Discordant High-Gradient Aortic Stenosis: A Systematic Review
Abstract
1. Introduction
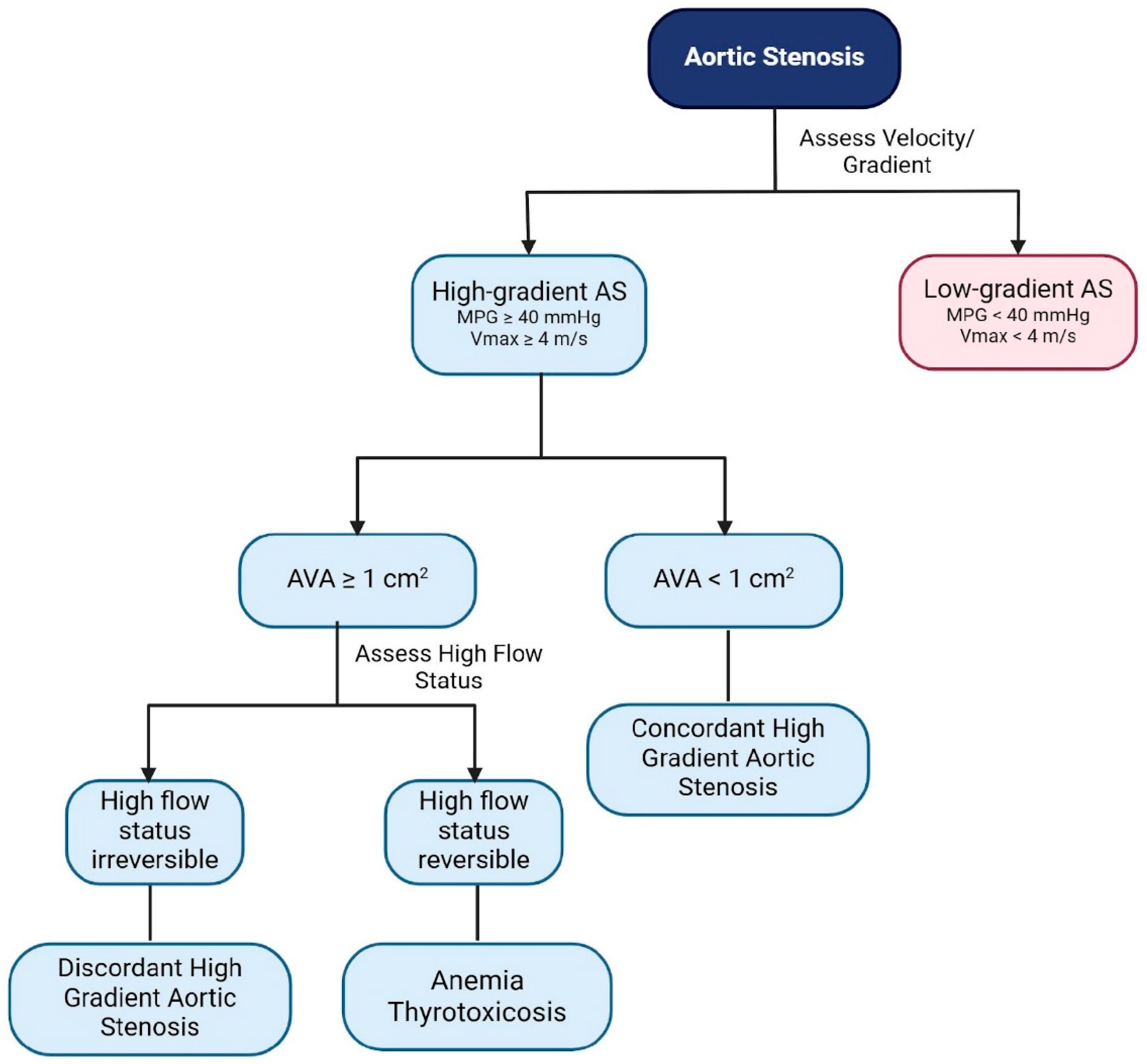
- Mild (AVA > 1.5 cm2, MPG < 25 mm Hg, or Vmax < 3.0 m/s);
- Moderate (AVA 1.0 to 1.5 cm2, MPG 25 to 40 mm Hg, or Vmax 3.0 to 4.0 m/s);
- Severe (AVA < 1.0 cm2, MPG > 40 mm Hg, or Vmax > 4.0 m/s) [6].
2. Methods
Literature Search Strategy and Inclusion Criteria
3. Results
3.1. Search Results and Quality Assessment of Included Articles
3.2. Prevalence of DHG-AS
3.3. Pathophysiology and Diagnostic Criteria
3.3.1. Pathophysiology
3.3.2. Signs and Symptoms
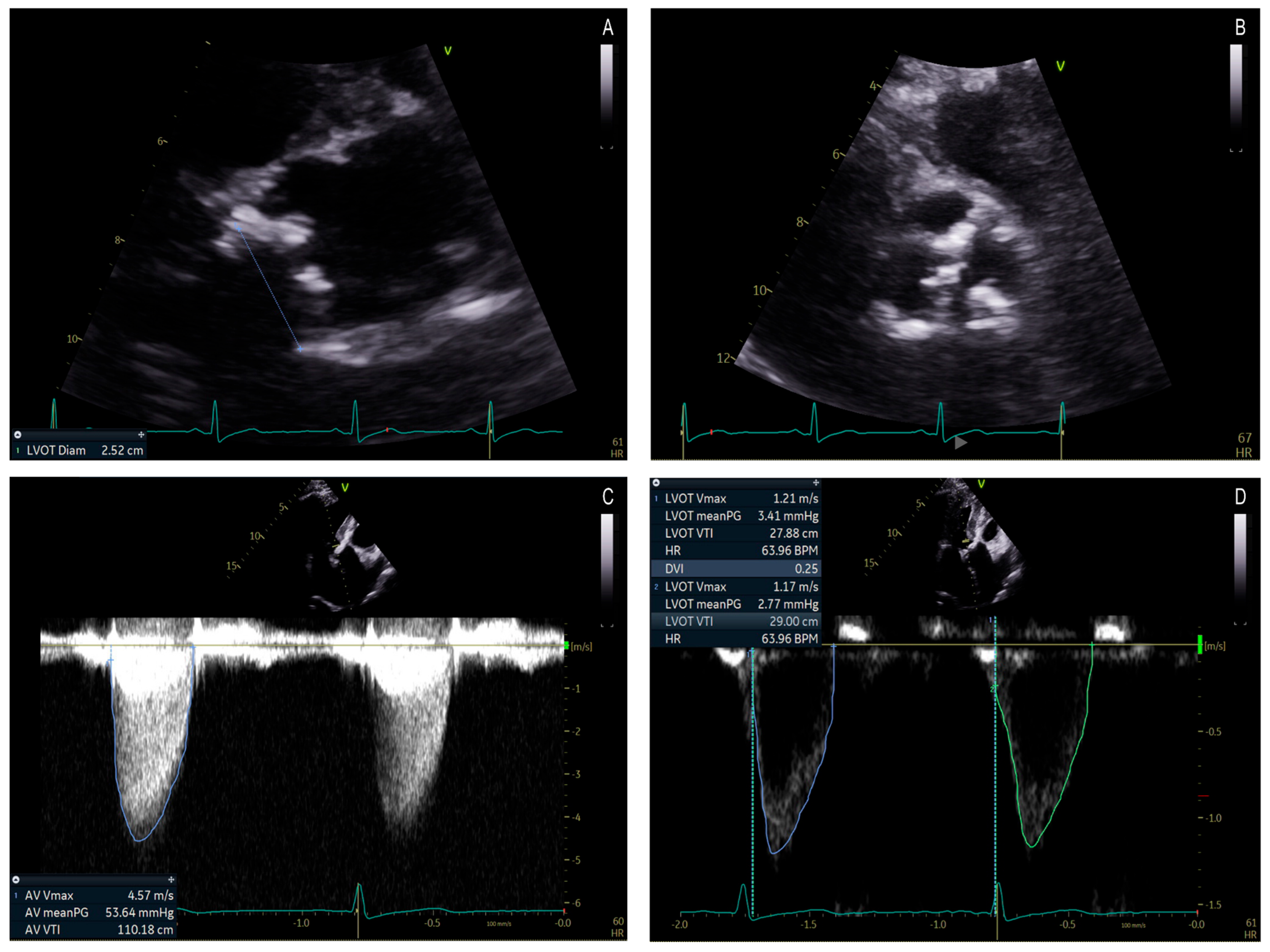
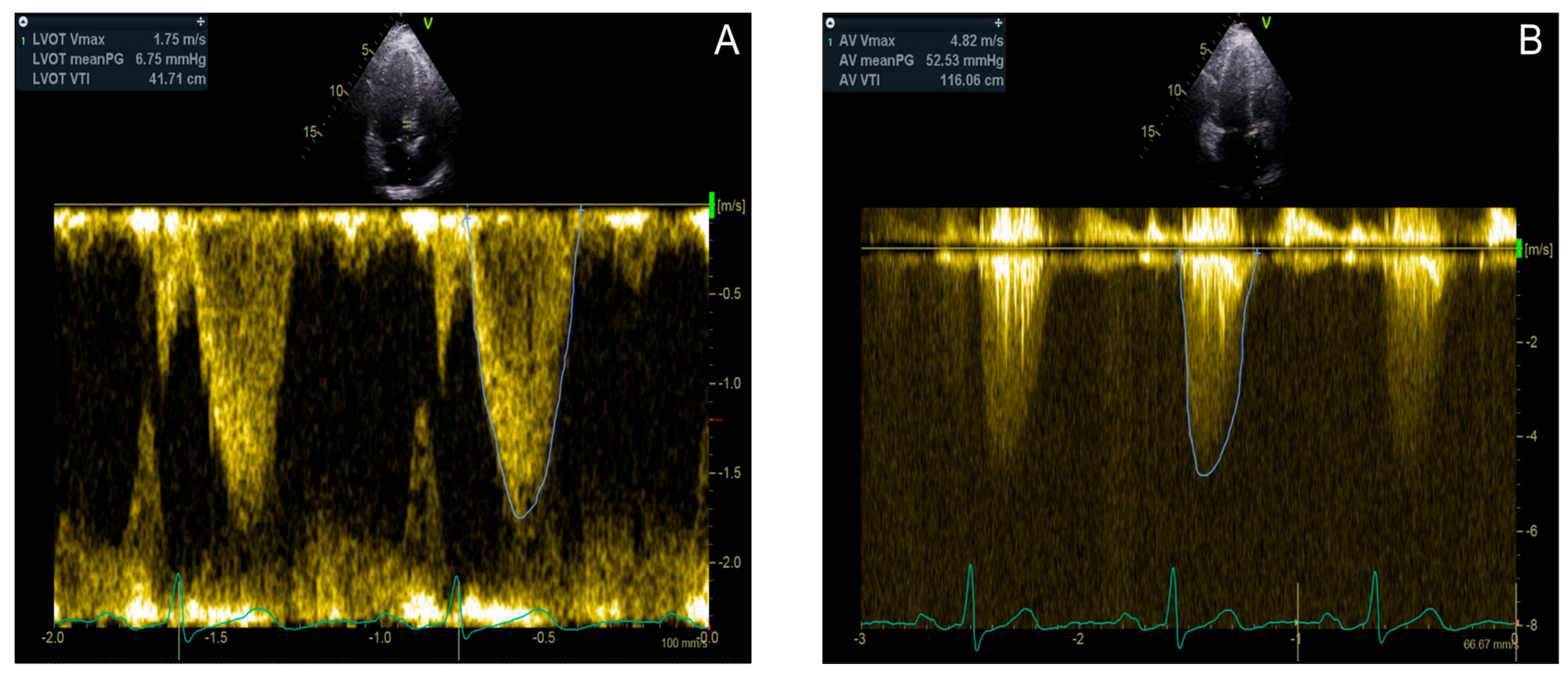
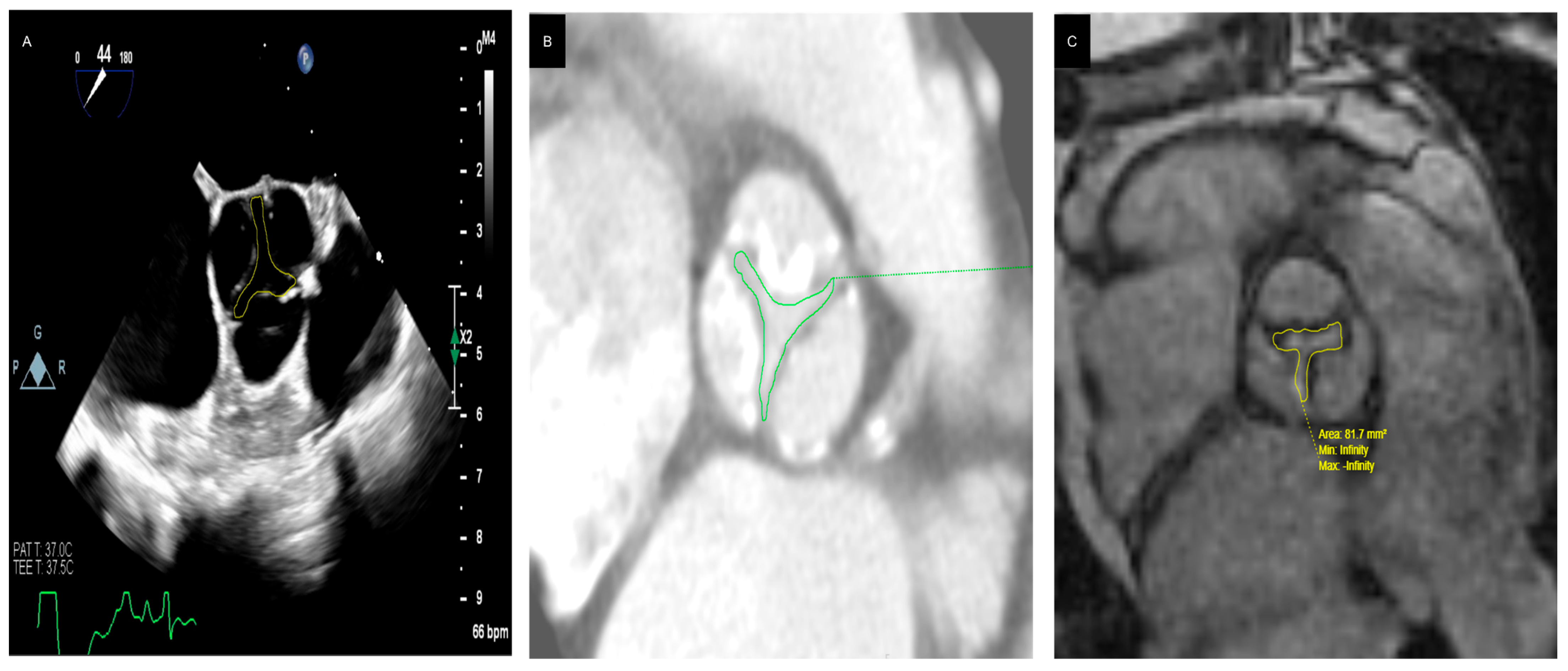
3.3.3. Diagnostic Tools and Techniques
| Imaging Modality | Clinical Utility | Prognostic Relevance | Relevance in Discordant High-Gradient Aortic Stenosis (DHG-AS) | Limitations |
|---|---|---|---|---|
| Transesophageal Echocardiography (TEE) |
|
|
|
|
| Computed Tomography (CT) |
|
|
|
|
| Cardiac Magnetic Resonance (CMR) |
|
|
|
|
| Study | Type of Study | Imaging Modality | Sample Size | Key Findings | Clinical Implications |
|---|---|---|---|---|---|
| Habis et al. [43] | Prospective Observational Study | 64-slice CT vs. TTE | 52 | The aortic orifice area measured by 64-slice CT correlated well with the effective area (r = 0.76; p < 0.0001), but was significantly greater, with a systematic overestimation (0.132 cm2). | CT planimetry allows accurate classification of AS severity comparable to echocardiographic methods. |
| John et al. [44] | Prospective Observational Study | MRI vs. TEE vs. Catheterization | 40 | Mean absolute differences in AVA were 0.02 cm2 for MRI versus TEE, 0.27 cm2 for MRI versus catheter, and 0.25 cm2 for TEE versus catheter. Correlations for AVAmax were r = 0.96 between MRI and TEE, r = 0.47 between TEE and catheter, and r = 0.44 between MRI and catheter. | Magnetic resonance planimetry of the AVA correlates well with TEE and less well with the catheter-derived AVA. MRI planimetry of the AVA may provide an accurate and noninvasive alternative to invasive techniques and TTE. |
| Feuchtner et al. [45] | Prospective Observational Study | 64-slice CT vs. TTE and TEE | 36 | CT AVA planimetry (1.11 ± 0.42 cm2) showed a good correlation with TTE (1.05 ± 0.42 cm2) (r = 0.88, p < 0.001) as well as with TEE (1.41 ± 1.61 cm2) (r = 0.99, p < 0.0001). | MSCT allows accurate planimetry of the AVA in patients with aortic stenosis comparable to both TTE and TEE. |
| Westermann et al. [46] | Prospective Observational Study | MSCT and MRI vs. TEE | 27 | Excellent correlation between MSCT and MRI (r = 0.99). The mean AVAs on both MSCT and MRI were systematically larger than on TTE (0.88 ± 0.28 cm2, p < 0.001 each) | MSCT and MRI have shown excellent correlation in AVA planimetry and similar accuracy in grading aortic valve stenosis. |
| Knobelsdorff-Brenkenhoff et al. [47] | Prospective Observational Study | CMR vs. TTE and TEE | 65 | Correlations of the AVA by CMR with TTE (r = 0.82) and CMR with TEE (r = 0.92) were significant. | CMR provides estimation of AVA with a close correlation to echocardiography and has low observer dependency. |
| Paelinck et al. [48] | Prospective Observational Study | MRI vs Catheterization vs. TTE and TEE | 24 | No differences in AVA were found among MRI, Doppler echocardiography, and three-dimensional TTE compared with catheterization (p = NS). | MRI planimetry, Doppler, and three-dimensional TTE provide an accurate estimate of the AVA compared to catheterization. |
| Alkadhi et al. [49] | Prospective Observational Study | 16-detector row CT vs. TTE and TEE | 40 | Significant correlations were present between AVA(CT) and AVA(TEE) (r = 0.99, p < 0.001), AVA(CT) and AVA(TTE) (r = 0.95, p < 0.001). Mean Differences were −0.08 cm2 for AVA(CT) vs. AVA(TEE) and 0.06 cm2 for AVA(CT) vs. AVA(TTE). | Planimetric measurements of AVA using a 16-detector row CT allow for the classification of AS, similar to echocardiographic methods. |
3.4. Prognosis and Outcomes of DHG-AS
Comparative Studies on DHG-AS and CHG-AS
3.5. Management of DHG-AS
| American Guidelines a | European Guidelines b | Canadian Guidelines c | Japanese Guidelines d | |
|---|---|---|---|---|
| Diagnosis |
|
|
|
|
| Management | Guidelines lack specific DHG-AS recommendations, so CHG-AS criteria are used. Severity is primarily based on the mean gradient and peak velocity, rather than AVA.
|
|
| |
|
| |||
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AS | Aortic stenosis |
| DHG-AS | Discordant high-gradient aortic stenosis |
| CHG-AS | Concordant high-gradient aortic stenosis |
| AVA | Aortic valve area |
| MPG | Mean transaortic pressure gradient |
| Vmax | Peak aortic jet velocity |
References
- Tribouilloy, C.; Rusinaru, D.; Bohbot, Y.; Maréchaux, S.; Vanoverschelde, J.L.; Enriquez-Sarano, M. How Should Very Severe Aortic Stenosis Be Defined in Asymptomatic Individuals? J. Am. Heart Assoc. 2019, 8, e011724. [Google Scholar] [CrossRef]
- Unger, P.; Powers, A.; Le Nezet, E.; Lacasse-Rioux, E.; Galloo, X.; Clavel, M.A. Prevalence and Outcomes of Patients with Discordant High-Gradient Aortic Stenosis. J. Am. Coll. Cardiol. 2024, 83, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.C.; Hung, J.C.-C.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 254–275. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, 2440–2492. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.J.; O’Sullivan, D. Discordant High-Gradient Aortic Stenosis: Trust the Gradient*. J. Am. Coll. Cardiol. 2024, 83, 1120–1122. [Google Scholar] [CrossRef]
- Bonow, R.O.; Carabello, B.A.; Chatterjee, K.; de Leon, A.C., Jr.; Faxon, D.P.; Freed, M.D.; Gaasch, W.H.; Lytle, B.W.; Nishimura, R.A.; O’Gara, P.T.; et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2008, 52, e1–e142. [Google Scholar] [CrossRef]
- Messika-Zeitoun, D.; Lloyd, G. Aortic valve stenosis: Evaluation and management of patients with discordant grading. E-J. Cardiol. Pract. 2018, 15, 26. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-15 (accessed on 14 March 2025).
- Chew, N.W.S.; Ho, Y.J.; Ngiam, J.H.N.; Kong, G.; Chin, Y.H.; Lim, O.Z.H.; Lin, C.; Sia, C.H.; Loh, P.H.; Kuntjoro, I.; et al. Clinical, echocardiographic and prognostic outcomes of patients with concordant and discordant high-gradient aortic stenosis in an Asian cohort. Int. J. Cardiovasc. Imaging 2022, 38, 1351–1360. [Google Scholar] [CrossRef]
- Bohbot, Y.; Kubala, M.; Rusinaru, D.; Maréchaux, S.; Vanoverschelde, J.-L.; Tribouilloy, C. Survival and Management of Patients With Discordant High-Gradient Aortic Stenosis: A Propensity-Matched Study. JACC Cardiovasc. Imaging 2021, 14, 1672–1674. [Google Scholar] [CrossRef]
- Vulesevic, B.; Burwash, I.G.; Beauchesne, L.M.; Cimadevilla, C.; Siouti, L.; Tubiana, S.; Duval, X.; Nguyen, V.; Arangalage, D.; Chan, K.L.; et al. Outcomes of Patients With Discordant High-Gradient Aortic Valve Stenosis. JACC Cardiovasc. Imaging 2020, 13, 1636–1638. [Google Scholar] [CrossRef]
- Pio, S.M.; Amanullah, M.R.; Butcher, S.C.; Sin, K.Y.; Ajmone Marsan, N.; Pibarot, P.; Van Mieghem, N.M.; Ding, Z.P.; Genereux, P.; Leon, M.B.; et al. Discordant severity criteria in patients with moderate aortic stenosis: Prognostic implications. Open Heart 2021, 8, e001639. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute. Updated 3 May 2021. 2025. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 14 March 2025).
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Ito, S.; Oh, J.K.; Michelena, H.I.; Egbe, A.C.; Connolly, H.M.; Pellikka, P.A.; Nkomo, V.T.; Lewis, B.R.; Miranda, W.R. High-Gradient Aortic Stenosis with Valve Area >1.0 cm2: The “Forgotten” Discordant Hemodynamic Phenotype. JACC Cardiovasc. Imaging 2024, 18, 166–176. [Google Scholar] [CrossRef]
- Ullah, W.; Gowda, S.N.; Khan, M.S.; Sattar, Y.; Al-Khadra, Y.; Rashid, M.; Mohamed, M.O.; Alkhouli, M.; Kapadia, S.; Bagur, R.; et al. Early intervention or watchful waiting for asymptomatic severe aortic valve stenosis: A systematic review and meta-analysis. J. Cardiovasc. Med. 2020, 21, 897–904. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Carabello, B.A. Clinical practice. Aortic stenosis. N. Engl. J. Med. 2002, 346, 677–682. [Google Scholar] [CrossRef]
- Metivier, F.; Marchais, S.J.; Guerin, A.P.; Pannier, B.; London, G.M. Pathophysiology of anaemia: Focus on the heart and blood vessels. Nephrol. Dial. Transplant. 2000, 15 (Suppl. S3), 14–18. [Google Scholar] [CrossRef]
- Fort, J. Chronic renal failure: A cardiovascular risk factor. Kidney Int. Suppl. 2005, 68, S25–S29. [Google Scholar] [CrossRef]
- Gopinathannair, R.; Olshansky, B. Management of tachycardia. F1000Prime Rep. 2015, 7, 60. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Bartalena, L.; Hegedus, L.; Leenhardt, L.; Poppe, K.; Pearce, S.H. 2018 European Thyroid Association Guideline for the Management of Graves’ Hyperthyroidism. Eur. Thyroid J. 2018, 7, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Fede, G.; Privitera, G.; Tomaselli, T.; Spadaro, L.; Purrello, F. Cardiovascular dysfunction in patients with liver cirrhosis. Ann. Gastroenterol. 2015, 28, 31–40. [Google Scholar] [PubMed]
- Peng, Y.; Qi, X.; Guo, X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine 2016, 95, e2877. [Google Scholar] [CrossRef] [PubMed]
- Jander, N.; Gohlke-Bärwolf, C.; Bahlmann, E.; Gerdts, E.; Boman, K.; Chambers, J.B.; Egstrup, K.; Nienaber, C.A.; Pedersen, T.R.; Ray, S.; et al. Indexing aortic valve area by body surface area increases the prevalence of severe aortic stenosis. Heart 2014, 100, 28–33. [Google Scholar] [CrossRef]
- Minners, J.; Allgeier, M.; Gohlke-Baerwolf, C.; Kienzle, R.-P.; Neumann, F.-J.; Jander, N. Inconsistent grading of aortic valve stenosis by current guidelines: Haemodynamic studies in patients with apparently normal left ventricular function. Heart 2010, 96, 1463–1468. [Google Scholar] [CrossRef]
- Grimard, B.H.; Safford, R.E.; Burns, E.L. Aortic Stenosis: Diagnosis and Treatment. Am. Fam. Physician 2016, 93, 371–378. [Google Scholar]
- Sakthi, C.; Yee, H.; Kotlewski, A. Overestimation of aortic valve gradient measured by Doppler echocardiography in patients with aortic stenosis. Catheter. Cardiovasc. Interv. 2005, 65, 176–179. [Google Scholar] [CrossRef]
- Baumgartner, H.; Stefenelli, T.; Niederberger, J.; Schima, H.; Maurer, G. “overestimation” of catheter gradients by doppler ultrasound in patients with aortic stenosis: A predictable manifestation of pressure recovery. J. Am. Coll. Cardiol. 1999, 33, 1655–1661. [Google Scholar] [CrossRef]
- Mancusi, C.; Bahlmann, E.; Basile, C.; Gerdts, E. New Evidence About Aortic Valve Stenosis and Cardiovascular Hemodynamics. High Blood Press. Cardiovasc. Prev. 2022, 29, 231–237. [Google Scholar] [CrossRef]
- Gjertsson, P.; Caidahl, K.; Svensson, G.; Wallentin, I.; Bech-Hanssen, O. Important pressure recovery in patients with aortic stenosis and high Doppler gradients. Am. J. Cardiol. 2001, 88, 139–144. [Google Scholar] [CrossRef]
- Stoddard, M.F.; Arce, J.; Liddell, N.E.; Peters, G.; Dillon, S.; Kupersmith, J. Two-dimensional transesophageal echocardiographic determination of aortic valve area in adults with aortic stenosis. Am. Heart J. 1991, 122, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Kamperidis, V.; van Rosendael, P.J.; Katsanos, S.; van der Kley, F.; Regeer, M.; Al Amri, I.; Sianos, G.; Marsan, N.A.; Delgado, V.; Bax, J.J. Low gradient severe aortic stenosis with preserved ejection fraction: Reclassification of severity by fusion of Doppler and computed tomographic data. Eur. Heart J. 2015, 36, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Klass, O.; Walker, M.J.; Olszewski, M.E.; Bahner, J.; Feuerlein, S.; Hoffmann, M.H.K.; Lang, A. Quantification of aortic valve area at 256-slice computed tomography: Comparison with transesophageal echocardiography and cardiac catheterization in subjects with high-grade aortic valve stenosis prior to percutaneous valve replacement. Eur. J. Radiol. 2011, 80, 151–157. [Google Scholar] [CrossRef]
- Mittal, T.K.; Marcus, N. Imaging diagnosis of aortic stenosis. Clin. Radiol. 2021, 76, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Schwartzenberg, S.; Vaturi, M.; Kazum, S.; Yedidya, I.; Monakier, D.; Ofek, H.; Sagie, A.; Kornowski, R.; Shapira, Y. Comparison of Simultaneous Transthoracic Versus Transesophageal Echocardiography for Assessment of Aortic Stenosis. Am. J. Cardiol. 2022, 163, 77–84. [Google Scholar] [CrossRef]
- Tastet, L.; Ali, M.; Pibarot, P.; Capoulade, R.; Øvrehus, K.A.; Arsenault, M.; Haujir, A.; Bédard, É.; Diederichsen, A.C.P.; Dahl, J.S.; et al. Grading of Aortic Valve Calcification Severity and Risk Stratification in Aortic Stenosis. J. Am. Heart Assoc. 2024, 13, e035605. [Google Scholar] [CrossRef]
- Pawade, T.; Clavel, M.-A.; Tribouilloy, C.; Dreyfus, J.; Mathieu, T.; Tastet, L.; Renard, C.; Gun, M.; Jenkins, W.S.A.; Macron, L.; et al. Computed Tomography Aortic Valve Calcium Scoring in Patients With Aortic Stenosis. Circ. Cardiovasc. Imaging 2018, 11, e007146. [Google Scholar] [CrossRef]
- Garcia, J.; Barker, A.J.; Markl, M. The Role of Imaging of Flow Patterns by 4D Flow MRI in Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 252–266. [Google Scholar] [CrossRef]
- Nickl, W.; Füth, R.; Smettan, J.; Köhler, T.; Lankisch, M.; Kramer, F.; Krahn, T.; Barroso, M.C.; Klein, R.M.; Dinh, W. Assessment of aortic valve area combining echocardiography and magnetic resonance imaging. Arq. Bras. Cardiol. 2012, 98, 234–242. [Google Scholar] [CrossRef]
- Debl, K.; Djavidani, B.; Seitz, J.; Nitz, W.; Schmid, F.-X.; Muders, F.; Buchner, S.; Feuerbach, S.; Riegger, G.; Luchner, A. Planimetry of Aortic Valve Area in Aortic Stenosis by Magnetic Resonance Imaging. Investig. Radiol. 2005, 40, 631–636. [Google Scholar] [CrossRef]
- Tzolos, E.; Andrews, J.P.; Dweck, M.R. Aortic valve stenosis-multimodality assessment with PET/CT and PET/MRI. Br. J. Radiol. 2020, 93, 20190688. [Google Scholar] [CrossRef] [PubMed]
- Habis, M.; Daoud, B.; Roger, V.L.; Ghostine, S.; Caussin, C.; Ramadan, R.; Nottin, R.; Lancelin, B.; Angel, C.Y.; Capderou, A.; et al. Comparison of 64-slice computed tomography planimetry and Doppler echocardiography in the assessment of aortic valve stenosis. J. Heart Valve Dis. 2007, 16, 216–224. [Google Scholar]
- John, A.S.; Dill, T.; Brandt, R.R.; Rau, M.; Ricken, W.; Bachmann, G.; Hamm, C.W. Magnetic resonance to assess the aortic valve area in aortic stenosis: How does it compare to current diagnostic standards? J. Am. Coll. Cardiol. 2003, 42, 519–526. [Google Scholar] [CrossRef]
- Feuchtner, G.M.; Muller, S.; Bonatti, J.; Schachner, T.; Velik-Salchner, C.; Pachinger, O.; Dichtl, W. Sixty-four slice CT evaluation of aortic stenosis using planimetry of the aortic valve area. AJR Am. J. Roentgenol. 2007, 189, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Westermann, Y.; Geigenmuller, A.; Elgeti, T.; Wagner, M.; Dushe, S.; Borges, A.C.; Dohmen, P.M.; Hein, P.A.; Lembcke, A. Planimetry of the aortic valve orifice area: Comparison of multislice spiral computed tomography and magnetic resonance imaging. Eur. J. Radiol. 2011, 77, 426–435. [Google Scholar] [CrossRef]
- von Knobelsdorff-Brenkenhoff, F.; Rudolph, A.; Wassmuth, R.; Bohl, S.; Buschmann, E.E.; Abdel-Aty, H.; Dietz, R.; Schulz-Menger, J. Feasibility of cardiovascular magnetic resonance to assess the orifice area of aortic bioprostheses. Circ. Cardiovasc. Imaging 2009, 2, 397–404. [Google Scholar] [CrossRef]
- Paelinck, B.P.; Van Herck, P.L.; Rodrigus, I.; Claeys, M.J.; Laborde, J.C.; Parizel, P.M.; Vrints, C.J.; Bosmans, J.M. Comparison of magnetic resonance imaging of aortic valve stenosis and aortic root to multimodality imaging for selection of transcatheter aortic valve implantation candidates. Am. J. Cardiol. 2011, 108, 92–98. [Google Scholar] [CrossRef]
- Alkadhi, H.; Wildermuth, S.; Plass, A.; Bettex, D.; Baumert, B.; Leschka, S.; Desbiolles, L.M.; Marincek, B.; Boehm, T. Aortic stenosis: Comparative evaluation of 16-detector row CT and echocardiography. Radiology 2006, 240, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Aluru, J.S.; Barsouk, A.; Saginala, K.; Rawla, P.; Barsouk, A. Valvular Heart Disease Epidemiology. Med. Sci. 2022, 10, 32. [Google Scholar] [CrossRef]
- Zilberszac, R.; Gabriel, H.; Schemper, M.; Zahler, D.; Czerny, M.; Maurer, G.; Rosenhek, R. Outcome of Combined Stenotic and Regurgitant Aortic Valve Disease. J. Am. Coll. Cardiol. 2013, 61, 1489–1495. [Google Scholar] [CrossRef]
- Généreux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; Jaber, W.A.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef]
- Pawade, T.; Sheth, T.; Guzzetti, E.; Dweck Marc, R.; Clavel, M.-A. Why and How to Measure Aortic Valve Calcification in Patients With Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Cueff, C.; Serfaty, J.-M.; Cimadevilla, C.; Laissy, J.-P.; Himbert, D.; Tubach, F.; Duval, X.; Iung, B.; Enriquez-Sarano, M.; Vahanian, A.; et al. Measurement of aortic valve calcification using multislice computed tomography: Correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart 2011, 97, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Scalia, I.G.; Farina, J.M.; Padang, R.; Jokerst, C.E.; Pereyra, M.; Mahmoud, A.K.; Naqvi, T.Z.; Chao, C.J.; Oh, J.K.; Arsanjani, R.; et al. Aortic Valve Calcium Score by Computed Tomography as an Adjunct to Echocardiographic Assessment-A Review of Clinical Utility and Applications. J. Imaging 2023, 9, 250. [Google Scholar] [CrossRef]
- Coisne, A.; Lancellotti, P.; Habib, G.; Garbi, M.; Dahl, J.S.; Barbanti, M.; Vannan, M.A.; Vassiliou, V.S.; Dudek, D.; Chioncel, O.; et al. ACC/AHA and ESC/EACTS Guidelines for the Management of Valvular Heart Diseases: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2023, 82, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Mazin, I.; Kuperstein, R.; Beigel, R.; Vaturi, O.; Feinberg, M.S.; Raanani, E.; Ben Zekry, S. Bicuspid aortic valve area in normal heart. Echocardiography 2020, 37, 439–444. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Ribeiro, H.B.; Mohammadi, S.; Serra, V.; Al-Atassi, T.; Iñiguez, A.; Vilalta, V.; Nombela-Franco, L.; Sáez de Ibarra Sánchez, J.I.; Auffret, V.; et al. Transcatheter or Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis and Small Aortic Annulus: A Randomized Clinical Trial. Circulation 2024, 149, 644–655. [Google Scholar] [CrossRef]
- Bismee, N.N.; Javadi, N.; Khedr, A.; Omar, F.; Awad, K.; Abbas, M.T.; Scalia, I.G.; Pereyra, M.; Bcharah, G.; Farina, J.M.; et al. Bioprosthetic Aortic Valve Degeneration After TAVR and SAVR: Incidence, Diagnosis, Predictors, and Management. J. Cardiovasc. Dev. Dis. 2024, 11, 384. [Google Scholar] [CrossRef]
- Asgar, A.W.; Ouzounian, M.; Adams, C.; Afilalo, J.; Fremes, S.; Lauck, S.; Leipsic, J.; Piazza, N.; Rodes-Cabau, J.; Welsh, R.; et al. 2019 Canadian Cardiovascular Society Position Statement for Transcatheter Aortic Valve Implantation. Can. J. Cardiol. 2019, 35, 1437–1448. [Google Scholar] [CrossRef]
- Izumi, C.; Eishi, K.; Ashihara, K.; Arita, T.; Otsuji, Y.; Kunihara, T.; Komiya, T.; Shibata, T.; Seo, Y.; Daimon, M.; et al. JCS/JSCS/JATS/JSVS 2020 Guidelines on the Management of Valvular Heart Disease. Circ. J. 2020, 84, 2037–2119. [Google Scholar] [CrossRef]

| Study | Selection | Comparability | Outcome | |||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration of the Outcome of Interest Was Not Present at the Start of the Study | Comparability of Cohorts on the Basis of the Design or Analysis | Assessment of Outcome | Was Follow-Up Long Enough for Outcomes to Occur? | Adequacy of Follow-Up of Cohorts | |
| Ito, S. et al. (2024) [14] | * | * | * | * | ** | * | * | * |
| Unger, P. et al. (2024) [2] | * | * | * | * | ** | * | * | * |
| Chew, N. W. S. et al. (2022) [8] | * | * | * | * | ** | * | * | * |
| Bohbot, Y. et al. (2021) [9] | * | * | * | * | ** | Not reported | * | * |
| Vulesevic, B. et al. (2020) [10] | * | * | * | * | ** | Not reported | * | * |
| Causes of High Flow Status | The Threshold at Which There Is a Significant Increase in Cardiac Index (>4 L/min/m2) |
|---|---|
| Anemia | Severe anemia (Hb < 7 g/dL a) |
| Renal failure | Stage 4–5 chronic kidney disease (eGFR < 30 mL/min b) |
| Tachycardia | >110 bpm c |
| Hyperthyroidism | Moderate hyperthyroidism: TSH < 0.01 mIU/L Free T4 > 2.5 ng/dL Free T3 > 8 pg/mL d |
| Liver failure | Stage 4 liver disease (Child-Pugh class C): e,f INR > 2.3 Albumin < 2.8 g/dL Bilirubin > 3 mg/dL |
| Study | Type of Study | Population Size (% of Dhg-As Among High-Gradient As) | Outcome |
|---|---|---|---|
| Ito, S. et al. (2024) [14] | Retrospective observational, single-center study | 3209 (13.5%) | All-cause mortality was higher in CHG-AS patients compared to patients with DHG-AS (unadjusted HR: 1.4; 95% CI: 1.1 to 1.7) |
| Unger, P. et al. (2024) [2] | Retrospective observational, single-center study | 3547 (11.6%) | The mortality rate for DHG-AS patients was similar to that of those with CHG-AS (adjusted HR: 0.98, 95% CI: 0.66 to 1.44; p = 0.91) |
| Chew, N. W. S. et al. (2022) [8] | Retrospective observational, single-center study | 467 (30.8%) | CHG-AS patients were significantly associated with all-cause mortality (adjusted HR: 3.082, 95% CI: 1.479 to 6.420; p = 0.003) and CHF admissions (adjusted HR: 12.728, 95% CI: 2.922 to 55.440; p = 0.001), but DHG-AS patients were not in reference to moderate AS |
| Bohbot, Y. et al. (2021) [9] | Retrospective observational, multicenter study | 2724 (4.3%) | DHG-AS patients had higher mortality rates compared to CHG-AS (adjusted HR: 1.59, 95% CI:1.04 to 2.56) |
| Vulesevic, B. et al. (2020) [10] | Prospective observational, single-center study | 234 (29.1%) | Event-free (sudden death, congestive heart failure, or new onset of symptoms of dyspnea, angina, or syncope) survival of patients with CHG-AS and DHG-AS was similar (p = 0.45) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bismee, N.N.; Abbas, M.T.; Sheashaa, H.; Abdelfattah, F.E.; Farina, J.M.; Awad, K.; Scalia, I.G.; Pietri, M.P.; Ali, N.B.; Esfahani, S.A.; et al. Discordant High-Gradient Aortic Stenosis: A Systematic Review. J. Cardiovasc. Dev. Dis. 2025, 12, 255. https://doi.org/10.3390/jcdd12070255
Bismee NN, Abbas MT, Sheashaa H, Abdelfattah FE, Farina JM, Awad K, Scalia IG, Pietri MP, Ali NB, Esfahani SA, et al. Discordant High-Gradient Aortic Stenosis: A Systematic Review. Journal of Cardiovascular Development and Disease. 2025; 12(7):255. https://doi.org/10.3390/jcdd12070255
Chicago/Turabian StyleBismee, Nadera N., Mohammed Tiseer Abbas, Hesham Sheashaa, Fatmaelzahraa E. Abdelfattah, Juan M. Farina, Kamal Awad, Isabel G. Scalia, Milagros Pereyra Pietri, Nima Baba Ali, Sogol Attaripour Esfahani, and et al. 2025. "Discordant High-Gradient Aortic Stenosis: A Systematic Review" Journal of Cardiovascular Development and Disease 12, no. 7: 255. https://doi.org/10.3390/jcdd12070255
APA StyleBismee, N. N., Abbas, M. T., Sheashaa, H., Abdelfattah, F. E., Farina, J. M., Awad, K., Scalia, I. G., Pietri, M. P., Ali, N. B., Esfahani, S. A., Ibrahim, O. H., Lester, S. J., Alsidawi, S., Ayoub, C., & Arsanjani, R. (2025). Discordant High-Gradient Aortic Stenosis: A Systematic Review. Journal of Cardiovascular Development and Disease, 12(7), 255. https://doi.org/10.3390/jcdd12070255








