.K. Extracorporeal Membrane Oxygenation—Hemostatic Complications. Transfus. Med. Rev. 2015, 29, 90–101. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.B.V.; Ryerson, L.M.; Ratano, D.; Fan, E.; Faraoni, D.; Annich, G.M. 2021 ELSO Adult and Pediatric Anticoagulation Guidelines. ASAIO J. 2022, 68, 303–310. [Google Scholar] [CrossRef]
- Helms, J.; Frere, C.; Thiele, T.; Tanaka, K.A.; Neal, M.D.; Steiner, M.E.; Connors, J.M.; Levy, J.H. Anticoagulation in Adult Patients Supported with Extracorporeal Membrane Oxygenation: Guidance from the Scientific and Standardization Committees on Perioperative and Critical Care Haemostasis and Thrombosis of the International Society on Thrombosis and Haemostasis. J. Thromb. Haemost. 2023, 21, 373–396. [Google Scholar] [CrossRef]
- Protti, A.; Iapichino, G.E.; Di Nardo, M.; Panigada, M.; Gattinoni, L. Anticoagulation Management and Antithrombin Supplementation Practice during Veno-Venous Extracorporeal Membrane Oxygenation: A Worldwide Survey. Anesthesiology 2020, 132, 562–570. [Google Scholar] [CrossRef]
- Sun, J.; Ma, Y.; Su, W.; Miao, H.; Guo, Z.; Chen, Q.; Zhang, Y.; Ma, X.; Chen, S.; Ding, R. Comparison of Anticoagulation Monitoring Strategies for Adults Supported on Extracorporeal Membrane Oxygenation: A Systematic Review. Heart Lung 2023, 61, 72–83. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Health Research Institute: Ottawa, ON, Canada, 2014; Volume 7. [Google Scholar]
- Al-Jazairi, A.; Raslan, S.; Al-mehizia, R.; Dalaty, H.A.; Vol, E.B.D.; Saad, E.; Alanazi, M.; Owaidah, T. Performance Assessment of a Multifaceted Unfractionated Heparin Dosing Protocol in Adult Patients on Extracorporeal Membrane Oxygenator. Ann. Pharmacother. 2021, 55, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Anton-Martin, P.; Journeycake, J.; Modem, V.; Golla, S.; Raman, L.; Tweed, J.; Darnell-Bowens, C. Coagulation Profile Is Not a Predictor of Acute Cerebrovascular Events in Pediatric Extracorporeal Membrane Oxygenation Patients. ASAIO J. 2017, 63, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Atallah, S.; Liebl, M.; Fitousis, K.; Bostan, F.; Masud, F. Evaluation of the Activated Clotting Time and Activated Partial Thromboplastin Time for the Monitoring of Heparin in Adult Extracorporeal Membrane Oxygenation Patients. Perfusion 2014, 29, 456–461. [Google Scholar] [CrossRef]
- Bailly, D.K.; Reeder, R.W.; Muszynski, J.A.; Meert, K.L.; Ankola, A.A.; Alexander, P.M.; Pollack, M.M.; Moler, F.W.; Berg, R.A.; Carcillo, J.; et al. Anticoagulation Practices Associated with Bleeding and Thrombosis in Pediatric Extracorporeal Membrane Oxygenation; a Multi-Center Secondary Analysis. Perfusion 2023, 38, 363–372. [Google Scholar] [CrossRef]
- Deshpande, S.J.; Vitali, S.; Thiagarajan, R.; Brediger, S.; McManus, M.; Geva, A. Coagulations Studies Do Not Correlate with Each Other or with Hematologic Complications During Pediatric Extracorporeal Membrane Oxygenation*. Pediatr. Crit. Care Med. 2021, 22, 542–552. [Google Scholar] [CrossRef]
- Doymaz, S.; Zinger, M.; Sweberg, T. Risk Factors Associated with Intracranial Hemorrhage in Neonates with Persistent Pulmonary Hypertension on ECMO. J. Intensive Care 2015, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Feih, J.T.; Wallskog, K.E.; Rinka, J.R.G.; Juul, J.J.; Rein, L.; Gaglianello, N.; Kreuziger, L.M.B.; Joyce, D.L.; Tawil, J.N. Heparin Monitoring with an Anti-Xa Protocol Compared to Activated Clotting Time in Patients on Temporary Mechanical Circulatory Support. Ann. Pharmacother. 2022, 56, 513–523. [Google Scholar] [CrossRef]
- Villalba, C.A.F.; Brogan, T.V.; McMullan, D.M.; Yalon, L.; Jordan, D.I.; Chandler, W.L. Conversion From Activated Clotting Time to Anti-Xa Heparin Activity Assay for Heparin Monitoring During Extracorporeal Membrane Oxygenation*. Crit. Care Med. 2020, 48, e1179–e1184. [Google Scholar] [CrossRef]
- Fitousis, K.; Klasek, R.; Mason, P.E.; Masud, F. Evaluation of a Pharmacy Managed Heparin Protocol for Extracorporeal Membrane Oxygenation Patients. Perfusion 2017, 32, 238–244. [Google Scholar] [CrossRef]
- Galura, G.; Said, S.J.; Shah, P.A.; Hissong, A.M.; Chokshi, N.K.; Fauman, K.R.; Rose, R.; Bondi, D.S. Comparison of Extracorporeal Life Support Anticoagulation Using Activated Clotting Time Only to a Multimodal Approach in Pediatric Patients. J. Pediatr. Pharmacol. Ther. 2022, 27, 517–523. [Google Scholar] [CrossRef]
- Henderson, N.; Sullivan, J.E.; Myers, J.; Wells, T.; Calhoun, A.; Berkenbosch, J.; Tzanetos, D.T. Use of Thromboelastography to Predict Thrombotic Complications in Pediatric and Neonatal Extracorporeal Membranous Oxygenation. J. Extra Corpor. Technol. 2018, 50, 149–154. [Google Scholar] [CrossRef]
- Hong, J.I.; Hwang, J.; Shin, H.J. Satisfactory Outcome with Low Activated Clotting Time in Extracorporeal Membrane Oxygenation. Rev. Cardiovasc. Med. 2021, 22, 1589. [Google Scholar] [CrossRef]
- Irby, K.; Swearingen, C.; Byrnes, J.; Bryant, J.; Prodhan, P.; Fiser, R. Unfractionated Heparin Activity Measured by Anti-Factor Xa Levels Is Associated with the Need for Extracorporeal Membrane Oxygenation Circuit/Membrane Oxygenator Change. Pediatr. Crit. Care Med. 2014, 15, e175–e182. [Google Scholar] [CrossRef] [PubMed]
- Kasirajan, V.; Smedira, N.G.; McCarthy, J.F.; Casselman, F.; Boparai, N.; McCarthy, P.M. Risk Factors for Intracranial Hemorrhage in Adults on Extracorporeal Membrane Oxygenation1. Eur. J. Cardio-Thorac. Surg. 1999, 15, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, Z.; Han, X.; Song, K.; Xing, J. A Comparison of Activated Partial Thromboplastin Time and Activated Coagulation Time for Anticoagulation Monitoring during Extracorporeal Membrane Oxygenation Therapy. Hämostaseologie 2023, 43, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Maul, T.M.; Wolff, E.L.; Kuch, B.A.; Rosendorff, A.; Morell, V.O.; Wearden, P.D. Activated Partial Thromboplastin Time Is a Better Trending Tool in Pediatric Extracorporeal Membrane Oxygenation. Pediatr. Crit. Care Med. 2012, 13, e363–e371. [Google Scholar] [CrossRef]
- Mazzeffi, M.A.; Tanaka, K.; Roberts, A.; Rector, R.; Menaker, J.; Kon, Z.; Deatrick, K.B.; Kaczorowski, D.; Griffith, B.; Herr, D. Bleeding, Thrombosis, and Transfusion with Two Heparin Anticoagulation Protocols in Venoarterial ECMO Patients. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1216–1220. [Google Scholar] [CrossRef]
- Moynihan, K.; Johnson, K.; Straney, L.; Stocker, C.; Anderson, B.; Venugopal, P.; Roy, J. Coagulation Monitoring Correlation with Heparin Dose in Pediatric Extracorporeal Life Support. Perfusion 2017, 32, 675–685. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Phan, X.T.; Nguyen, T.H.; Huynh, D.Q.; Tran, L.T.; Pham, H.M.; Nguyen, T.N.; Kieu, H.T.; Ngoc Pham, T.T. Major Bleeding in Adults Undergoing Peripheral Extracorporeal Membrane Oxygenation (ECMO): Prognosis and Predictors. Crit. Care Res. Pract. 2022, 2022, 5348835. [Google Scholar] [CrossRef]
- Niebler, R.A.; Parker, H.; Hoffman, G.M. Impact of Anticoagulation and Circuit Technology on Complications During Extracorporeal Membrane Oxygenation. ASAIO J. 2019, 65, 270–276. [Google Scholar] [CrossRef]
- Northam, K.A.; Nguyen, B.; Chen, S.-L.; Sredzienski, E.; Charles, A. Evaluation of a Multimodal Heparin Laboratory Monitoring Protocol in Adult Extracorporeal Membrane Oxygenation Patients. J. Pharm. Pract. 2023, 36, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.R.; Mirsaeidi, M.; Mangar, D.; Camporesi, E.M. Duration of ECMO Is an Independent Predictor of Intracranial Hemorrhage Occurring During ECMO Support. ASAIO J. 2016, 62, 634–636. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, L.C.; Alten, J.A.; Goldberg, K.G.; Timpa, J.G.; Phillips, J.; Laney, D.; Borasino, S. Anti-Xa Directed Protocol for Anticoagulation Management in Children Supported with Extracorporeal Membrane Oxygenation. ASAIO J. 2015, 61, 339–344. [Google Scholar] [CrossRef]
- Perez Ortiz, A.; Dempfle, C.E.; Jung, T.; Doniga, T.; Weiß, C.; Hetjens, S.; Schaible, T.; Rafat, N. Assessing Anticoagulation in Neonates with Congenital Diaphragmatic Hernia During Extracorporeal Membrane Oxygenation: Does Anti-Factor Xa or Thromboelastometry Provide Additional Benefit? Front. Pediatr. 2021, 9, 685906. [Google Scholar] [CrossRef]
- Rama, G.; Middlesworth, W.; Neunert, C.; Streltsova, S.; Cheung, E.W. Antifactor Xa Monitoring and Hematologic Complications of Pediatric Extracorporeal Membrane Oxygenation. ASAIO J. 2021, 67, 91–95. [Google Scholar] [CrossRef]
- Reed, R.C.; Rutledge, J.C. Laboratory and Clinical Predictors of Thrombosis and Hemorrhage in 29 Pediatric Extracorporeal Membrane Oxygenation Nonsurvivors. Pediatr. Dev. Pathol. 2010, 13, 385–392. [Google Scholar] [CrossRef]
- Riley, J.B.; Schears, G.J.; Nuttall, G.A.; Oliver, W.C.; Ereth, M.H.; Dearani, J.A. Coagulation Parameter Thresholds Associated with Non-Bleeding in the Eighth Hour of Adult Cardiac Surgical Post-Cardiotomy Extracorporeal Membrane Oxygenation. J. Extracorpor. Technol. 2016, 48, 71–78. [Google Scholar] [CrossRef]
- Saini, A.; Hartman, M.E.; Gage, B.F.; Said, A.; Gazit, A.Z.; Eghtesady, P.; Boston, U.S.; Spinella, P.C. Incidence of Platelet Dysfunction by Thromboelastography–Platelet Mapping in Children Supported with ECMO: A Pilot Retrospective Study. Front. Pediatr. 2016, 3, 116. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Pasrija, C.; Kronfli, A.; Essien, E.-O.; Zhou, Y.; Brigante, F.; Bittle, G.; Menaker, J.; Herr, D.; Mazzeffi, M.A.; et al. A Comparison of Anticoagulation Strategies in Veno-Venous Extracorporeal Membrane Oxygenation. ASAIO J. 2022, 68, 738–743. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, X.; Guo, Z.; Zhang, W.; Shen, J.; Wang, W. Risk Factors of Pediatric Venoarterial Extracorporeal Membrane Oxygenation-related Gastrointestinal Bleeding after Open-heart Surgeries. Artif. Organs 2022, 46, 1682–1688. [Google Scholar] [CrossRef]
- Maclaren, G.; Brodie, D.; Lorusso, R.; Peek, G.; Thiagarajan, R.; Vercaemst, L. Extracorporeal Life Support: The ELSO Red Book, 6th ed.; Extracorporeal Life Support Organization: Ann Arbor, MI, USA, 2022. [Google Scholar]
- Rajsic, S.; Treml, B. Anticoagulation Monitoring During ECMO Support: Monitor or Flip a Coin? Clin. Cardiol. 2024, 47, e70061. [Google Scholar] [CrossRef]
- Bembea, M.M.; Annich, G.; Rycus, P.; Oldenburg, G.; Berkowitz, I.; Pronovost, P. Variability in Anticoagulation Management of Patients on Extracorporeal Membrane Oxygenation: An International Survey*. Pediatr. Crit. Care Med. 2013, 14, e77. [Google Scholar] [CrossRef]
- Schwaiger, D.; Schausberger, L.; Treml, B.; Jadzic, D.; Innerhofer, N.; Oberleitner, C.; Bukumiric, Z.; Rajsic, S. Association of Activated Clotting Time–Guided Anticoagulation with Complications during Extracorporeal Membrane Oxygenation Support: A Systematic Review and Meta-Analysis. J. Cardiothorac. Vasc. Anesth. 2024, 38, 3034–3042. [Google Scholar] [CrossRef] [PubMed]
- Rajsic, S.; Schwaiger, D.; Schausberger, L.; Breitkopf, R.; Treml, B.; Jadzic, D.; Oberleitner, C.; Bukumiric, Z. Anticoagulation Monitoring Using Activated Clotting Time in Patients Receiving Extracorporeal Membrane Oxygenation: A Meta-Analysis of Correlation Coefficients. J. Cardiothorac. Vasc. Anesth. 2024, 38, 2651–2660. [Google Scholar] [CrossRef] [PubMed]
- Wehner, J.E.; Boehne, M.; David, S.; Brand, K.; Tiede, A.; Bikker, R. Activated Clotting Time (ACT) for Monitoring of Low-Dose Heparin: Performance Characteristics in Healthy Adults and Critically Ill Patients. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620975494. [Google Scholar] [CrossRef]
- Martindale, S.J.; Shayevitz, J.R.; D’Errico, C. The Activated Coagulation Time: Suitability for Monitoring Heparin Effect and Neutralization during Pediatric Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 1996, 10, 458–463. [Google Scholar] [CrossRef]
- Colby, C.E.; Sheehan, A.; Benitz, W.; Van Meurs, K.; Halamek, L.P.; Moss, R.L. Maintaining Adequate Anticoagulation on Extracorporeal Membrane Oxygenation Therapy: Hemochron Junior Low Range versus Hemochron 400. J. Extracorpor. Technol. 2003, 35, 35–38. [Google Scholar] [CrossRef]
- Rajsic, S.; Treml, B.; Jadzic, D.; Breitkopf, R.; Oberleitner, C.; Krneta, M.P.; Bukumiric, Z. Extracorporeal Membrane Oxygenation for Cardiogenic Shock: A Meta-Analysis of Mortality and Complications. Ann. Intensive Care 2022, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Vaquer, S.; De Haro, C.; Peruga, P.; Oliva, J.C.; Artigas, A. Systematic Review and Meta-Analysis of Complications and Mortality of Veno-Venous Extracorporeal Membrane Oxygenation for Refractory Acute Respiratory Distress Syndrome. Ann. Intensive Care 2017, 7, 51. [Google Scholar] [CrossRef]
- Zangrillo, A.; Landoni, G.; Biondi-Zoccai, G.; Greco, M.; Greco, T.; Frati, G.; Patroniti, N.; Antonelli, M.; Pesenti, A.; Pappalardo, F. A Meta-Analysis of Complications and Mortality of Extracorporeal Membrane Oxygenation. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2013, 15, 172–178. [Google Scholar] [CrossRef]
- Cheng, R.; Hachamovitch, R.; Kittleson, M.; Patel, J.; Arabia, F.; Moriguchi, J.; Esmailian, F.; Azarbal, B. Complications of Extracorporeal Membrane Oxygenation for Treatment of Cardiogenic Shock and Cardiac Arrest: A Meta-Analysis of 1,866 Adult Patients. Ann. Thorac. Surg. 2014, 97, 610–616. [Google Scholar] [CrossRef]
- Rastan, A.J.; Lachmann, N.; Walther, T.; Doll, N.; Gradistanac, T.; Gommert, J.F.; Lehmann, S.; Wittekind, C.; Mohr, F.W. Autopsy Findings in Patients on Postcardiotomy Extracorporeal Membrane Oxygenation (ECMO). Int. J. Artif. Organs 2006, 29, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Rajsic, S.; Treml, B.; Jadzic, D.; Breitkopf, R.; Oberleitner, C.; Bachler, M.; Bösch, J.; Bukumiric, Z. aPTT-Guided Anticoagulation Monitoring during ECMO Support: A Systematic Review and Meta-Analysis. J. Crit. Care 2023, 77, 154332. [Google Scholar] [CrossRef]
- ELSO International Summary of Statistics|ECMO|ECLS. Available online: https://www.elso.org/registry/internationalsummaryandreports/internationalsummary.aspx (accessed on 18 November 2024).
- Willems, A.; Roeleveld, P.P.; Labarinas, S.; Cyrus, J.W.; Muszynski, J.A.; Nellis, M.E.; Karam, O. Anti-Xa versus Time-Guided Anticoagulation Strategies in Extracorporeal Membrane Oxygenation: A Systematic Review and Meta-Analysis. Perfusion 2021, 36, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, M.M.; Baltagi, S.; Carlson, M.; Levy, J.H.; Spinella, P.C. Clinical Controversies in Anticoagulation Monitoring and Antithrombin Supplementation for ECMO. Crit. Care 2020, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Arnouk, S.; Altshuler, D.; Lewis, T.C.; Merchan, C.; Smith, D.E.; Toy, B.; Zakhary, B.; Papadopoulos, J. Evaluation of Anti-Xa and Activated Partial Thromboplastin Time Monitoring of Heparin in Adult Patients Receiving Extracorporeal Membrane Oxygenation Support. ASAIO J. 2020, 66, 300–306. [Google Scholar] [CrossRef]
- Kulig, C.E.; Schomer, K.J.; Black, H.B.; Dager, W.E. Activated Partial Thromboplastin Time Versus Anti-Factor Xa Monitoring of Heparin Anticoagulation in Adult Venoarterial Extracorporeal Membrane Oxygenation Patients. ASAIO J. 2021, 67, 411–415. [Google Scholar] [CrossRef]
- Descamps, R.; Moussa, M.D.; Besnier, E.; Fischer, M.-O.; Preau, S.; Tamion, F.; Daubin, C.; Cousin, N.; Vincentelli, A.; Goutay, J.; et al. Anti-Xa Activity and Hemorrhagic Events under Extracorporeal Membrane Oxygenation (ECMO): A Multicenter Cohort Study. Crit. Care 2021, 25, 127. [Google Scholar] [CrossRef]
- Weitz, J.I.; Hudoba, M.; Massel, D.; Maraganore, J.; Hirsh, J. Clot-Bound Thrombin Is Protected from Inhibition by Heparin-Antithrombin III but Is Susceptible to Inactivation by Antithrombin III-Independent Inhibitors. J. Clin. Investig. 1990, 86, 385–391. [Google Scholar] [CrossRef]
- Fina, D.; Matteucci, M.; Jiritano, F.; Meani, P.; Kowalewski, M.; Ballotta, A.; Ranucci, M.; Lorusso, R. Extracorporeal Membrane Oxygenation Without Systemic Anticoagulation: A Case-Series in Challenging Conditions. J. Thorac. Dis. 2020, 12, 2113–2119. [Google Scholar] [CrossRef]
- Olson, S.R.; Murphree, C.R.; Zonies, D.; Meyer, A.D.; Mccarty, O.J.T.; Deloughery, T.G.; Shatzel, J.J. Thrombosis and Bleeding in Extracorporeal Membrane Oxygenation (ECMO) Without Anticoagulation: A Systematic Review. ASAIO J. 2021, 67, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.L.; Ayers, B.; Gosev, I.; Kumar, N.; Melvin, A.L.; Barrus, B.; Prasad, S. Venoarterial-Extracorporeal Membrane Oxygenation Without Routine Systemic Anticoagulation Decreases Adverse Events. Ann. Thorac. Surg. 2020, 109, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
| Author Country (Study Period) | Population Study Type | Number of Patients | ECMO Type | ECMO Duration (Days) | Main ECMO Indications | Main Study Aim | NOS |
|---|---|---|---|---|---|---|---|
| Al-Jazairi et al. [12], Saudi Arabia (-) | Adult PR and RT | 20 | VA: 16 VV: 4 | 15 (7–28) | Cardiac arrest, intraoperative wean-off failure, acute respiratory failure, bridge to transplant, and others | Correlation between anticoagulation monitoring and UFH infusion dose | Good |
| Anton-Martin et al. [13], USA (2009–2014) | Paediatric RT | 36 | VA: 31 VV: 5 | 154 A (25–1047) | Pulmonary, cardiac, and eCPR | Predictors for ICH or stroke | Good |
| Atallah et al. [14], USA (2011–2012) | Adult RT | 46 | - | 11 ± 14.6 | Cardiac and respiratory | Evaluation of the correlation between the UFH dose and ACT or aPTT | Good |
| Bailly et al. [15], USA (2012–2014) | Paediatric PR | 481 | VA: 400 VV: 81 | 5.4 (3.0–9.8) | Respiratory, cardiac, and eCPR | Association of anticoagulation practices with bleeding and thrombosis | Good |
| Deshpande et al. [16], USA (2010–2016) | Paediatric RT | 133 | VA: 92 VV: 41 | - | ARDS, CDH, lower respiratory tract infection, sepsis, etc. | Association of anticoagulation monitoring tools with haemostatic adverse events | Good |
| Doymaz et al. [17], USA (1997–2010) | Paediatric RT | 32 | VA: 19 VV: 13 | - | Persistent pulmonary hypertension | Incidence and risk factors for ICH | Good |
| Feih et al. [18], USA (2012–2018) | Adult RT | 45 | - | 99.0 A (51.0–169.3) | Respiratory failure, cardiogenic shock, and bypass weaning failure | Identification of risk factors for haemostatic complications | Good |
| Figueroa Villalba et al. [19], USA (2015–2018) | Paediatric RT | 65 | VA: 50 VV: 15 | - | Congenital heart disease, postcardiac surgery, cardiac arrest, respiratory failure, CDH, and others | Effect of monitoring change from ACT to anti-factor Xa | Good |
| Fitousis et al. [20], USA (2011–2014) | Adult RT | 61 | VA: 24 VV: 37 | 244 ± 326.1 A | - | Comparison of the efficacy and safety of aPTT- and ACT-based UFH anticoagulation | Good |
| Galura et al. [21], USA (2014–2020) | Paediatric RT | 27 | VA: 24 VV: 3 | 136 A (95, 192) | Cardiac and respiratory failure | Comparison of anticoagulation monitoring with ACT to a multimodal strategy (ACT, aPTT, anti-factor Xa, and TEG) | Good |
| Henderson et al. [22], USA (2013–2015) | Paediatric RT | 30 | VA: 26 VV: 4 | 146.8 ± 38.5 A | Cardiac arrest, cardiogenic shock, and respiratory failure | Analysis of anticoagulation goals for predicting haemostatic adverse events | Good |
| Hong et al. [23], Korea (2017–2019) | Adult RT | 43 | VA: 31 VV: 12 | - | - | Analysis of lower and conventional ACT target (<150 vs. 180–200 s) and its impact on safety and outcome | Good |
| Irby et al. [24] USA (2009–2011) | Paediatric RT | 62 | - | - | CBP weaning failure, eCPR, sepsis, respiratory failure, cardiac, bridge to transplant, etc. | Association of anti-factor Xa with ECMO circuit changes | Good |
| Kasirajan et al. [25], USA (1992–1996) | Adult RT | 74 | VA: 74 | - | Respiratory failure, myocardial infarction, post-cardiotomy, myocarditis, post-heart transplant | Prevalence and risk factors for ICH | Good |
| Liu et al. [26], China (2019–2020) | Adult RT | 17 | VA: 11 VV: 6 | 10 (8, 15) | Respiratory and circulatory support | Comparison between ACT/aPTT and UFH infusion dose | Good |
| Maul et al. [27], USA (2007–2010) | Paediatric RT | 47 | - | - | Respiratory or cardiac distress | Comparison of ACT and aPTT for UFH infusion monitoring | Good |
| Mazzeffi et al. [28], USA (2010–2015) | Adult RT | 50 | VA: 50 | 5 (2–8) | Cardiogenic shock, post-cardiotomy shock, and respiratory failure with cardiac dysfunction | Incidence of bleeding and thrombosis (ACT vs. aPTT) | Good |
| Moynihan et al. [29], Australia (2015–2016) | Paediatric RT | 31 | VA: 29 VV: 5 | 144.2 A (87.3–221.2) | Respiratory failure, sepsis, postoperative cardiac, eCPR, etc. | Correlation between anticoagulation monitoring methods and UFH dose | Good |
| Nguyen et al. [30], Vietnam (2019–2020) | Adult RT | 105 | VA: 61 VV: 38 VAV: 6 | - | Acute myocarditis, severe anaphylaxis, myocardial infarction, and ARDS | Risk factors for bleeding | Good |
| Niebler et al. [31], USA (2006–2016) | Paediatric RT | 129 | - | 94.5 A (59.5–154.5) | Cardiac and noncardiac surgery | Association of ACT and anti-factor Xa with haemostatic complications | Good |
| Northam et al. [32], USA (2014–2019) | Adult RT | 26 | VV: 26 | 5.0 (3.0–9.5) | Acute respiratory distress syndrome | Comparison of multimodal approach (aPTT/anti-factor Xa) and ACT for UFH monitoring | Good |
| Omar et al. [33], USA (2007–2013) | Adult RT | 154 | VA: 125 VV: 29 | 5.7 ± 6.8 | eCPR, respiratory failure, pulmonary embolism, cardiogenic shock, post-cardiac or lung surgery, etc. | Predictors and incidence of ICH | Good |
| O’Meara et al. [34], USA (2012–2012) | Paediatric RT | 10 | - | - | eCPR, cardiorespiratory failure, and pulmonary hypertension | Change from ACT to anti-factor Xa monitoring and impact on the oxygenator/circuit change | Fair |
| Perez Ortiz et al. [35], Germany (2018–2019) | Paediatric PR | 23 | VA; 23 | 10.3 (1–20) | CDH | Correlation between anticoagulation monitoring methods and UFH dose | Fair |
| Rama et al. [36], USA (2010–2016) | Paediatric RT | 96 | VA: 80 VV:16 | 112 A (73.4–165.6) | Cardiac, respiratory, and eCPR | Incidence of haemostatic complications based on ACT or anti-factor Xa monitoring | Fair |
| Reed et al. [37], USA (2004–2008) | Paediatric RT | 29 | - | - | Congenital or acquired cardiac or pulmonary diseases | Incidence and predictors of haemostatic complications | Fair |
| Riley et al. [38], USA (2007–2010) | Adult RT | 53 | VA: 53 | - | Post-cardiotomy | Acceptable blood loss and the sensitivity to detect haemorrhage for different coagulation monitoring methods measured in the first hours of ECMO | Good |
| Saini et al. [39], USA (2011–2012) | Paediatric RT | 24 | VA: 19 VV: 5 | - | Myocarditis, postoperative support, ARDS, pulmonary hypertension, CDH, etc. | Laboratory predictors for haemorrhage and mortality | Good |
| Shah et al. [40], USA (2009–2014) | Adult RT | 53 | VV: 53 | 10 (5–17) | ARDS and bridge to lung transplant | Change in monitoring from ACT to aPTT in relation to haemostatic complications and overall patient outcome | Fair |
| Yang et al. [41], China (2017–2020) | Paediatric RT | 148 | VA: 148 | - | Congenital heart disease | Gastrointestinal bleeding risk factors | Good |
| Outcome | Number of Studies Reporting Data (Events) | Pooled Rate (95% CI) | I2 (p-Value) |
|---|---|---|---|
| Mortality | |||
| In-hospital mortality | 17 (693) | 51.3 (44.0; 58.7) | 84% (<0.001) |
| ICU mortality | 5 (90) | 42.2 (26.6; 59.4) | 82% (<0.001) |
| Death during ECMO | 5 (81) | 36.8 (21.1; 55.9) | 84% (<0.001) |
| Bleeding | |||
| Major bleeding | 13 (501) | 49.2 (36.7; 61.9) | 90% (<0.001) |
| Any bleeding | 17 (700) | 47.8 (38.5; 57.2) | 88% (<0.001) |
| Cerebral haemorrhage | 15 (218) | 13.8 (10.3; 18.3) | 73% (<0.001) |
| Gastrointestinal bleeding | 5 (51) | 12.1 (5.8; 23.5) | 77% (0.001) |
| Pulmonary bleeding | 4 (61) | 5.3 (2.1; 12.8) | 72% (0.014) |
| Other bleeding | 6 (92) | 33.3 (18.3; 52.6) | 87% (<0.001) |
| Thrombosis | |||
| Any thrombosis | 16 (309) | 25.1 (17.6; 34.4) | 88% (<0.001) |
| ECMO circuit and membrane clot | 9 (168) | 16.6 (10.4; 25.4) | 81% (<0.001) |
| Deep venous thrombosis | 3 (12) | 12.1 (7.0; 20.2) | 16% (0.304) |
| Limb ischemia | 2 (26) | 7.5 (1.5; 30.8) | 94% (<0.001) |
| Ischemic stroke | 7 (50) | 5.5 (4.2; 7.2) | 0% (0.465) |
| Other thrombosis | 4 (30) | 14.4 (1.5; 65.5) | 95% (<0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwaiger, D.; Schausberger, L.; Treml, B.; Jadzic, D.; Innerhofer, N.; Oberleitner, C.; Bukumirić, Z.; Spurnić, I.; Rajsic, S. Activated Clotting Time and Haemostatic Complications in Patients Receiving ECMO Support: A Systematic Review. J. Cardiovasc. Dev. Dis. 2025, 12, 267. https://doi.org/10.3390/jcdd12070267
Schwaiger D, Schausberger L, Treml B, Jadzic D, Innerhofer N, Oberleitner C, Bukumirić Z, Spurnić I, Rajsic S. Activated Clotting Time and Haemostatic Complications in Patients Receiving ECMO Support: A Systematic Review. Journal of Cardiovascular Development and Disease. 2025; 12(7):267. https://doi.org/10.3390/jcdd12070267
Chicago/Turabian StyleSchwaiger, Daniel, Lukas Schausberger, Benedikt Treml, Dragana Jadzic, Nicole Innerhofer, Christoph Oberleitner, Zoran Bukumirić, Igor Spurnić, and Sasa Rajsic. 2025. "Activated Clotting Time and Haemostatic Complications in Patients Receiving ECMO Support: A Systematic Review" Journal of Cardiovascular Development and Disease 12, no. 7: 267. https://doi.org/10.3390/jcdd12070267
APA StyleSchwaiger, D., Schausberger, L., Treml, B., Jadzic, D., Innerhofer, N., Oberleitner, C., Bukumirić, Z., Spurnić, I., & Rajsic, S. (2025). Activated Clotting Time and Haemostatic Complications in Patients Receiving ECMO Support: A Systematic Review. Journal of Cardiovascular Development and Disease, 12(7), 267. https://doi.org/10.3390/jcdd12070267






