Divergent Cardiac Adaptations in Endurance Sport: Atrial Fibrillation Markers in Marathon Versus Ultramarathon Athletes
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
- “atrial fibrillation” AND (“marathon runners” OR “ultramarathon runners” OR “endurance athletes”) AND (“left atrial remodeling” OR “right atrial strain” OR “cardiac fibrosis” OR “arrhythmia incidence” OR “biomarkers”)
2.2. Eligibility Criteria
- Involved adult participants (≥18 years old).
- Included populations of marathon (≥42.2 km) or ultramarathon (>42.2 km) runners
- Reported at least one marker associated with AF, including but not limited to atrial size, myocardial strain, P-wave duration, inflammatory or fibrotic biomarkers, or arrhythmia outcomes
- Employed a comparative or observational study design (cross-sectional, case–control, prospective, or retrospective cohort);
- Focused on chronic cardiac adaptations, excluding acute responses immediately after isolated exercise bouts
- Included either a non-running control group or compared different endurance distances
- Published in English-language peer-reviewed journals.
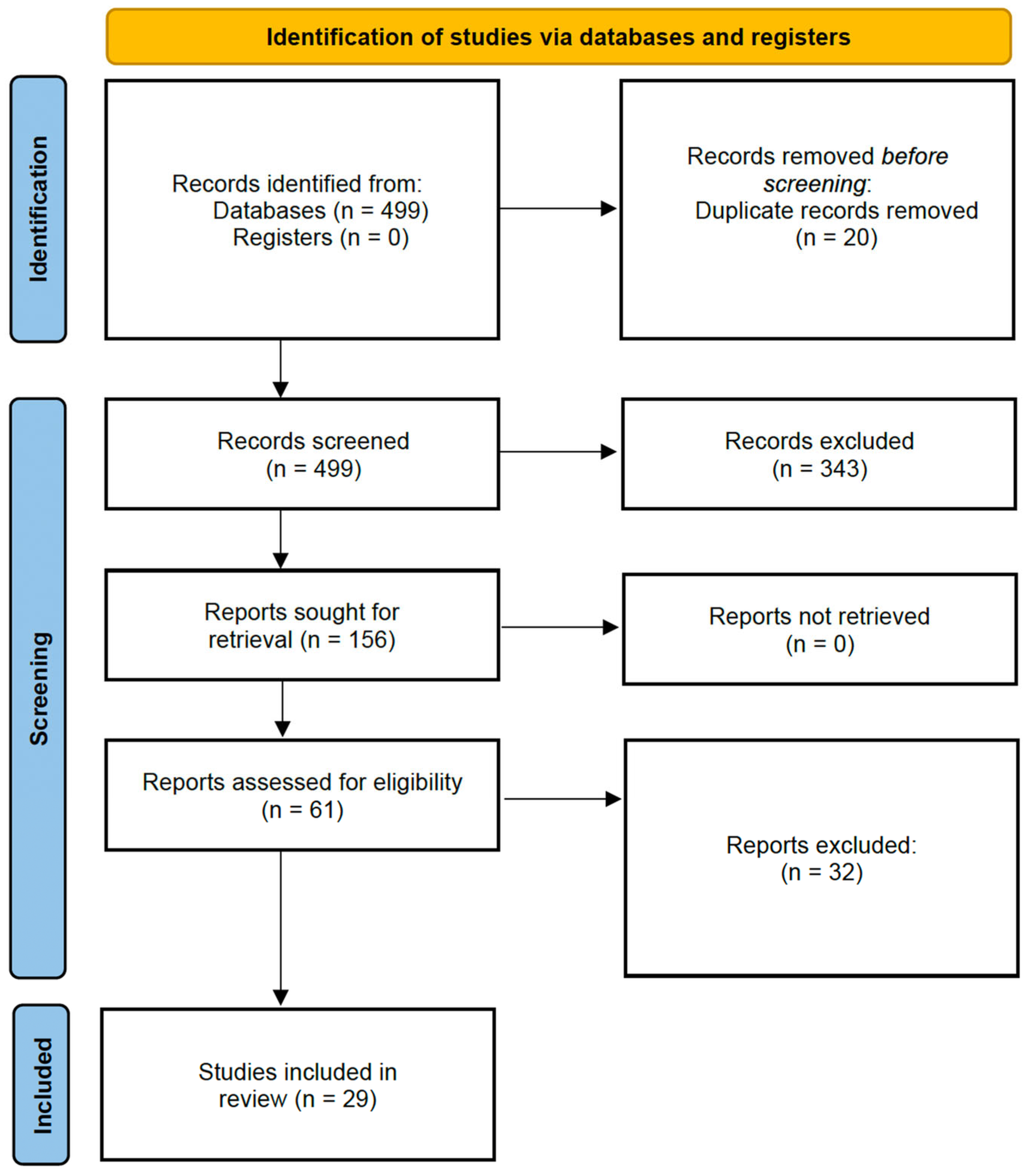
2.3. Study Screening and Selection
2.4. Data Extraction
- Study design (e.g., cross-sectional, prospective cohort).
- Sample characteristics (total number, gender distribution, age, training background, event type).
- AF markers: Structural (e.g., LA/RA size, strain), functional (e.g., ECG features), and biochemical (e.g., sVCAM-1, hs-CRP, Galectin-3).
- AF incidence or prevalence, when reported, including hazard ratios (HRs), confidence intervals (CIs), and statistical significance values.
- Measurement techniques (e.g., echocardiography, cardiac MRI, biomarker assay methods).
- Follow-up duration for longitudinal studies.
2.5. Data Synthesis
2.6. Risk of Bias and Quality Assessment
- Selection (maximum 4 points): including the representativeness of the sample and ascertainment of exposure.
- Comparability (maximum 2 points): based on the control of potential confounders such as age, sex, or training level.
- Outcome/Exposure (maximum 3 points): assessing the method and accuracy of outcome ascertainment, duration of follow-up, and reporting transparency.
3. Results
3.1. Risk of Bias and Methodological Quality
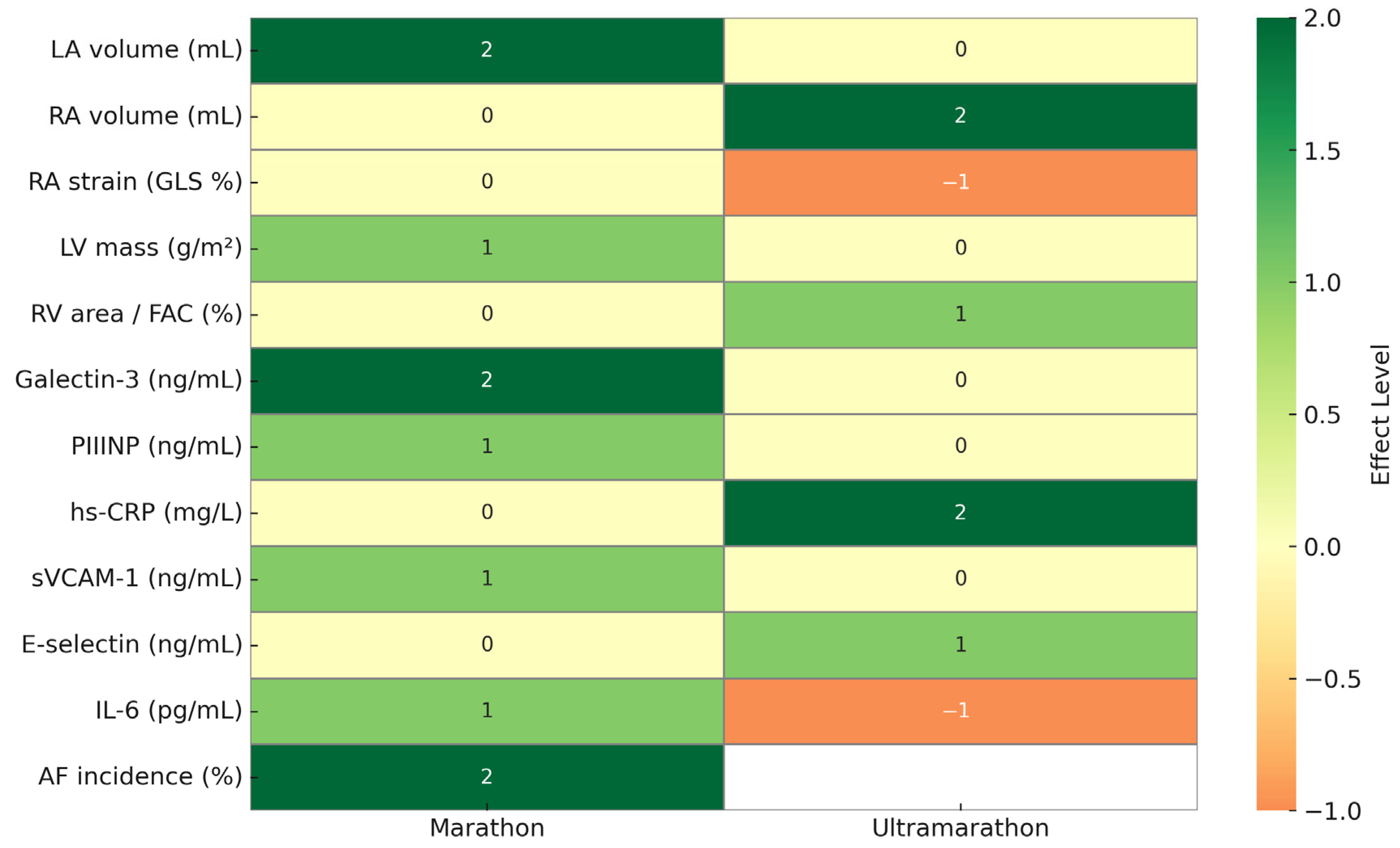
3.2. Cardiac Structural Adaptations
3.2.1. Atrial Remodeling
3.2.2. Ventricular Remodeling
| Category | Sample Size | LV Change | RV Change | Clinical Relevance |
|---|---|---|---|---|
| Marathon runners | ~100–160 [1,5] | ↑ LVEDV and LV mass (p < 0.05) [5] | No acute dilation or dysfunction [1] | Adaptive remodeling without dysfunction |
| Ultramarathon runners | ~60–80 [7,17] | Transient ↓ LV EF post-race (p < 0.01) [17] | ↑ RVEDV; ↓ RV FAC and strain (p < 0.01) [7] | RV strain and temporary systolic impairment |
| Mixed endurance cohorts | 100–200 [11,12] | ↑ LV mass indexed to BSA [12] | Not consistently reported | Remodeling may affect atrial stretch/load |
3.3. Functional Markers and Arrhythmia Incidence
3.4. Biomarker Profiles
3.4.1. Endothelial Function
3.4.2. Inflammatory Markers
3.5. Long-Term Cardiovascular Implications
3.6. Risk Stratification
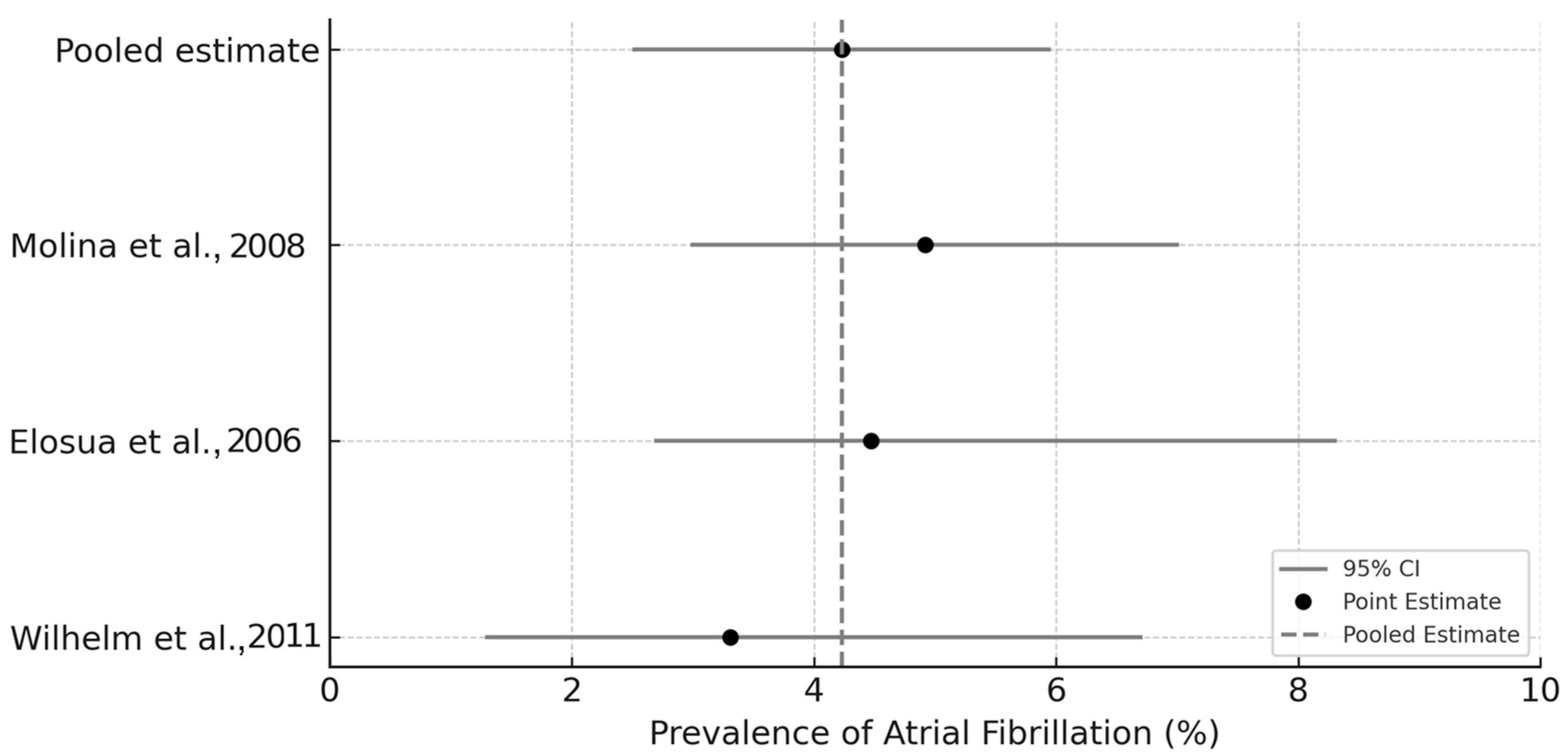
3.7. Exploratory Meta-Analysis of Atrial Fibrillation Prevalence
4. Discussion
4.1. Structural Remodeling and Modality-Specific Adaptation
4.2. Electrophysiological Risk and Arrhythmia Incidence
4.3. Molecular and Inflammatory Pathways
4.4. Risk Stratification and Clinical Implications
4.5. Supporting Observations from Supplementary Studies
4.6. Limitations and Future Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Molina, L.; Mont, L.; Marrugat, J.; Berruezo, A.; Brugada, J.; Bruguera, J.; Rebato, C.; Elosúa, R. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: A follow-up study. Europace 2008, 10, 618–623. [Google Scholar] [CrossRef]
- Wilhelm, M.; Roten, L.; Tanner, H.; Wilhelm, I.; Schmid, J.; Saner, H. Gender differences of atrial and ventricular remodeling and autonomic tone in nonelite athletes. Am. J. Cardiol. 2011, 108, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Nuoffer, J.; Schmid, J.; Wilhelm, I.; Saner, H. Comparison of pro-atrial natriuretic peptide and atrial remodeling in marathon versus non-marathon runners. Am. J. Cardiol. 2012, 109, 1498–1502. [Google Scholar] [CrossRef]
- Fernández, J.A.; Ocaranza, M.P.; Herrera, S.; Vega, J.; Salinas, M.; Contreras-Briceño, F.; Llevaneras, S.; Jalil, J.E.; Yañez, F.; Godoy, P.; et al. Biomarcadores de fibrosis y función ventricular derecha en maratonistas con distinto grado de entrenamiento: Estudio en la maratón de Santiago. Rev. Chil. Cardiol. 2019, 38, 15–23. [Google Scholar] [CrossRef]
- Gabrielli, L.; García, L.; Fernández, R.; Vega, J.; Ocaranza, M.; Contreras, F.; Salinas, M.; Chiong, M.; Jalil, J.; Munoz, M.; et al. Increased circulating levels of VCAM-1 correlate with left atrial remodeling in highly trained athletes. Eur. Heart J. 2019, 40 (Suppl. S1), ehz745.0823. [Google Scholar] [CrossRef]
- Contreras-Briceño, F.; Herrera, S.; Vega-Adauy, J.; Salinas, M.; Ocaranza, M.; Jalil, J.; Mandiola, J.R.; García, L.; Chiong, M.; Castro, P.F.; et al. Circulating vascular cell adhesion molecule-1 (sVCAM-1) is associated with left atrial remodeling in long-distance runners. Front. Cardiovasc. Med. 2021, 8, 737285. [Google Scholar] [CrossRef] [PubMed]
- Ujka, K.; Bruno, R.M.; Bastiani, L.; Tonacci, A.; D’Angelo, G.; Mrakic-Sposta, S.; Vezzoli, A.; Giardini, G.; Pratali, L. Enhanced right-chamber remodeling in endurance ultra-trail athletes compared to marathon runners detected by standard and speckle-tracking echocardiography. Front. Physiol. 2017, 8, 527. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2014; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 January 2025).
- Wilhelm, M.; Roten, L.; Tanner, H.; Schmid, J.; Wilhelm, I.; Saner, H. Long-term cardiac remodeling and arrhythmias in nonelite marathon runners. Am. J. Cardiol. 2012, 109, 608–613. [Google Scholar] [CrossRef]
- Clauss, S.; Wakili, R.; Hildebrand, B.; Kääb, S.; Hoster, E.; Klier, I.; Martens, E.; Hanley, A.; Hanssen, H.; Halle, M.; et al. MicroRNAs as biomarkers for acute atrial remodeling in marathon runners (The miRathon Study). PLoS ONE 2016, 11, e0148599. [Google Scholar] [CrossRef]
- Cipriani, A.; Vio, R.; Mastella, G.; Ciarmatori, N.; Del Monte, A.; Trovato, D.; Iliceto, S.; Schiavon, M.; Bertaglia, E.; Corrado, D.; et al. Burden of premature atrial beats in middle-aged endurance athletes with and without lone atrial fibrillation versus sedentary controls. Eur. J. Prev. Cardiol. 2020, 27, 379–386. [Google Scholar] [CrossRef]
- Li, Y.-S.; Ao, Y.-W.; Zhu, D.; Zhang, L.; Yang, R.; Zhao, Y.-L.; Zha, Y.-F. Reduced myocardial strain of interventricular septum among male amateur marathon runners: A cardiac magnetic resonance study. J. Sci. Med. Sport 2023, 26, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Picco, J.; Wolff, S.; González Dávila, E.; Wolff, D. Sex differences between pre- and post-effort ultramarathon athletes. Rev. Argent. Cardiol. 2021, 89, 12–17. [Google Scholar] [CrossRef]
- Shin, K.; Park, Y.; Kim, Y.-J. Effects of super-ultramarathon races (622 km) on cardiac biomarkers and markers of muscle damage. Korean J. Sports Med. 2018, 36, 135–143. [Google Scholar] [CrossRef]
- Breuckmann, F.; Möhlenkamp, S.; Nassenstein, K.; Lehmann, N.; Ladd, S.; Schmermund, A.; Sievers, B.; Chlosser, T.; Jöckel, K.-H.; Heusch, G.; et al. Myocardial late gadolinium enhancement: Prevalence, pattern, and prognostic relevance in marathon runners. Radiology 2009, 251, 50–57. [Google Scholar] [CrossRef]
- Trivax, J.E.; Franklin, B.A.; Goldstein, J.A.; Chinnaiyan, K.M.; Gallagher, M.J.; de Jong, A.T.; Colar, J.M.; Haines, D.E.; McCullough, P.A. Acute cardiac effects of marathon running. J. Appl. Physiol. 2010, 108, 1148–1153. [Google Scholar] [CrossRef]
- Drca, N.; Larsson, S.; Jensen-Urstad, M. Endurance training increases the risk of atrial fibrillation in women: A matched-cohort study. EP Europace 2022, 24 (Suppl. S1), euac053.139. [Google Scholar] [CrossRef]
- Drca, N.; Larsson, S.; Grannas, D.; Jensen-Urstad, M. Elite female endurance athletes are at increased risk of atrial fibrillation compared to the general population: A matched cohort study. Br. J. Sports Med. 2023, 57, 129–134. [Google Scholar] [CrossRef]
- Haeusler, K.G.; Herm, J.; Kunze, C.; Krüll, M.; Brechtel, L.; Lock, J.; Hohenhaus, M.; Heuschmann, P.U.; Fiebach, J.B.; Haverkamp, W.; et al. Rate of cardiac arrhythmias and silent brain lesions in experienced marathon runners: Rationale, design and baseline data of the Berlin Beat of Running study. BMC Cardiovasc. Disord. 2012, 12, 69. [Google Scholar] [CrossRef]
- Jee, H.; Park, J.; Oh, J.-G.; Lee, Y.; Shin, K.; Kim, Y.-J. Effect of a prolonged endurance marathon on vascular endothelial and inflammation markers in runners with exercise-induced hypertension. Am. J. Phys. Med. Rehabil. 2013, 92, 628–637. [Google Scholar] [CrossRef]
- Fernández, B.O.; Garrido, V. Evolution of Galectin-3 and ST2 cardiac fibrosis biomarkers during endurance running events. Clin. Chem. Lab. Med. 2019, 57, 1875–1882. [Google Scholar] [CrossRef]
- Yalcin, M.U.; Gurses, K.M.; Kocyigit, D.; Canpinar, H.; Canpolat, U.; Evranos, B.; Yorgun, H. The association of serum galectin-3 levels with atrial electrical and structural remodeling. J. Cardiovasc. Electrophysiol. 2015, 26, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Ragab, H.; Lund, G.; Breitsprecher, L.; Sinn, M.; Muellerleile, K.; Cavus, E.; Stehning, C.; Tahir, E.; Blankenberg, S.; Patten, M.; et al. Prevalence and pattern of focal and potential diffuse myocardial fibrosis in male and female marathon runners using contrast-enhanced cardiac magnetic resonance. Eur. Radiol. 2023, 33, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Ujka, K.; Bastiani, L.; D’Angelo, G.; Catuzzo, B.; Tonacci, A.; Mrakic-Sposta, S.; Vezzoli, A.; Giardini, G.; Pratali, L. Right cardiac chambers remodeling in marathon and ultra-trail athletes detected by speckle-tracking echocardiography. Eur. Heart J.—Cardiovasc. Imaging 2016, 17 (Suppl. S2), ii45–ii48. [Google Scholar] [CrossRef]
- Konwerski, M.; Postuła, M.; Barczuk-Falęcka, M.; Czajkowska, A.; Mróz, A.; Witek, K.; Bakalarski, W.; Gąsecka, A.; Małek, Ł.A.; Mazurek, T. Epicardial adipose tissue and cardiovascular risk assessment in ultra-marathon runners: A pilot study. Int. J. Environ. Res. Public Health 2021, 18, 3136. [Google Scholar] [CrossRef]
- Elosua, R.; Arquer, A.; Mont, L.; Sambola, A.; Molina, L.; García-Morán, E.; Brugada, J.; Marrugat, J. Sport practice and the risk of lone atrial fibrillation: A case–control study. Int. J. Cardiol. 2006, 108, 332–337. [Google Scholar] [CrossRef]
- Doni, F.; Ammadeo, P.; Doni, A.; Ventura, V. The effects of different training schedules on cardiac size and function and on arrhythmic heartbeat in amateur marathon runners. Med. Dello Sport 2022, 75, 463–471. [Google Scholar] [CrossRef]
- Louis-Georges, G.; Ndongo, J.M.; Richard, G.W.; Ndemba, A.; Brice, P.; Ernest, T.; Claude, B. Electrocardiographic patterns in mountain race athletes. Eur. J. Phys. Educ. Sport Sci. 2019, 5, 30–45. [Google Scholar]
- Madalosso, M.; Raviele, A. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: A follow-up study. J. Atr. Fibrillation 2008, 1, 51. [Google Scholar] [CrossRef][Green Version]
- Madalosso, M.; Raviele, A. Review of “Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: A follow-up study”. J. Atr. Fibrillation 2008, 1, 416. [Google Scholar] [CrossRef][Green Version]
- Maqueda, M.; Roca, E.; Brotons, D.; Soria, J.; Perera-Lluna, A. Training-induced gene expression plasticity in cardiac function and neural regulation for ultra-trail runners. Int. Conf. Comput. Cardiol. 2015, 42, 1057–1060. [Google Scholar] [CrossRef]
- Martínez-Mas, M.L.; Chorro, F.J.; Sanchis, J.; Asensi, J.; Losada, J.A.; Ruiz, R.; López-Merino, V. Doppler assessment of left ventricular diastolic function in marathon runners. J. Sports Med. Phys. Fit. 1994, 34, 147–151. [Google Scholar]
- Möckel, M.; Störk, T.; Eichstädt, H.; Müller, R.; Bortfeldt, R.; Hochrein, H. Effects of endurance training on left ventricular filling dynamics: A study using stress Doppler echocardiography. Z. Für Kardiol. 1992, 81, 399–405. [Google Scholar]
- Störk, T.; Möckel, M.; Müller, R.; Eichstädt, H.; Hochrein, H. Left ventricular filling behaviour in ultra-endurance and amateur athletes: A stress Doppler-echo study. Int. J. Sports Med. 1992, 13, 123–127. [Google Scholar] [CrossRef]
| Category | Sample Size | LA Volume Change | RA Volume Change | Clinical Relevance |
|---|---|---|---|---|
| Marathon runners | ~121–200 [3,5] | ↑ 42 ± 8 vs. 25 ± 9 mL/m2 (p = 0.001) [3]; ↑ 56 ± 13 vs. 49 ± 10 mL (p = 0.001) [5] | Not reported/↑ with cumulative exposure [3] | LA enlargement associated with AF risk [10] |
| Ultramarathon runners | n = 45 [7] | Not reported | ↑ RA volume; ↓ RA GLS (p < 0.05) [7] | RA remodeling may promote arrhythmogenesis [7] |
| Mixed endurance cohorts | ~134–200 [11,12] | ↑ LA diameter/volume associated with AF (p < 0.05) [11,12] | Not reported | LA metrics predictive of AF incidence [12] |
| Study | Sample Size | AF Estimate | Key Arrhythmic Finding | Interpretation |
|---|---|---|---|---|
| Drca et al. [18,19] | 12,045 athletes | 4.4% incidence; HR 3.63–3.67 | AF risk in elite female endurance athletes | Significantly higher risk compared to matched general population |
| Molina et al. [1] | 1006 marathon runners | 0.43/100 PY; HR 8.80 (95% CI: 1.26–61.29) | Long-term training associated with increased AF | Cumulative exposure raises lone AF incidence |
| Wilhelm et al. [2] | 121 non-elite male runners | 3.3% incidence; p = 0.042 | AF in males; none observed in females | Sex-specific vulnerability and atrial remodeling |
| Cipriani et al. [12] | 130 athletes/130 controls | 17% PABs vs. 22% in controls; p = 0.61 | No significant difference in ectopy prevalence | AF surrogate markers not elevated in athletes |
| Haeusler et al. [20] | Survey-based (estimated n > 500) | Estimated prevalence: 5.1–9.8% | Elevated self-reported AF prevalence | Suggests underdiagnosed burden in marathon runners |
| Biomarker | Marathon Runners | Ultramarathon Runners | Interpretation |
|---|---|---|---|
| sVCAM-1 | ↑ post-marathon (651 ± 350 → 905 ± 373 ng/mL, p = 0.002) [6] | Not reported | Associated with LA volume and atrial remodeling |
| VCAM-1 | Correlated with LA volume (rho = 0.483, p = 0.007) [5] | Not reported | Potential biomarker for AF risk in trained athletes |
| Soluble E-selectin | Not reported | ↑ in EIH runners [21] | Marker of endothelial activation during ultra-endurance exposure |
| Biomarker | Marathon Runners | Ultramarathon Runners | Interpretation |
|---|---|---|---|
| Galectin-3 | ↑ post-marathon (6.8 ± 2.2 → 19.7 ± 4.9 ng/mL, p = 0.012) [22] | Not reported | Marker of myocardial fibrosis linked to LA remodeling |
| PIIINP | ↑ in less-trained runners (61 ± 16 → 94 ± 24 ng/mL, p = 0.01) [22] | Not reported | Reflects collagen turnover and fibrotic signaling |
| IL-6 | Not reported | ↓ elevated IL-6 levels vs. controls (17% vs. 56%, p < 0.05) [21] | Possible anti-inflammatory adaptation in ultrarunners |
| hs-CRP | Not reported | ↑ throughout race and at day 3 post-race [15] | Indicator of systemic inflammation and physiological stress |
| Study | Marathon Runners | Ultramarathon Runners | Clinical Implications |
|---|---|---|---|
| Wilhelm et al. [3] | Progressive LA and RA enlargement based on race volume | Not reported | Suggests cumulative remodeling from repetitive endurance exposure |
| Wilhelm et al. [10] | ↑ pro-ANP levels correlated with atrial size | Not reported | Indicates chronic atrial wall stress with endurance training |
| Ragab et al. [24] | Higher LGE prevalence in male marathoners | Not reported | Suggests subclinical myocardial fibrosis and potential long-term arrhythmic substrate |
| Drca et al. [19] | HR for AF = 3.63 (95% CI: 1.56–8.41) in female runners | Not reported | Long-term AF risk elevated in female endurance athletes |
| Ujka et al. [7] | Not reported | Greater RA remodeling than in marathon runners | Suggests endurance-specific remodeling of the right atrium under ultra-endurance conditions |
| Study | Marathon Runners | Ultramarathon Runners | Interpretation |
|---|---|---|---|
| Molina et al. [1] | LA size positively associated with AF incidence | Not reported | Structural markers such as LA volume are predictive of arrhythmia |
| Wilhelm et al. [11] | Prolonged P-wave duration; increased sympatho-vagal tone; ↑ LA volume | Not reported | Multiple interacting factors influence AF risk |
| Drca et al. [18] | Training volume correlates with increased AF risk | Not reported | Highlights cumulative burden of endurance exercise |
| Clauss et al. [11] | MicroRNA expression associated with LA diameter in elite runners | Not reported | Potential role for epigenetic markers in individualized AF screening |
| Konwerski et al. [26] | Not reported | Lower epicardial adipose tissue linked to favorable CV profile | May suggest cardioprotective adaptation in ultra-endurance runners |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waśkiewicz, Z.; Bezuglov, E.; Talibov, O.; Gajda, R.; Mukhambetov, Z.; Azerbaev, D.; Bondarev, S. Divergent Cardiac Adaptations in Endurance Sport: Atrial Fibrillation Markers in Marathon Versus Ultramarathon Athletes. J. Cardiovasc. Dev. Dis. 2025, 12, 260. https://doi.org/10.3390/jcdd12070260
Waśkiewicz Z, Bezuglov E, Talibov O, Gajda R, Mukhambetov Z, Azerbaev D, Bondarev S. Divergent Cardiac Adaptations in Endurance Sport: Atrial Fibrillation Markers in Marathon Versus Ultramarathon Athletes. Journal of Cardiovascular Development and Disease. 2025; 12(7):260. https://doi.org/10.3390/jcdd12070260
Chicago/Turabian StyleWaśkiewicz, Zbigniew, Eduard Bezuglov, Oleg Talibov, Robert Gajda, Zhassyn Mukhambetov, Daulet Azerbaev, and Sergei Bondarev. 2025. "Divergent Cardiac Adaptations in Endurance Sport: Atrial Fibrillation Markers in Marathon Versus Ultramarathon Athletes" Journal of Cardiovascular Development and Disease 12, no. 7: 260. https://doi.org/10.3390/jcdd12070260
APA StyleWaśkiewicz, Z., Bezuglov, E., Talibov, O., Gajda, R., Mukhambetov, Z., Azerbaev, D., & Bondarev, S. (2025). Divergent Cardiac Adaptations in Endurance Sport: Atrial Fibrillation Markers in Marathon Versus Ultramarathon Athletes. Journal of Cardiovascular Development and Disease, 12(7), 260. https://doi.org/10.3390/jcdd12070260









