Aorto-Esophageal Fistula Caused by Vascular Malformation: A Case Description and an Analysis of the Literature
Abstract
1. Introduction
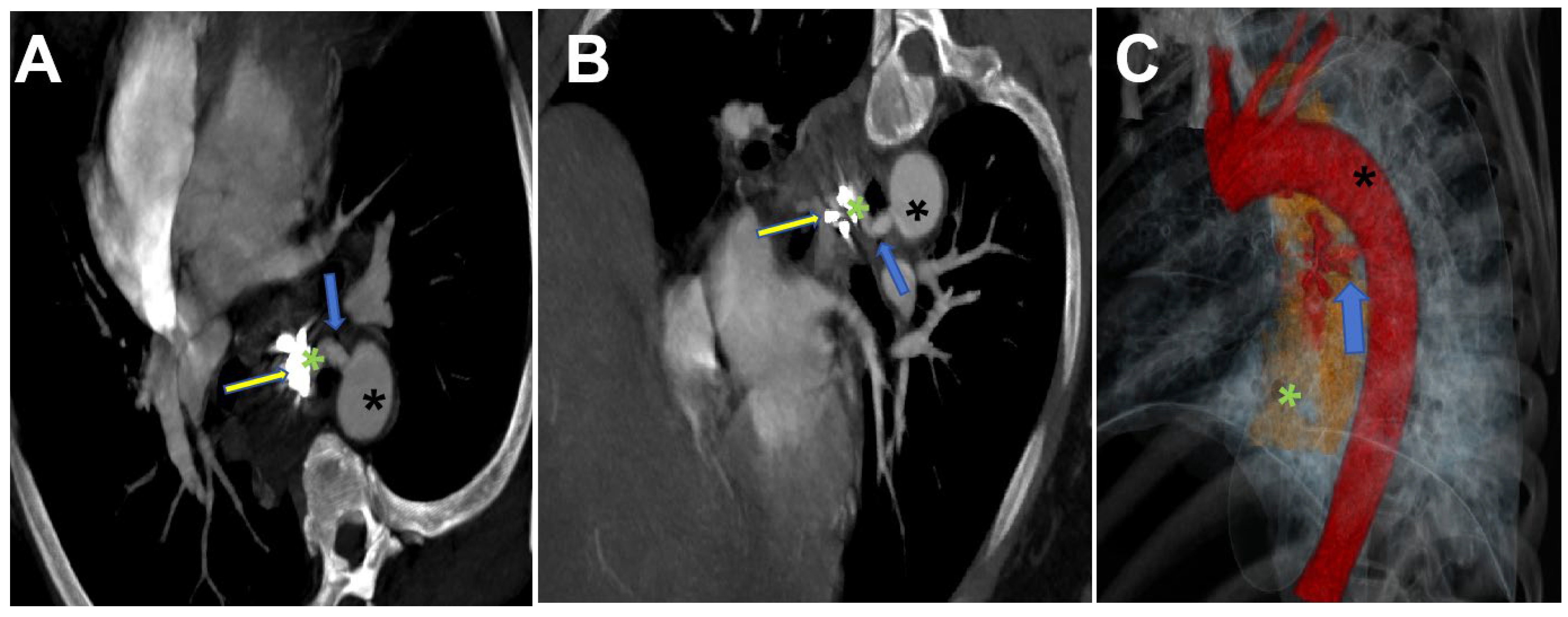
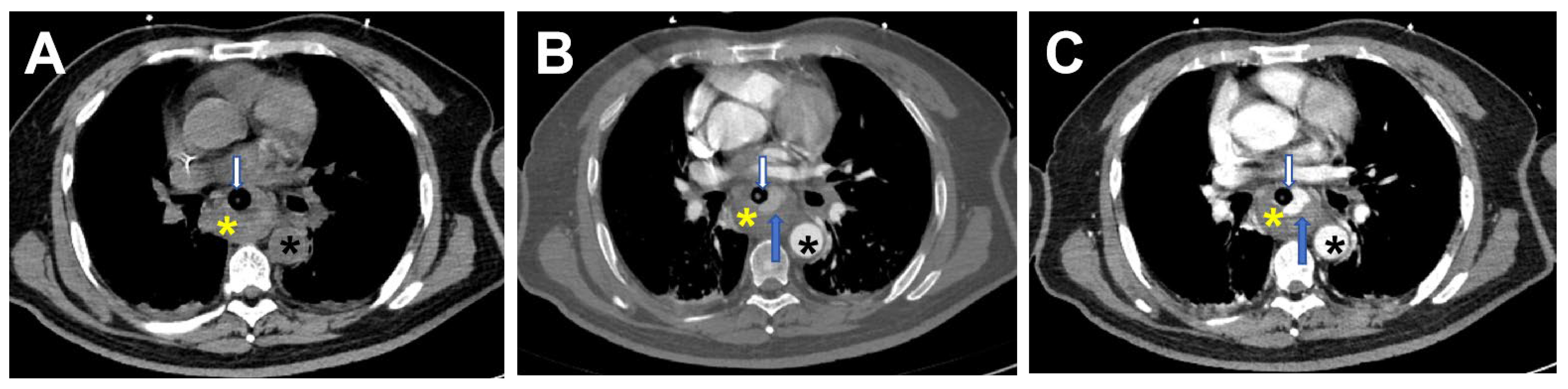
2. Case Report
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ctercteko, G.; Mok, C.K. Aorta-esophageal fistula induced by a foreign body: The first recorded survival. J. Thorac. Cardiovasc. Surg. 1980, 80, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.E.; Quick, G. Aortoesophageal fistula: A comprehensive review of the literature. Am. J. Med. 1991, 91, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Jonker, F.H.; Heijmen, R.; Trimarchi, S.; Verhagen, H.J.; Moll, F.L.; Muhs, B.E. Acute management of aortobronchial and aortoe-sophageal fistulas using thoracic endovascular aortic repair. J. Vasc. Surg. 2009, 50, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.L.; Peters, P.; Ogg, M.J.; Li, A.; Smithers, B.M. Successful management of an aortoesophageal fistula caused by a fishbone—Case report and review of literature. J. Cardiothorac. Surg. 2009, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Rawala, M.S.; Badami, V.; Rizvi, S.B.; Nanjundappa, A. Aortoesophageal fistula: A fatal complication of thoracic endovascular aorticstent-graft placement. Am. J. Case Rep. 2018, 19, 1258–1261. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Ueda, H.; Fujita, H.; Takeuchi, S. Successful endovascular repair of mycotic aortic pseudoaneurysm followed by aortoesophageal fistula. Ann. Vasc. Dis. 2017, 10, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.R.; Lee, C.C.; Lin, Y.C. Aortoesophageal fistula causing massive gastrointestinal bleeding and death in a patient with dermatomyositis: A case report. Am. J. Case Rep. 2018, 19, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, L.; Wang, Y.; Yu, D.; Peng, J.; Xu, J. Proposed management protocol for ingested esophageal foreign body and aortoesophageal fistula: A single-center experience. Int. J. Clin. Exp. Med. 2015, 8, 607–615. [Google Scholar] [PubMed]
- Hsu, W.F.; Lin, C.C.; Chang, K.M.; Lee, T.H. Primary aortoesophageal fistula: A rare but fatal cause of upper gastrointestinal bleeding. J. Dig. Dis. 2013, 14, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, E.; Chiche, L.; Gomes, D. Aortoesophageal fistula: Value of in situaortic allograft replacement. Ann. Surg. 2003, 238, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Li, J.; Hu, J.; Yu, F.; Li, S.; Yang, X. Diagnosis and treatment of 32 cases with aortoesophageal fistula due to esophageal foreign body. Laryngoscope 2011, 121, 267–272. [Google Scholar] [CrossRef] [PubMed]
- So, K.; Smith, C.R.; Faroqui, N.M.; Simone, N.; Senkowski, A.; Patel, S.K.; Bailey, B.M. Control of aortoesophageal fistula using endoscopicand endovascular techniques: A palliative intervention. Am. Surg. 2018, 84, e47–e49. [Google Scholar] [CrossRef] [PubMed]
- Akashi, H.; Kawamoto, S.; Saiki, Y.; Sakamoto, T.; Sawa, Y.; Tsukube, T.; Kubota, S.; Matsui, Y.; Karube, N.; Imoto, K.; et al. Therapeutic strategy for treating aortoesophageal fistulas. Gen. Thorac. Cardiovasc. Surg. 2014, 62, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Egashira, H.; Tokoro, S.; Ichita, C.; Takizawa, S.; Tsuki-yama, T.; Ogino, H.; Kawachi, J.; Shimoyama, R.; Kako, M. Thoracic endovascular aortic repair of esophageal cancer-associated aortoesophageal fistula: A case report and literature review. Case Rep. Oncol. Med. 2018, 2018, 9851397. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.Y.; Zhang, H.W.; Fan, K.J.; Liao, H.; Zhang, E.Y.; Hu, J. Aortoesophageal fistula and arch pseudoaneurysm after removing of a swallowed chicken bone: A case report of one-stage hybridtreatment. BMC Surg. 2018, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Takei, N.; Kunieda, T.; Kumada, Y.; Murayama, M. Perigraft abscess subsequent to aortoesophageal fistula. Intern. Med. 2018, 57, 3255–3259. [Google Scholar] [CrossRef] [PubMed]
- Takeno, S.; Ishii, H.; Nanashima, A.; Nakamura, K. Aortoesophageal fistula: Review of trends in the last decade. Surg. Today 2020, 50, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, F.; Hu, H.O.; Shi, J.; Zhang, J. Risk Factors for Mortality in Patients with Aortoesophageal Fistula Related to Aortic Lesions. Gastroenterol. Res. Pract. 2020, 2020, 4850287. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Hu, X.; Yu, J. Aorto-Esophageal Fistula Caused by Vascular Malformation: A Case Description and an Analysis of the Literature. J. Cardiovasc. Dev. Dis. 2025, 12, 262. https://doi.org/10.3390/jcdd12070262
Zhang W, Hu X, Yu J. Aorto-Esophageal Fistula Caused by Vascular Malformation: A Case Description and an Analysis of the Literature. Journal of Cardiovascular Development and Disease. 2025; 12(7):262. https://doi.org/10.3390/jcdd12070262
Chicago/Turabian StyleZhang, Wenzhao, Xu Hu, and Jianqun Yu. 2025. "Aorto-Esophageal Fistula Caused by Vascular Malformation: A Case Description and an Analysis of the Literature" Journal of Cardiovascular Development and Disease 12, no. 7: 262. https://doi.org/10.3390/jcdd12070262
APA StyleZhang, W., Hu, X., & Yu, J. (2025). Aorto-Esophageal Fistula Caused by Vascular Malformation: A Case Description and an Analysis of the Literature. Journal of Cardiovascular Development and Disease, 12(7), 262. https://doi.org/10.3390/jcdd12070262




