Abstract
Perioperative and long-term postoperative major adverse cardiovascular events (MACE) are a leading cause of morbidity and mortality in patients undergoing noncardiac surgery. In selected high-risk patients, when information about cardiovascular status may influence surgical decisions, preoperative risk stratification is reasonable, with stress imaging being the preferred method. Coronary computed angiography (CCTA) and coronary artery calcium score (CACS) offer direct anatomical assessment of atherosclerotic coronary arteries and help gauge the extent and severity of coronary artery disease. Strong evidence supports that CCTA and CACS, either alone or in combination, are reliable methods for assessing the risk of both perioperative and long-term postoperative MACE, often demonstrating equal or superior prognostic performance compared to traditional imaging tools. Moreover, integrating CCTA or CACS into standard preoperative imaging protocols further enhances perioperative risk prediction and improves the ability to accurately stratify patients. Future research is needed to better define the role of CCTA and CACS in preoperative cardiovascular risk evaluation of patients undergoing noncardiac surgery.
1. Introduction
Cardiovascular perioperative complications are common and are associated with increased 1-year postoperative mortality [1]. The incidence of postoperative major adverse cardiac events (MACE) varies widely between different examined cohorts, ranging from 1.8% to 25% [2,3,4]. Older patients with existing cardiovascular disease or comorbidities (diabetes mellitus, kidney dysfunction, etc.) are at higher risk for perioperative cardiovascular complications and cardiac death [5,6,7]. Importantly, poor functional status and reduced exercise capacity—defined as metabolic equivalents (MET) of less than 4—have been consistently linked with increased risk of poor postoperative outcomes [8]. This high-risk population should undergo preoperative risk evaluation to determine whether further strategies (guideline-directed medical therapy, revascularization, etc.) should be employed to reduce the risk and if the benefits of the surgery override the relevant risks. The ACC/AHA guidelines state that stress testing can help stratify risk and guide management in patients suspected of high-risk ischemia [8]. In a similar manner, the 2022 European Society of Cardiology (ESC) supports stress imaging in high-risk noncardiac surgery with poor functional capacity, high likelihood of coronary artery disease (CAD), high clinical risk according to the Revised Cardiac Risk Index (RCRI), or previous coronary revascularization [9].
Coronary computed tomography angiography (CCTA)—an important diagnostic tool for noninvasive anatomic evaluation of CAD—has been extensively investigated as an imaging modality for cardiovascular risk stratification. Both the Appropriate Use Criteria for Cardiac Computed Tomography (2010) [10] and the 2014 ACC/AHA guidelines [11] stated that there are no appropriate indications for using CCTA in preoperative evaluations for noncardiac surgery. However, in light of new evidence, the latest 2022 ESC guidelines and most recent 2024 ACC/AHA guidelines recognize the role of CCTA in assessing suspected CAD in select patients [8,9]. Indeed, numerous observational studies have demonstrated that CCTA as well as coronary artery calcium score (CACS) measurement are reliable options for perioperative risk stratification, yielding outcomes comparable to those of traditional imaging methods [12,13,14,15]. In this narrative review, we explore whether CCTA or CACS can effectively stratify the risk of patients prior to noncardiac surgery.
2. Perioperative Risk Stratification
2.1. CCTA
CCTA is recommended as the initial test for evaluation of patients with low-to-intermediate risk for CAD [16]. Thanks to its high negative predictive value (NPV), CCTA acts as a gatekeeper for invasive coronary angiography (ICA) [17].
In the surgical population, given that no existing evidence has demonstrated that preoperative revascularization reduces postoperative MACE and cardiac mortality [18], the 2024 ACC/AHA guidelines recommend against routine CCTA preoperative imaging and support the use of CCTA in select high-risk patients [8]. CCTA has shown comparable or even superior performance compared to tools like the RCRI and other imaging modalities, such as dobutamine stress echocardiography (DSE) or myocardial perfusion imaging (MPI), for accurately estimating cardiovascular risk and predicting postoperative MACE [19,20].
Previous studies have demonstrated a higher risk of MACE in patients diagnosed with obstructive CAD (stenosis > 50% or >70% on CCTA) [19,21,22,23]. Ahn et al. investigated 239 patients undergoing intermediate risk noncardiac surgery and reported a more than five-fold increase in postoperative events, including cardiac death, acute coronary syndrome, pulmonary edema, and arrythmias, in patients with significant (>50%) coronary stenosis on CCTA [21]. Similarly, Hwang et al. reported an increased risk of perioperative major cardiac events in patients with significant stenosis. There was an exponential risk with higher stenosis severity (OR: 6.1, 95% CI: 2.7–14 for stenosis > 50% vs. OR: 8.4, 95% CI: 3.6–19.6 for stenosis > 70%) [22].
Significant stenosis on CCTA has also been found to have an additive value on the prognostic performance of RCRI, a commonly used risk model that uses six different clinical risk predictors of perioperative cardiovascular events [24]. Hwang et al. found that the combination of segment involvement score > 3, Duke jeopardy score > 0 and RCRI resulted in a significantly increased area under the receiver operating characteristic curve (AUC) compared to RCRI alone for diagnosing perioperative cardiac events (AUC: 0.757 vs. AUC: 0.631, p = 0.003) [22]. Furthermore, CCTA findings can significantly reclassify the risk of perioperative major cardiac events in patients categorized as having RCRI risk levels 2 and 3. For those with significant CCTA findings, the odds of experiencing such events can increase 3- to 17-fold compared to patients with non-significant findings (p < 0.05). Based on these findings, the authors highlighted the usefulness of CCTA in further stratifying the risk of patients with an RCRI of 2 or 3 [22].
Li et al. investigated the impact of CCTA findings on surgery cancellation in a study involving 841 older patients (mean age 69.5 ± 5.8 years) with unknown or suspected CAD. Their findings revealed that the likelihood of canceling scheduled surgeries increased with the severity of coronary stenosis and the number of affected coronary arteries [23].
On the other hand, while CCTA possesses excellent ability to exclude nonsignificant CAD, false-positive findings due to calcium blooming artifacts and coronary lumen caliber underestimation are common [25]. Sheth et al. investigated the composite outcome of perioperative cardiovascular death and non-fatal myocardial infarction (MI) in patients with or at risk of atherosclerotic CAD undergoing noncardiac surgery [19]. The study found that combining CCTA findings with the RCRI, compared with RCRI alone, correctly reclassified as being at higher risk 17 out of 77 patients who experienced a perioperative event (p < 0.001). However, using CCTA also led to an overestimation of the risk of 98 out of 923 patients who did not experience a perioperative event. The last finding is of great importance since overestimation of the cardiovascular risk in patients scheduled for surgery imposes great risks, including redundant further cardiovascular evaluation and potentially detrimental surgery delay or even cancellation. Studies that have evaluated diagnostic and prognostic significance of preoperative CCTA are described in Table 1.

Table 1.
Studies evaluating the preoperative use of coronary computed tomography angiography.
2.2. Coronary Artery Calcium Score
CACS is a measure of atherosclerosis burden in the coronary arteries and acts as an indicator of CAD. Closely correlated with the severity of CAD, elevated CACS serves as an independent predictor for mortality and MACE in both symptomatic and asymptomatic patients [34,35].
CACS—alone or in combination with CCTA findings—has been also investigated as a method of preoperative risk stratification. The existing literature attests that an elevated CACS is associated with an increased risk of perioperative cardiovascular events. Studies that have evaluated diagnostic and prognostic significance of preoperative CACS are described in Table 2.

Table 2.
Studies evaluating the preoperative use of coronary artery calcium score.
In 2001, Mahla et al. examined the preoperative CACS, measured by electron beam computed tomography, in 51 patients undergoing elective vascular surgery. Six patients with elevated postoperative cardiac troponin T levels had a median CACS 2.5 times higher than the remaining 45 troponin-negative patients (2080 vs. 810; p = 0.021), suggesting a possible association between preoperative CACS and postoperative cardiac cell injury [42].
Choi et al. investigated 2554 patients having a non-gated chest CT performed within one year before surgery [37]. The authors employed an estimated coronary calcium burden (ECCB) score ranging from 0 to 9 with ECCB: 3–9 scores indicating severe disease (≥50% of the total artery length calcified) in one or two vessels. The authors found that higher coronary calcium burden was associated with a stepwise increase in the rate of postoperative major clinical events (MCE), defined as perioperative mortality and MI (ECCB 0: 2.9%, ECCB 1–2: 3.7%, ECCB 3–5: 8.0%; ECCB 6–9: 12.6%, p < 0.001). The odds for perioperative clinical events were twice as high in patients with ECCB > 3 compared to patients with ECCB < 3 (adjusted OR: 2.11, 95% CI: 1.42–3.12). Finally, the study demonstrated that implementing ECCB score in an RCRI model resulted in a significant improvement of the AUC for prediction of MCE (from 0.675 to 0.712, p = 0.018), with a net reclassification improvement of 0.428 (95% CI, 0.254–0.601, p < 0.0001). Of note, all-cause death rather than cardiac death was included in the composite primary outcome.
In another study of 4491 patients with lung cancer who underwent intermediate-risk surgery, the presence of coronary calcification (CACS ≥ 1) was associated with increased risk for perioperative cardiovascular events (OR: 1.75, 95% CI: 1.14–2.68) after adjustment for relevant variables [38]. Finally, in a meta-analysis of eleven studies including 3480 patients undergoing noncardiac surgery, a ten-fold increase in risk for perioperative MACE was seen in patients having severe coronary calcifications (CACS ≥ 1000 vs. CACS < 1000, OR:10.4, 95% CI 1.6–69.7) [43].
In terms of prognostic performance, a previously used CACS cut-off of 113 demonstrated an NPV of 97% for postoperative cardiac events in patients undergoing intermediate-risk noncardiac surgery, highlighting the strong ability of CACS to identify patients at low risk for perioperative events [21].
It remains unclear whether preoperative CACS measurement has different predictive value in men versus women. This is an important gap, as prior large cohorts have shown that women with CACS > 100 have significantly higher cardiovascular mortality compared to men [44]. No available studies in the existing literature have directly compared CAC burden and postoperative outcomes stratified by gender, but most available analyses were adjusted for sex using multivariable Cox models, which may partially account for these differences. Notably, women are more likely to present with ischemic equivalents (e.g., fatigue, dyspnea, etc.), making symptom-based evaluation challenging [45]. In such cases, CACS and CCTA may provide more reliable risk assessment and improve diagnostic accuracy.
There are insufficient data in the literature to determine whether CACS, CCTA, or a combination of both is superior for risk stratification of perioperative cardiovascular events. While CACS possesses several advantages over CCTA (easier to perform, no risk for contrast induced allergy/nephropathy, etc.), it cannot adequately evaluate the coronary anatomy and the presence and the extent of non-calcified stenoses. Future comparative studies are needed to address this question.
Example images of calcified and non-calcified coronary arteries are shown in Figure 1 and Figure 2, respectively.
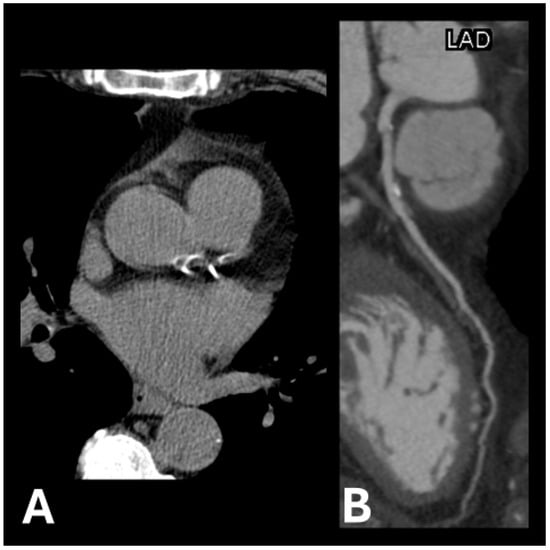
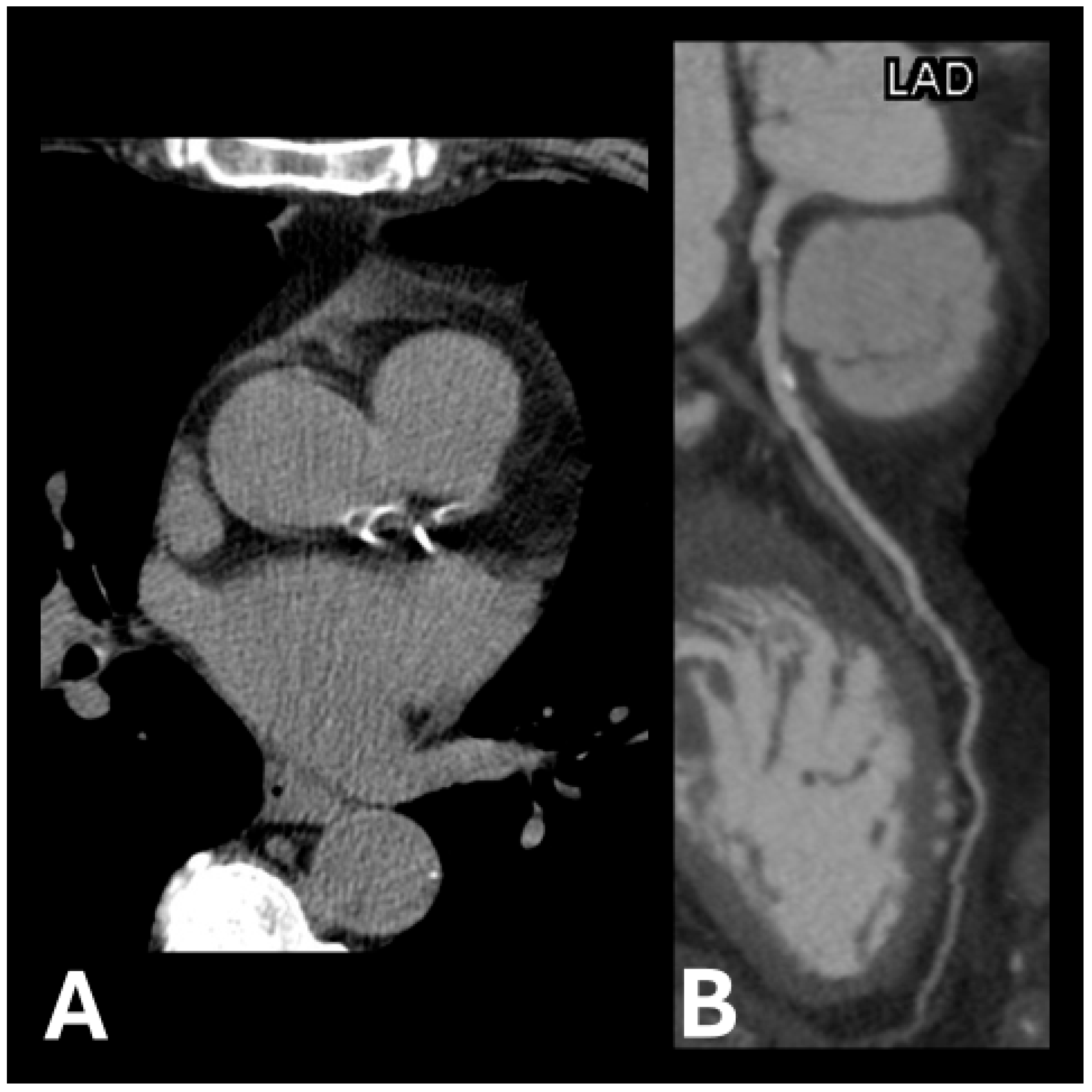
Figure 1.
Examples of calcification plaque(s) in (A) left anterior descending artery and left circumflex artery and (B) left anterior descending artery.
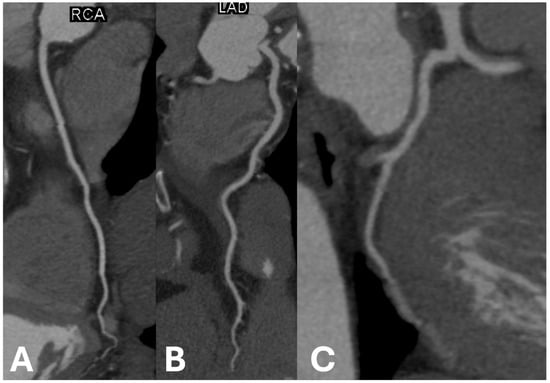
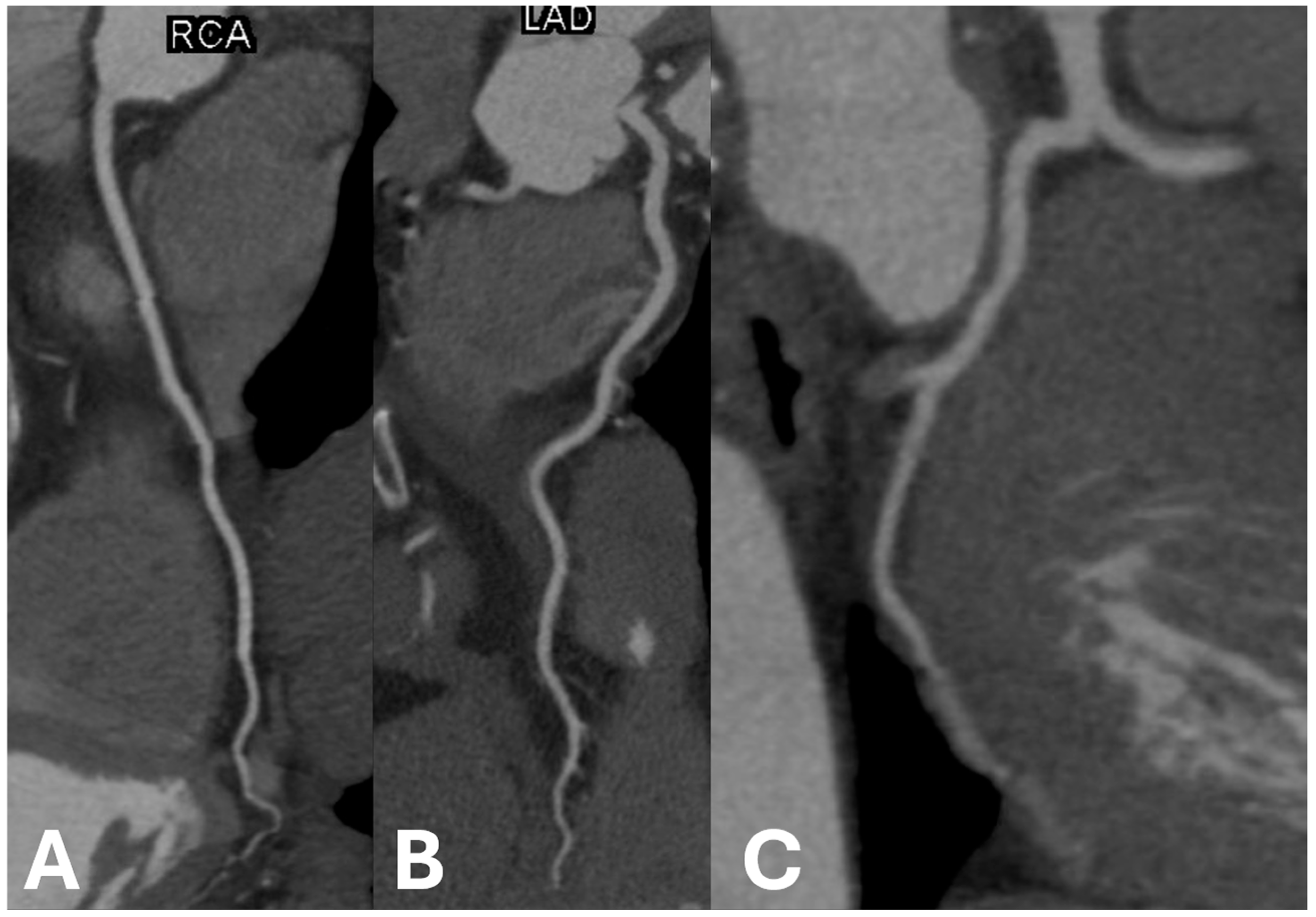
Figure 2.
Examples of non-calcified coronary arteries in (A) right coronary artery, (B) left anterior descending artery, and (C) left circumflex artery.
2.3. Fractional Flow Reserve–Computed Tomography (FFR-CT)
FFR-CT is a noninvasive diagnostic tool with great correlation and agreement with invasive FFR and better performance than CCTA alone in diagnosing CAD [46]. Ma et al. investigated the predictive value of machine-learning based FFR-CT in patients scheduled for lung cancer surgery [47]. FFR-CT ≤ 0.8 (compared to FFR-CT > 0.8) was associated with 10-fold increased odds for MACE in the perioperative period. Furthermore, when assessing the accuracy for prediction of MACE, FFR-CT outperformed CCTA findings (AUC: 0.737 vs. AUC: 0.524). Given its higher NPV for CAD, FFR-CT could potentially act as a gatekeeper for preoperative ICA when DSE or CCTA results are suspected to be false-positive.
3. Long-Term Outcomes
CCTA findings and CACS have been studied for risk stratification and prediction of long-term cardiovascular events, with coronary atherosclerosis burden emerging as a negative predictor. With a median follow-up of 4 years, Sampaio Rodrigues et al. reported a hazard ratio of 5.8 (95% CI 1.6–20.6) for MACE when significant CAD (stenosis > 50%) was identified preoperatively on CCTA [14]. Another study found a 12-fold increase (OR: 11.97, 95% CI: 5.79–24.75) in MACE for patients with severe coronary calcification (CACS > 400) or obstructive CAD in a follow-up period of 5 years [13]. Finally, when assessing the diagnostic value of CCTA, Cassagneau et al. found a 95% and 100% NPV of CCTA for MACE and coronary events (increase in creatine kinase or troponin, MI, postoperative coronary artery revascularization, etc.), respectively [30]. No significant differences were observed between the diagnostic performances of DSE and CCTA.
4. Revascularization Prior to Noncardiac Surgery
The usefulness of CCTA and CACS findings should be carefully considered given the lack of evidence supporting a strategy of routine revascularization before noncardiac surgery in stable CAD patients [48]. Two large trials (CARP and DECREASE-V) showed no benefit of prophylactic revascularization for short-term outcomes (death, MI, etc.) or long-term survival in vascular surgery patients [18,49]. Of note, left main CAD patients were excluded from both trials. A sub-analysis of the CARP trial, however, showed that revascularization in patients with anterior ischemia on stress imaging reduced long-term death and MI risk [50]. The 2024 ACC/AHA guidelines advise against routine revascularization before noncardiac surgery and recommend it only for traditional indications (e.g., unstable angina, left main disease, or high-risk anatomy) [8]. Perhaps, better stratification of preoperative cardiovascular risk, implementing clinical variables and imaging results, could help identify which patients might benefit from revascularization versus those who might be harmed by unnecessary procedures or delayed surgery.
5. Specific Types of Surgeries
Besides research on patients undergoing noncardiac surgery in general, there are also studies examining specific operation types. One population that has been particularly well studied is patients preparing for liver transplantation (LT) surgery. Patients with cirrhosis (especially metabolic-associated fatty liver disease-associated) are considered as high risk for CAD due to both traditional (obesity, metabolic syndrome, diabetes, and hypertension) and nontraditional risk factors (related to end-stage liver disease) [51]. LT recipients face a higher risk of cardiovascular death and ischemic events compared to the general population matched for age and sex [52]. For this reason and to select the most suitable candidates, LT patients undergo routine cardiac preoperative evaluation, commonly with stress electrocardiography, stress echocardiography, or MPI studies [53].
CCTA has been studied as an alternative imaging modality for preoperative evaluation of LT patients, with adequate ability of ruling out obstructive coronary stenosis [31,32]. More importantly, CACS has been shown to have great predictive ability of perioperative cardiovascular events. Kong et al. reported that patients with severe calcification (CACS > 400) had significantly increased odds for cardiovascular complications one month after LT (OR: 4.62, 95% CI: 1.14–18.72) [40]. Another study with 66 LT recipients undergoing both CACS measurement and ICA showed that a CACS of less than 400 (less than severe calcification) appeared to have a 100% NPV for obstructive CAD [36]. Consequently, the authors suggested there is no need for further CAD evaluation in patients with CACS < 400. A cutoff of 400 was also used by another study which reported a 100% NPV and estimated that 24% of their cohort could have avoided catheterization without missing any obstructive coronary disease [39].
Regarding vascular surgeries, patients undergoing intermediate-risk surgeries like carotid endarterectomy or endovascular aneurysm repair and high-risk surgeries like open aortic surgeries are usually risk-stratified based on traditional testing with echocardiography and MPI [29]. Prior studies evaluating the role of ICA and selective revascularization in patients undergoing vascular surgeries did not show significant benefit in outcomes [18,54]. However, compared to LT, fewer studies on the use of CACS and CCTA have been performed in this population. In a recent retrospective study of patients undergoing open peripheral artery bypass surgery, patients with known CAD (history of myocardial infarction or revascularization) and those with subclinical atherosclerosis determined by a positive CAC had similar postoperative troponin elevation and heart failure events [55]. Chang et al. evaluated the use of CCTA combined with stress perfusion CT in patients undergoing various vascular surgeries [29]. While CAD was common in these patients, there were only a few cardiovascular events overall, and perfusion defects did not predict perioperative cardiac events. Lastly, Vallier et al. showed that postoperative myocardial injury was better predicted by CCTA when compared to functional testing [56].
Studies on other specific surgery populations, such as bariatric surgery [27,33] and foot amputation in type 2 diabetics [12,28] have been also demonstrated sufficient CCTA and CACS performance in predicting postoperative outcomes.
6. CCTA and CACS in Relation to Other Imaging Modalities
6.1. Dobutamine Stress Echocardiography (DSE)
Data comparing CCTA with other imaging modalities for preoperative assessment were limited until the publication of the PANDA trial in 2020 [57]. In this prospective observational study, 215 patients with more than one clinical risk factor for perioperative cardiovascular events received both DSE and CCTA prior to noncardiac surgery. The authors employed different models for prediction of events, implementing RCRI scores, CCTA findings (≥50% stenosis and/or CACS ≥ 203), or DSE abnormal results. They found that combining CCTA findings with the RCRI score significantly enhanced risk stratification compared to the RCRI alone (p < 0.001), an improvement not observed with DSE. When DSE plus RCRI were compared head-to-head with significant stenosis on CCTA plus CACS > 203 plus RCRI, the latter model provided significantly better prognostic performance (AUC: 0.713 vs. AUC: 0.839, p = 0.027).
6.2. Single-Photon Emission Computed Tomography Myocardial Perfusion Imaging (SPECT MPI) and Stress Cardiovascular Magnetic Resonance Imaging (CMR)
Ghadri et al. assessed the added value of using CACS in combination with SPECT MPI. The study showed that using a cut-off of CACS ≥ 1314 improved the risk discrimination both when perfusion studies were normal and abnormal. Most importantly, CACS < 1314 combined with a normal perfusion study predicted lower risk for perioperative events than a normal perfusion study alone. However, this study did not directly compare the predictive value of CCTA with that of SPECT MPI [41]. Similarly, the VISION-CTA trial showed that the predictive accuracy of SPECT MPI for MACE improved significantly when CCTA results (stenosis ≥ 70%) were integrated into the risk evaluation [20]. Addition of pathological CCTA results to SPECT MPI led to an increase in NPV for MACE from 75% (CI 34.9–96.8) to 100% (CI 79.4–100). These findings highlight the potential additive value of incorporating CCTA and CACS into routine imaging methods.
CMR is another tool for evaluating myocardial ischemia and has demonstrated higher diagnostic sensitivity than SPECT in detecting angiographically significant CAD [58]. In a recent study of 669 patients undergoing MPI before intermediate- to high-risk noncardiac surgery, postoperative cardiac event rates were similar between patients who underwent stress CMR and those who had SPECT-MPI [59]. Due to the limited availability of supporting evidence, the 2024 ACC/AHA guidelines do not yet define a clear role for stress CMR in preoperative risk stratification [8]. Overall, there are insufficient data to conclude whether an anatomy-based approach (e.g., CCTA) or an ischemia-based approach (e.g., stress CMR or SPECT) provides superior prognostic value in the preoperative setting.
7. Conclusions
CCTA and CACS have consistently been shown to effectively predict the risk of both perioperative and long-term cardiovascular events. Future studies directly comparing CCTA with standard imaging methods are needed to clarify the optimal role of CCTA in preoperative cardiovascular evaluation for patients undergoing noncardiac surgery.
Author Contributions
Conceptualization, D.G.K.; methodology, I.K.; software, I.K.; validation, S.S.K., G.D.L. and D.S.; formal analysis, I.K.; investigation, I.K. and S.S.K.; resources, D.G.K.; data curation, S.S.K. and D.S.; writing—original draft preparation, I.K.; writing—review and editing, D.G.K. and D.S.; visualization, G.D.L.; supervision, D.G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Oh, A.R.; Park, J.; Lee, J.H.; Kim, H.; Yang, K.; Choi, J.H.; Ahn, J.; Sung, J.D.; Lee, S.H. Association Between Perioperative Adverse Cardiac Events and Mortality During One-Year Follow-Up After Noncardiac Surgery. J. Am. Heart Assoc. 2022, 11, e024325. [Google Scholar] [CrossRef] [PubMed]
- Lurati Buse, G.A.; Mauermann, E.; Ionescu, D.; Szczeklik, W.; De Hert, S.; Filipovic, M.; Beck-Schimmer, B.; Spadaro, S.; Matute, P.; Bolliger, D.; et al. Risk assessment for major adverse cardiovascular events after noncardiac surgery using self-reported functional capacity: International prospective cohort study. Br. J. Anaesth. 2023, 130, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Sazgary, L.; Puelacher, C.; Lurati Buse, G.; Glarner, N.; Lampart, A.; Bolliger, D.; Steiner, L.; Gurke, L.; Wolff, T.; Mujagic, E.; et al. Incidence of major adverse cardiac events following non-cardiac surgery. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, N.R.; Gupta, N.; Ramakrishna, H.; Guo, Y.; Berger, J.S.; Bangalore, S. Perioperative Major Adverse Cardiovascular and Cerebrovascular Events Associated with Noncardiac Surgery. JAMA Cardiol. 2017, 2, 181–187. [Google Scholar] [CrossRef]
- Davis, C.; Tait, G.; Carroll, J.; Wijeysundera, D.N.; Beattie, W.S. The Revised Cardiac Risk Index in the new millennium: A single-centre prospective cohort re-evaluation of the original variables in 9,519 consecutive elective surgical patients. Can. J. Anaesth. 2013, 60, 855–863. [Google Scholar] [CrossRef]
- Hallqvist, L.; Granath, F.; Bell, M. Myocardial infarction after noncardiac surgery in Sweden: A national, retrospective observational cohort study. Br. J. Anaesth. 2020, 125, 47–54. [Google Scholar] [CrossRef]
- Tashiro, T.; Pislaru, S.V.; Blustin, J.M.; Nkomo, V.T.; Abel, M.D.; Scott, C.G.; Pellikka, P.A. Perioperative risk of major non-cardiac surgery in patients with severe aortic stenosis: A reappraisal in contemporary practice. Eur. Heart J. 2014, 35, 2372–2381. [Google Scholar] [CrossRef]
- Thompson, A.; Fleischmann, K.E.; Smilowitz, N.R.; de Las Fuentes, L.; Mukherjee, D.; Aggarwal, N.R.; Ahmad, F.S.; Allen, R.B.; Altin, S.E.; Auerbach, A.; et al. 2024 AHA/ACC/ACS/ASNC/HRS/SCA/SCCT/SCMR/SVM Guideline for Perioperative Cardiovascular Management for Noncardiac Surgery: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 150, e351–e442. [Google Scholar] [CrossRef]
- Halvorsen, S.; Mehilli, J.; Cassese, S.; Hall, T.S.; Abdelhamid, M.; Barbato, E.; De Hert, S.; de Laval, I.; Geisler, T.; Hinterbuchner, L.; et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur. Heart J. 2022, 43, 3826–3924. [Google Scholar] [CrossRef]
- Taylor, A.J.; Cerqueira, M.; Hodgson, J.M.; Mark, D.; Min, J.; O’Gara, P.; Rubin, G.D. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 2010, 56, 1864–1894. [Google Scholar] [CrossRef]
- Fleisher, L.A.; Fleischmann, K.E.; Auerbach, A.D.; Barnason, S.A.; Beckman, J.A.; Bozkurt, B.; Davila-Roman, V.G.; Gerhard-Herman, M.D.; Holly, T.A.; Kane, G.C.; et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 130, 2215–2245. [Google Scholar] [CrossRef] [PubMed]
- Shalaeva, E.; Bano, A.; Kasimov, U.; Janabaev, B.; Baumgartner, I.; Laimer, M.; Saner, H. Coronary artery calcium score and coronary computed tomography angiography predict one-year mortality in patients with type 2 diabetes and peripheral artery disease undergoing partial foot amputation. Diab Vasc. Dis. Res. 2022, 19, 14791641221125190. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, Y.K.; Ha, T.Y.; Hwang, S.; Kim, W.; Koo, H.J.; Yang, D.H.; Kang, J.W.; Lee, S.G. Prognostic Value of Computed Tomographic Coronary Angiography for Long-Term Major Adverse Cardiac Events after Liver Transplantation. J. Clin. Med. 2021, 10, 3132. [Google Scholar] [CrossRef] [PubMed]
- Sampaio Rodrigues, T.; Koshy, A.N.; Gow, P.J.; Weinberg, L.; Cailes, B.; Testro, A.; Smith, G.; Lim, H.S.; Teh, A.W.; Lim, R.P.; et al. Atherosclerosis on CT coronary angiography and the risk of long-term cardiovascular events after liver transplantation. Liver Transpl. 2024, 30, 182–191. [Google Scholar] [CrossRef]
- Fathala, A. Coronary computed tomography angiography for risk stratification before noncardiac surgery. Ann. Card. Anaesth. 2016, 19, 31–37. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Doris, M.; Newby, D.E. Coronary CT Angiography as a Diagnostic and Prognostic Tool: Perspectives from the SCOT-HEART Trial. Curr. Cardiol. Rep. 2016, 18, 18. [Google Scholar] [CrossRef]
- McFalls, E.O.; Ward, H.B.; Moritz, T.E.; Goldman, S.; Krupski, W.C.; Littooy, F.; Pierpont, G.; Santilli, S.; Rapp, J.; Hattler, B.; et al. Coronary-artery revascularization before elective major vascular surgery. N. Engl. J. Med. 2004, 351, 2795–2804. [Google Scholar] [CrossRef]
- Sheth, T.; Chan, M.; Butler, C.; Chow, B.; Tandon, V.; Nagele, P.; Mitha, A.; Mrkobrada, M.; Szczeklik, W.; Faridah, Y.; et al. Prognostic capabilities of coronary computed tomographic angiography before non-cardiac surgery: Prospective cohort study. BMJ 2015, 350, h1907. [Google Scholar] [CrossRef]
- Dowsley, T.F.; Sheth, T.; Chow, B.J.W. Complementary pre-operative risk assessment using coronary computed tomography angiography and nuclear myocardial perfusion imaging in non-cardiac surgery: A VISION-CTA sub-study. J. Nucl. Cardiol. 2020, 27, 1331–1337. [Google Scholar] [CrossRef]
- Ahn, J.H.; Park, J.R.; Min, J.H.; Sohn, J.T.; Hwang, S.J.; Park, Y.; Koh, J.S.; Jeong, Y.H.; Kwak, C.H.; Hwang, J.Y. Risk stratification using computed tomography coronary angiography in patients undergoing intermediate-risk noncardiac surgery. J. Am. Coll. Cardiol. 2013, 61, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Kim, E.K.; Yang, J.H.; Chang, S.A.; Song, Y.B.; Hahn, J.Y.; Choi, S.H.; Gwon, H.C.; Lee, S.H.; Kim, S.M.; et al. Assessment of perioperative cardiac risk of patients undergoing noncardiac surgery using coronary computed tomographic angiography. Circ. Cardiovasc. Imaging 2015, 8, e002582. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Xu, Z.Z.; Wen, Z.P.; Pei, J.; Dai, W.; Wang, H.M.; Reng, J.; Zhou, P.; Xu, G.H. Usefulness of preoperative coronary computed tomography angiography in high risk non-cardiovascular surgery old patients with unknown or suspected coronary artery disease. BMC Cardiovasc. Disord. 2020, 20, 450. [Google Scholar] [CrossRef]
- Ford, M.K.; Beattie, W.S.; Wijeysundera, D.N. Systematic review: Prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann. Intern. Med. 2010, 152, 26–35. [Google Scholar] [CrossRef]
- Sun, Z.; Choo, G.H.; Ng, K.H. Coronary CT angiography: Current status and continuing challenges. Br. J. Radiol. 2012, 85, 495–510. [Google Scholar] [CrossRef]
- Walpot, J.; Massalha, S.; Jayasinghe, P.; Sadaf, M.; Clarkin, O.; Godkin, L.; Sharma, A.; Ratnayake, I.; Godkin, K.; Jia, K.; et al. Normalized Subendocardial Myocardial Attenuation on Coronary Computed Tomography Angiography Predicts Postoperative Adverse Cardiovascular Events: Coronary CTA VISION Substudy. Circ. Cardiovasc. Imaging 2022, 15, e012654. [Google Scholar] [CrossRef]
- Messerli, M.; Maywald, C.; Walti, S.; Warschkow, R.; Wildermuth, S.; Alkadhi, H.; Leschka, S.; Schiesser, M. Prognostic Value of Negative Coronary CT Angiography in Severely Obese Patients Prior to Bariatric Surgery: A Follow-Up After 6 Years. Obes. Surg. 2017, 27, 2044–2049. [Google Scholar] [CrossRef]
- Shalaeva, E.V.; Saner, H.; Janabaev, B.B.; Shalaeva, A.V. Coronary artery calcium score and coronary computed tomographic angiography for major perioperative cardiovascular complications in symptomatic diabetic patients undergoing trans-femoral amputation. Int. J. Cardiol. 2016, 221, 806–811. [Google Scholar] [CrossRef]
- Chang, S.A.; Kim, S.M.; Choi, S.H.; Choe, Y.H.; Kim, Y.W.; Kim, D.K. Clinical Utility of Coronary CT Angiography with Stress Perfusion CT in Preoperative Cardiac Risk Evaluation. Korean Circ. J. 2014, 44, 170–176. [Google Scholar] [CrossRef]
- Cassagneau, P.; Jacquier, A.; Giorgi, R.; Amabile, N.; Gaubert, J.Y.; Cohen, F.; Muller, C.; Jolibert, M.; Louis, G.; Varoquaux, A.; et al. Prognostic value of preoperative coronary computed tomography angiography in patients treated by orthotopic liver transplantation. Eur. J. Gastroenterol. Hepatol. 2012, 24, 558–562. [Google Scholar] [CrossRef]
- Chae, W.Y.; Hwang, S.; Yoon, Y.I.; Kang, M.C.; Moon, D.B.; Song, G.W.; Park, G.C.; Jung, D.H.; Namgoong, J.M.; Jung, S.W.; et al. Clinical value of preoperative coronary risk assessment by computed tomographic arteriography prior to adult living donor liver transplantation. Transplant. Proc. 2012, 44, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Jodocy, D.; Abbrederis, S.; Graziadei, I.W.; Vogel, W.; Pachinger, O.; Feuchtner, G.M.; Jaschke, W.; Friedrich, G. Coronary computer tomographic angiography for preoperative risk stratification in patients undergoing liver transplantation. Eur. J. Radiol. 2012, 81, 2260–2264. [Google Scholar] [CrossRef] [PubMed]
- Tognolini, A.; Arellano, C.S.; Marfori, W.; Sayre, J.W.; Hollada, J.L.; Goldin, J.G.; Dutson, E.P.; Ruehm, S.G. Cardiac dual-source CT for the preoperative assessment of patients undergoing bariatric surgery. Clin. Radiol. 2013, 68, e154–e163. [Google Scholar] [CrossRef] [PubMed]
- Coylewright, M.; Rice, K.; Budoff, M.J.; Blumenthal, R.S.; Greenland, P.; Kronmal, R.; Barr, R.G.; Burke, G.L.; Tracy, R.; Post, W.S. Differentiation of severe coronary artery calcification in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2011, 219, 616–622. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Yu, C.M.; Ji, Q.W.; Cai, M.; Zhao, Y.X.; Zhou, Y.J. Current understanding of coronary artery calcification. J. Geriatr. Cardiol. 2015, 12, 668–675. [Google Scholar] [CrossRef]
- Groen, R.A.; Barbero, F.L.; Fischer, S.E.; van Dijkman, P.R.M.; Bax, J.J.; Tushuizen, M.E.; Jukema, J.W.; Coenraad, M.J.; de Graaf, M.A. Coronary artery calcium assessment on non-gated chest CT to optimize pre-operative cardiac screening in liver transplantation. Int. J. Cardiol. 2024, 407, 132015. [Google Scholar] [CrossRef]
- Choi, D.Y.; Hayes, D.; Maidman, S.D.; Dhaduk, N.; Jacobs, J.E.; Shmukler, A.; Berger, J.S.; Cuff, G.; Rehe, D.; Lee, M.; et al. Existing Nongated CT Coronary Calcium Predicts Operative Risk in Patients Undergoing Noncardiac Surgeries (ENCORES). Circulation 2023, 148, 1154–1164. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Y.; Xie, M.; Wang, X.; Fang, W.; Luo, Q.; Zhou, Q.; Yao, F.; Yu, H.; Shen, L.; et al. Nongated Computed Tomography Predicts Perioperative Cardiovascular Risk in Lung Cancer Surgery. Ann. Thorac. Surg. 2022, 114, 2050–2057. [Google Scholar] [CrossRef]
- West, B.H.; Low, C.G.; Bista, B.B.; Yang, E.H.; Vorobiof, G.; Busuttil, R.W.; Budoff, M.J.; Elashoff, D.; Tobis, J.M.; Honda, H.M. Significance of Coronary Artery Calcium Found on Non-Electrocardiogram-Gated Computed Tomography During Preoperative Evaluation for Liver Transplant. Am. J. Cardiol. 2019, 124, 278–284. [Google Scholar] [CrossRef]
- Kong, Y.G.; Kang, J.W.; Kim, Y.K.; Seo, H.; Lim, T.H.; Hwang, S.; Hwang, G.S.; Lee, S.G. Preoperative coronary calcium score is predictive of early postoperative cardiovascular complications in liver transplant recipients. Br. J. Anaesth. 2015, 114, 437–443. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Fiechter, M.; Veraguth, K.; Gebhard, C.; Pazhenkottil, A.P.; Fuchs, T.A.; Templin, C.; Gaemperli, O.; Kaufmann, P.A. Coronary calcium score as an adjunct to nuclear myocardial perfusion imaging for risk stratification before noncardiac surgery. J. Nucl. Med. 2012, 53, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Mahla, E.; Vicenzi, M.N.; Schrottner, B.; Maier, R.; Tiesenhausen, K.; Watzinger, N.; Rienmuller, R.; Moser, R.L.; Metzler, H. Coronary artery plaque burden and perioperative cardiac risk. Anesthesiology 2001, 95, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Koshy, A.N.; Ha, F.J.; Gow, P.J.; Han, H.C.; Amirul-Islam, F.M.; Lim, H.S.; Teh, A.W.; Farouque, O. Computed tomographic coronary angiography in risk stratification prior to non-cardiac surgery: A systematic review and meta-analysis. Heart 2019, 105, 1335–1342. [Google Scholar] [CrossRef]
- Shaw, L.J.; Min, J.K.; Nasir, K.; Xie, J.X.; Berman, D.S.; Miedema, M.D.; Whelton, S.P.; Dardari, Z.A.; Rozanski, A.; Rumberger, J.; et al. Sex differences in calcified plaque and long-term cardiovascular mortality: Observations from the CAC Consortium. Eur. Heart J. 2018, 39, 3727–3735. [Google Scholar] [CrossRef]
- Angeli, F.; Ricci, F.; Moscucci, F.; Sciomer, S.; Bucciarelli, V.; Bianco, F.; Mattioli, A.V.; Pizzi, C.; Gallina, S. Sex- and gender-related disparities in chest pain syndromes: The feminine mystique of chest pain. Curr. Probl. Cardiol. 2024, 49, 102457. [Google Scholar] [CrossRef]
- Rajiah, P.; Cummings, K.W.; Williamson, E.; Young, P.M. CT Fractional Flow Reserve: A Practical Guide to Application, Interpretation, and Problem Solving. Radiographics 2022, 42, 340–358. [Google Scholar] [CrossRef]
- Ma, Z.; Dong, S.; Ou, S.; Ma, X.; Liu, L.; An, Z.; Xu, F.; Zhang, D.; Tu, C.; Song, X.; et al. The predictive value of coronary computed tomography angiography-derived fractional flow reserve for perioperative cardiac events in lung cancer surgery. Eur. J. Radiol. 2024, 180, 111688. [Google Scholar] [CrossRef]
- Garcia, S.; McFalls, E.O. Need for elective PCI prior to noncardiac surgery: High risk through the eyes of the beholder. J. Am. Heart Assoc. 2014, 3, e001068. [Google Scholar] [CrossRef]
- Poldermans, D.; Schouten, O.; Vidakovic, R.; Bax, J.J.; Thomson, I.R.; Hoeks, S.E.; Feringa, H.H.; Dunkelgrun, M.; de Jaegere, P.; Maat, A.; et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: The DECREASE-V Pilot Study. J. Am. Coll. Cardiol. 2007, 49, 1763–1769. [Google Scholar] [CrossRef]
- Garcia, S.; Rider, J.E.; Moritz, T.E.; Pierpont, G.; Goldman, S.; Larsen, G.C.; Shunk, K.; Littooy, F.; Santilli, S.; Rapp, J.; et al. Preoperative coronary artery revascularization and long-term outcomes following abdominal aortic vascular surgery in patients with abnormal myocardial perfusion scans: A subgroup analysis of the coronary artery revascularization prophylaxis trial. Catheter. Cardiovasc. Interv. 2011, 77, 134–141. [Google Scholar] [CrossRef]
- Therapondos, G.; Flapan, A.D.; Plevris, J.N.; Hayes, P.C. Cardiac morbidity and mortality related to orthotopic liver transplantation. Liver Transpl. 2004, 10, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.D.; Morris, J.K.; Cramb, R.; Gunson, B.K.; Neuberger, J. Cardiovascular morbidity and mortality after orthotopic liver transplantation. Transplantation 2002, 73, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; DiMartini, A.; Feng, S.; Brown, R., Jr.; Fallon, M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014, 59, 1144–1165. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.J.; Owens, D.K.; Harris, R.A.; Cooke, J.P.; Hlatky, M.A. The role of coronary angiography and coronary revascularization before noncardiac vascular surgery. JAMA 1995, 273, 1919–1925. [Google Scholar] [CrossRef]
- Pritchard, A.; Brunton, N.; Sharma, S.; Young, M.N.; Henkin, S. Subclinical coronary artery disease and perioperative cardiac events in patients undergoing peripheral artery bypass surgery. Vasc. Med. 2024, 29, 720–722. [Google Scholar] [CrossRef]
- Vaillier, A.; Deharo, P.; Cuisset, T.; Piquet, P.; Barral, P.A.; Jacquier, A.; Bonnet, G.; Bonnet, J.L. Impact of coronary computed tomography angiography (CCTA) in risk assessment of peri-operative myocardial ischemia for patients undergoing vascular surgery. Arch. Cardiovasc. Dis. Suppl. 2020, 12, 9. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jeong, Y.H.; Park, Y.; Kwak, C.H.; Jang, J.Y.; Hwang, J.Y.; Hwang, S.J.; Koh, J.S.; Kim, K.H.; Kang, M.G.; et al. Head-to-head comparison of prognostic accuracy in patients undergoing noncardiac surgery of dobutamine stress echocardiography versus computed tomography coronary angiography (PANDA trial): A prospective observational study. J. Cardiovasc. Comput. Tomogr. 2020, 14, 471–477. [Google Scholar] [CrossRef]
- Patel, A.R.; Salerno, M.; Kwong, R.Y.; Singh, A.; Heydari, B.; Kramer, C.M. Stress Cardiac Magnetic Resonance Myocardial Perfusion Imaging: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 78, 1655–1668. [Google Scholar] [CrossRef]
- Fazzari, F.; Lisi, C.; Catapano, F.; Cannata, F.; Brilli, F.; Figliozzi, S.; Bragato, R.M.; Stefanini, G.G.; Monti, L.; Francone, M. Prognostic value of stress CMR and SPECT-MPI in patients undergoing intermediate-to-high-risk non-cardiac surgery. Radiol. Med. 2024, 129, 1485–1498. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).