Feasibility, Added Value, and Radiation Dose of Combined Coronary CT Angiography and Stress Dynamic CT Myocardial Perfusion Imaging in Moderate Coronary Artery Disease: A Real-World Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Scan Protocol
2.3. CCTA Imaging Analysis
2.4. Dynamic CT-MPI Analysis
2.5. SPECT Examination
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. CAC and Pre-Test Probabilty of CAD
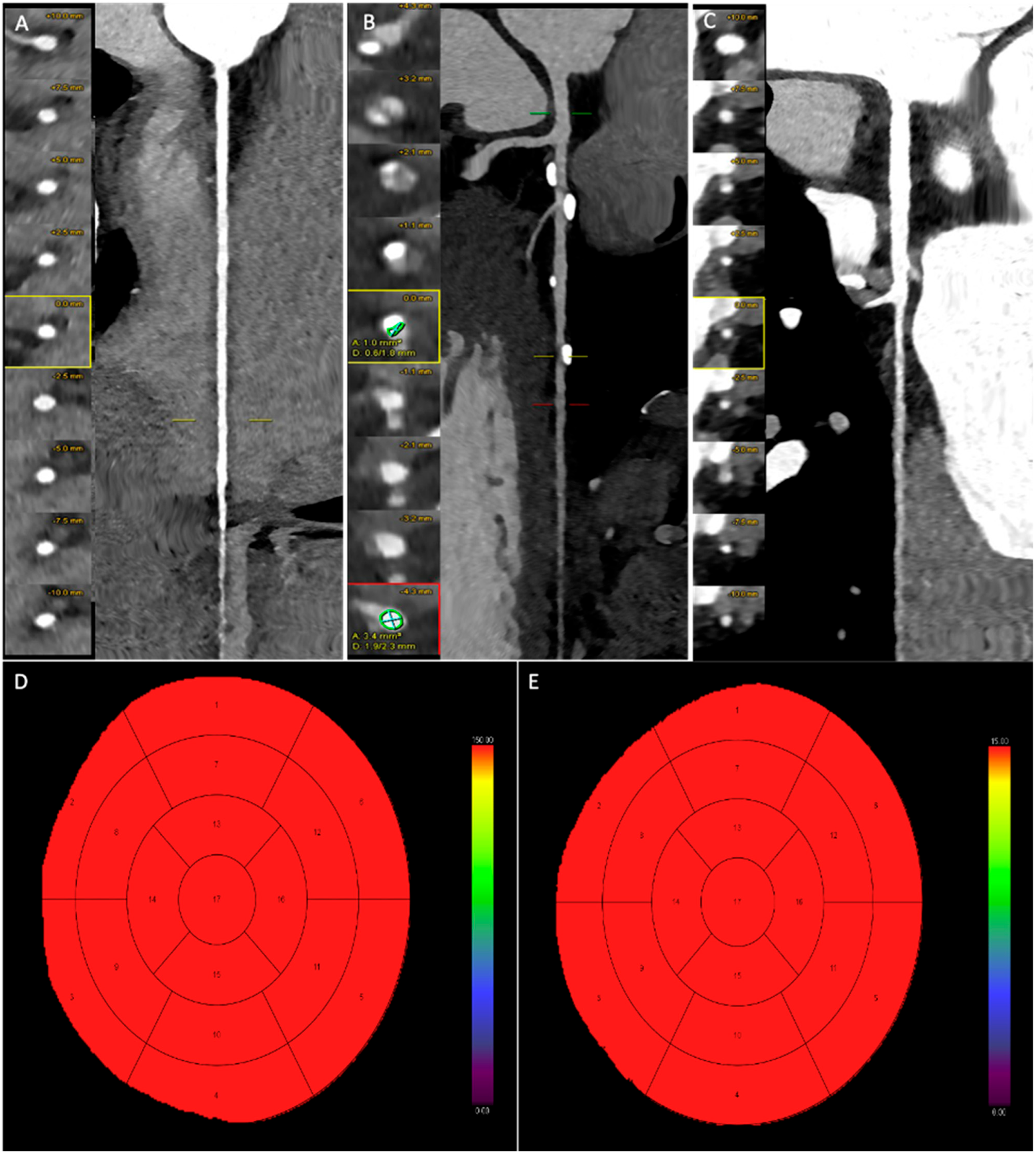
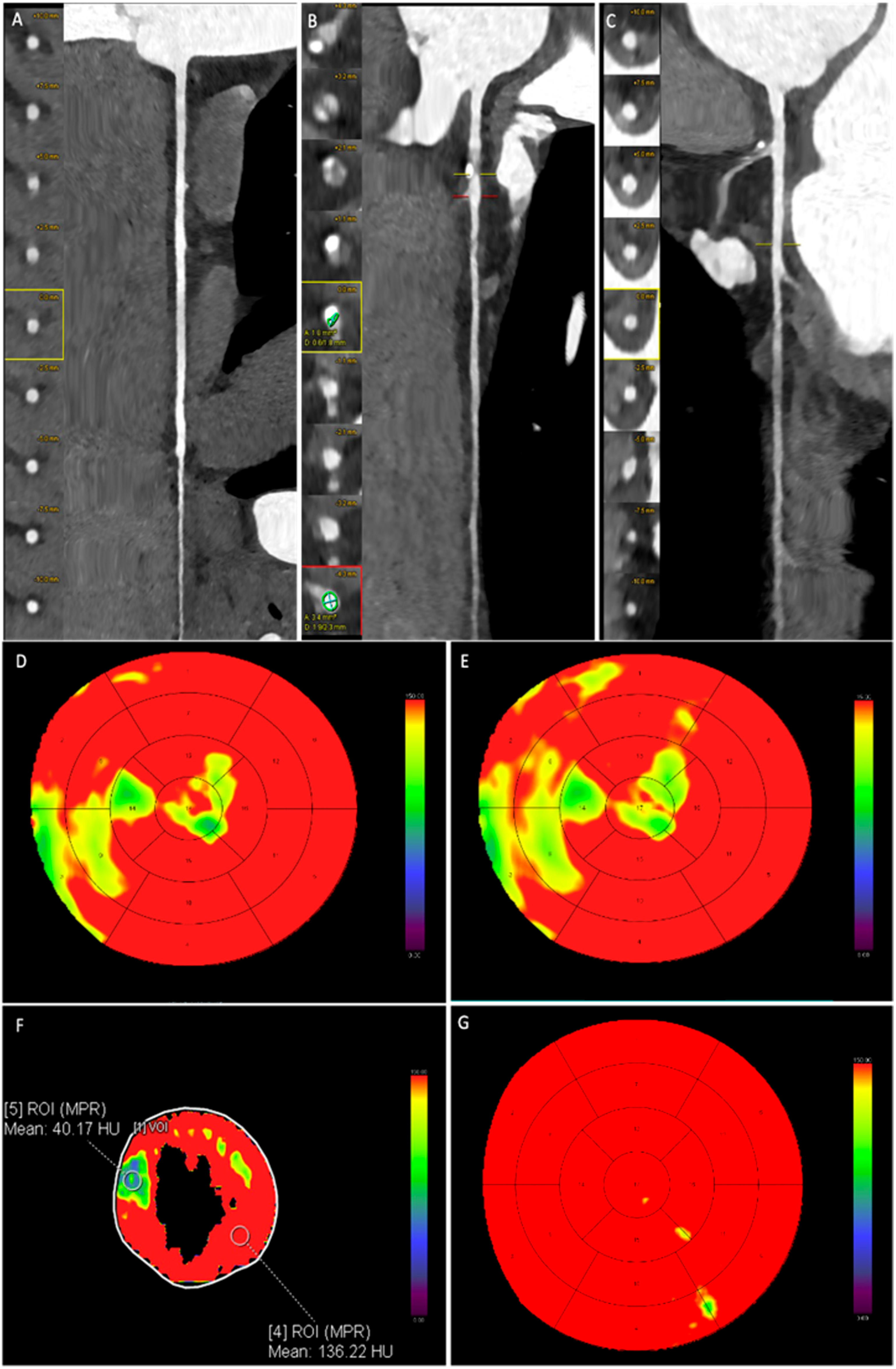
3.3. CCTA Imaging Analysis and CAD-RADS
3.4. Dynamic CT-MPI Analysis
4. Discussion
4.1. Radiation-Induced Malignancy Risk in Cardiac Imaging
4.2. Limitations to the Study
4.3. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruff, C.T.; Braunwald, E. The Evolving Epidemiology of Acute Coronary Syndromes. Nat. Rev. Cardiol. 2011, 8, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Al’Aref, S.J.; Berger, A.; Hartaigh, B.Ó.; Gransar, H.; Valenti, V.; Lin, F.Y.; Achenbach, S.; Berman, D.S.; Budoff, M.J.; et al. Prognostic Value of Coronary Computed Tomographic Angiography Findings in Asymptomatic Individuals: A 6-Year Follow-up from the Prospective Multicentre International CONFIRM Study. Eur. Heart J. 2018, 39, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Blanke, P.; Naoum, C.; Ahmadi, A.; Cheruvu, C.; Soon, J.; Arepalli, C.; Gransar, H.; Achenbach, S.; Berman, D.S.; Budoff, M.J.; et al. Long-Term Prognostic Utility of Coronary CT Angiography in Stable Patients with Diabetes Mellitus. JACC Cardiovasc. Imaging 2016, 9, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Andreini, D.; Guaricci, A.I.; Baggiano, A.; Fazzari, F.; Guglielmo, M.; Muscogiuri, G.; Berzovini, C.M.; Pasquini, A.; Mushtaq, S.; et al. Incremental Diagnostic Value of Stress Computed Tomography Myocardial Perfusion with Whole-Heart Coverage CT Scanner in Intermediate- to High-Risk Symptomatic Patients Suspected of Coronary Artery Disease. JACC Cardiovasc. Imaging 2019, 12, 338–349. [Google Scholar] [CrossRef]
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.W.J.M.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI Expert Consensus Recommendations for Multimodality Imaging in Cardiac Amyloidosis: Part 1 of 2-Evidence Base and Standardized Methods of Imaging. Circ. Cardiovasc. Imaging 2021, 14, e000029. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Li, C.; Xu, R.; Yao, K.; Zhang, J.; Chen, S.; Pang, L.; Lu, H.; Dai, Y.; Qian, J.; Shi, H.; et al. Functional Significance of Intermediate Coronary Stenosis in Patients with Single-Vessel Coronary Artery Disease: A Comparison of Dynamic SPECT Coronary Flow Reserve with Intracoronary Pressure-Derived Fractional Flow Reserve (FFR). J. Nucl. Cardiol. 2022, 29, 622–629. [Google Scholar] [CrossRef]
- Schicchi, N.; Fogante, M.; Paolini, E.; Cela, F.; Pirani, P.E.; Perna, G.P. Stress-Rest Dynamic-CT Myocardial Perfusion Imaging in the Management of Myocardial Bridging: A “One-Stop Shop” Exam. J. Cardiol. Cases 2023, 28, 229–232. [Google Scholar] [CrossRef]
- Nishiyama, H.; Tanabe, Y.; Kido, T.; Kurata, A.; Uetani, T.; Kido, T.; Ikeda, S.; Miyagawa, M.; Mochizuki, T. Incremental Diagnostic Value of Whole-Heart Dynamic Computed Tomography Perfusion Imaging for Detecting Obstructive Coronary Artery Disease. J. Cardiol. 2019, 73, 425–431. [Google Scholar] [CrossRef]
- Yang, D.H.; Kim, Y.-H. CT Myocardial Perfusion Imaging: Current Status and Future Perspectives. Int. J. Cardiovasc. Imaging 2017, 33, 1009–1020. [Google Scholar] [CrossRef]
- Rossi, A.; Merkus, D.; Klotz, E.; Mollet, N.; de Feyter, P.J.; Krestin, G.P. Stress Myocardial Perfusion: Imaging with Multidetector CT. Radiology 2014, 270, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Feger, S.; Rief, M.; Zimmermann, E.; Richter, F.; Roehle, R.; Dewey, M.; Schönenberger, E. Patient Satisfaction with Coronary CT Angiography, Myocardial CT Perfusion, Myocardial Perfusion MRI, SPECT Myocardial Perfusion Imaging and Conventional Coronary Angiography. Eur. Radiol. 2015, 25, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Minhas, A.; Dewey, M.; Vavere, A.L.; Tanami, Y.; Ostovaneh, M.R.; Laule, M.; Rochitte, C.E.; Niinuma, H.; Kofoed, K.F.; Geleijns, J.; et al. Patient Preferences for Coronary CT Angiography with Stress Perfusion, SPECT, or Invasive Coronary Angiography. Radiology 2019, 291, 340–348. [Google Scholar] [CrossRef]
- Seitun, S.; De Lorenzi, C.; Cademartiri, F.; Buscaglia, A.; Travaglio, N.; Balbi, M.; Bezante, G.P. CT Myocardial Perfusion Imaging: A New Frontier in Cardiac Imaging. Biomed. Res. Int. 2018, 2018, 7295460. [Google Scholar] [CrossRef]
- Rajiah, P.; Maroules, C.D. Myocardial Ischemia Testing with Computed Tomography: Emerging Strategies. Cardiovasc. Diagn. Ther. 2017, 7, 475–488. [Google Scholar] [CrossRef]
- Zdanowicz, A.; Guzinski, M.; Pula, M.; Witkowska, A.; Reczuch, K. Dynamic CT Myocardial Perfusion: The Role of Functional Evaluation in the Diagnosis of Coronary Artery Disease. J. Clin. Med. 2023, 12, 7062. [Google Scholar] [CrossRef]
- Nous, F.M.A.; Geisler, T.; Kruk, M.B.P.; Alkadhi, H.; Kitagawa, K.; Vliegenthart, R.; Hell, M.M.; Hausleiter, J.; Nguyen, P.K.; Budde, R.P.J.; et al. Dynamic Myocardial Perfusion CT for the Detection of Hemodynamically Significant Coronary Artery Disease. JACC Cardiovasc. Imaging 2022, 15, 75–87. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S.; et al. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart. A Statement for Healthcare Professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, M.; Yu, M.; Lu, Z.; Shen, C.; Wang, Y.; Lu, B.; Zhang, J. Prevalence of Decreased Myocardial Blood Flow in Symptomatic Patients with Patent Coronary Stents: Insights from Low-Dose Dynamic CT Myocardial Perfusion Imaging. Korean J. Radiol. 2019, 20, 621. [Google Scholar] [CrossRef]
- Parker, M.W.; Iskandar, A.; Limone, B.; Perugini, A.; Kim, H.; Jones, C.; Calamari, B.; Coleman, C.I.; Heller, G.V. Diagnostic Accuracy of Cardiac Positron Emission Tomography versus Single Photon Emission Computed Tomography for Coronary Artery Disease: A Bivariate Meta-Analysis. Circ. Cardiovasc. Imaging 2012, 5, 700–707. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, L.; Shi, X.; Zeng, Y.; Jing, H.; Schoepf, U.J.; Jin, Z. Adenosine-Stress Dynamic Myocardial Perfusion Imaging with Second-Generation Dual-Source CT: Comparison with Conventional Catheter Coronary Angiography and SPECT Nuclear Myocardial Perfusion Imaging. Am. J. Roentgenol. 2012, 198, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Cox, E.; Rothwell, B.; Berry, C.; McCann, G.P.; Bucciarelli-Ducci, C.; Dall’Armellina, E.; Prasad, A.; Foley, J.R.J.; Mangion, K.; et al. Cost-Effectiveness of Cardiovascular Imaging for Stable Coronary Heart Disease. Heart 2021, 107, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Pelgrim, G.J.; Dorrius, M.; Xie, X.; den Dekker, M.A.M.; Schoepf, U.J.; Henzler, T.; Oudkerk, M.; Vliegenthart, R. The Dream of a One-Stop-Shop: Meta-Analysis on Myocardial Perfusion CT. Eur. J. Radiol. 2015, 84, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Katikireddy, C.K.; Samim, A. Myocardial Viability Assessment and Utility in Contemporary Management of Ischemic Cardiomyopathy. Clin. Cardiol. 2022, 45, 152–161. [Google Scholar] [CrossRef]
- Yang, J.; Dou, G.; He, B.; Jin, Q.; Chen, Z.; Jing, J.; Di Carli, M.F.; Chen, Y.; Blankstein, R. Stress Myocardial Blood Flow Ratio by Dynamic CT Perfusion Identifies Hemodynamically Significant CAD. JACC Cardiovasc. Imaging 2020, 13, 966–976. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Angelopoulos, A.; Tsioufis, K.; Antoniades, C.; Tousoulis, D. Cardiovascular Risk Stratification by Coronary Computed Tomography Angiography Imaging: Current State-of-the-Art. Eur. J. Prev. Cardiol. 2022, 29, 608–624. [Google Scholar] [CrossRef]
- Ferencik, M.; Mayrhofer, T.; Bittner, D.O.; Emami, H.; Puchner, S.B.; Lu, M.T.; Meyersohn, N.M.; Ivanov, A.V.; Adami, E.C.; Patel, M.R.; et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients with Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 144–152. [Google Scholar] [CrossRef]
- Schicchi, N.; Fogante, M.; Palumbo, P.; Agliata, G.; Esposto Pirani, P.; Di Cesare, E.; Giovagnoni, A. The Sub-Millisievert Era in CTCA: The Technical Basis of the New Radiation Dose Approach. Radiol. Med. 2020, 125, 1024–1039. [Google Scholar] [CrossRef]
- Schicchi, N.; Fogante, M.; Esposto Pirani, P.; Agliata, G.; Basile, M.C.; Oliva, M.; Agostini, A.; Giovagnoni, A. Third-Generation Dual-Source Dual-Energy CT in Pediatric Congenital Heart Disease Patients: State-of-the-Art. Radiol. Med. 2019, 124, 1238–1252. [Google Scholar] [CrossRef]
- Fogante, M.; Esposto Pirani, P.; Cela, F.; Balardi, L.; Piva, T.; Argalia, G.; Schicchi, N. Ultra-Low Radiation Dose and Contrast Volume CT Protocol and TAVI-CT Score for TAVI Planning and Outcome. Br. J. Radiol. 2023, 96, 20221026. [Google Scholar] [CrossRef]
- Agliata, G.; Schicchi, N.; Agostini, A.; Fogante, M.; Mari, A.; Maggi, S.; Giovagnoni, A. Radiation Exposure Related to Cardiovascular CT Examination: Comparison between Conventional 64-MDCT and Third-Generation Dual-Source MDCT. Radiol. Med. 2019, 124, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Fogante, M.; Agliata, G.; Basile, M.C.; Compagnucci, P.; Volpato, G.; Falanga, U.; Stronati, G.; Guerra, F.; Vignale, D.; Esposito, A.; et al. Cardiac Imaging in Athlete’s Heart: The Role of the Radiologist. Medicina 2021, 7, 455. [Google Scholar] [CrossRef] [PubMed]
- Lecchi, M.; Malaspina, S.; Scabbio, C.; Gaudieri, V.; Del Sole, A. Myocardial Perfusion Scintigraphy Dosimetry: Optimal Use of SPECT and SPECT/CT Technologies in Stress-First Imaging Protocol. Clin. Transl. Imaging 2016, 4, 491–498. [Google Scholar] [CrossRef]
- Liu, K.; Diao, K.; Hu, S.; Xu, X.; Zhang, J.; Peng, W.; Xia, C.; Zhang, K.; Li, Y.; Guo, Y.; et al. Achieving Low Radiation Dose in “One-Stop” Myocardial Computed Tomography Perfusion Imaging in Coronary Artery Disease Using 16-cm Wide Detector CT. Acad. Radiol. 2020, 27, 1531–1539. [Google Scholar] [CrossRef]
- van Assen, M.; Duguay, T.M.; Litwin, S.E.; Bayer, R.R.; Nance, J.W.; Suranyi, P.; De Cecco, C.N.; Varga-Szemes, A.; Jacobs, B.E.; Johnson, A.A.; et al. The Feasibility, Tolerability, Safety, and Accuracy of Low-Radiation Dynamic Computed Tomography Myocardial Perfusion Imaging with Regadenoson Compared with Single-Photon Emission Computed Tomography. J. Thorac. Imaging 2021, 36, 345–352. [Google Scholar] [CrossRef]
- Ma, H.; Dai, X.; Yang, X.; Zhao, X.; Wang, R.; Zhang, J. Clinical and Imaging Predictors of Impaired Myocardial Perfusion in Symptomatic Patients after Percutaneous Coronary Intervention: Insights from Dynamic CT Myocardial Perfusion Imaging. Quant. Imaging Med. Surg. 2021, 11, 3327–3337. [Google Scholar] [CrossRef]
- Raja, J.; Seitz, M.P.; Yedlapati, N.; Khouzam, R.N. Can Computed Fractional Flow Reserve Coronary CT Angiography (FFRCT) Offer an Accurate Noninvasive Comparison to Invasive Coronary Angiography (ICA)? “The Noninvasive CATH.” A Comprehensive Review. Curr. Probl. Cardiol. 2021, 46, 100642. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Alabed, S.; Hoole, S.P.; Abraham, G.; Weir-McCall, J.R. Prognostic Value of Stress Perfusion Cardiac MRI in Cardiovascular Disease: A Systematic Review and Meta-Analysis of the Effects of the Scanner, Stress Agent, and Analysis Technique. Radiol. Cardiothorac. Imaging 2024, 6, e230382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Picano, E.; Vano, E. Updated Estimates of Radiation Risk for Cancer and Cardiovascular Disease: Implications for Cardiology Practice. J. Clin. Med. 2024, 2, 2066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Picano, E.; Vano, E.; Gale, R.P.; Serruys, P. Cardiac radiation exposure and incident cancer: Challenges and opportunities. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Gargani, L.; Picano, E. The risk of cumulative radiation exposure in chest imaging and the advantage of bedside ultrasound. Crit. Ultrasound J. 2015, 7, 4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith-Bindman, R.; Chu, P.W.; Azman Firdaus, H.; Stewart, C.; Malekhedayat, M.; Alber, S.; Bolch, W.E.; Mahendra, M.; Berrington de González, A.; Miglioretti, D.L. Projected Lifetime Cancer Risks from Current Computed Tomography Imaging. JAMA Intern. Med. 2025, 185, 710–719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Picano, E.; Vañó, E.; Rehani, M.M.; Cuocolo, A.; Mont, L.; Bodi, V.; Bar, O.; Maccia, C.; Pierard, L.; Sicari, R.; et al. The appropriate and justified use of medical radiation in cardiovascular imaging: A position document of the ESC Associations of Cardiovascular Imaging, Percutaneous Cardiovascular Interventions and Electrophysiology. Eur. Heart J. 2014, 35, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, M.; De Mauri, A.; Leva, L.; Carriero, A.; Picano, E. Cumulative radiation dose from medical imaging in chronic adult patients. Am. J. Med. 2013, 126, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Vano, E. The radiation issue in cardiology: The time for action is now. Cardiovasc. Ultrasound 2011, 9, 35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Picano, E. The risks of inappropriateness in cardiac imaging. Int. J. Environ. Res. Public Health 2009, 6, 1649–1664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Einstein, A.J.; Henzlova, M.J.; Rajagopalan, S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007, 298, 317–323. [Google Scholar] [CrossRef] [PubMed]

| First Acquisition CCTA | Second Acquisition Dynamic CTMPI | |
|---|---|---|
| ECG-protocol | Prospective ECG-triggering set at 35% of the RR interval (HR ≤ 65 bpm) Prospective ECG-gating set from 40% to 60% of the RR interval (HR > 65 bmp) | Prospective shuttle technique acquisition |
| Scan range | Carina–cardiac apex | Carina–cardiac apex |
| Pitch | 3.2 | 3.2 |
| Tube voltage | Automatic modulation (CARE kV, Siemens) | 70 kV |
| Tube current | Automatic modulation (CAREDose4D, Siemens) | Automatic modulation (CAREDose4D, Siemens) |
| Rotation time | 0.25 | 0.25 |
| Temporal resolution | 66 ms | 66 ms |
| Collimation | 2 × 192 × 0.6 mm | 2 × 192 × 0.6 mm |
| Section thickness/increment | 0.6 mm/0.6 mm | 3 mm/2 mm |
| Reconstruction kernel | Body-vascular 40–Body-vascular 44 | Body-vascular 40 |
| Iterative reconstruction algorithm | ADMIRE (Siemens)—strength 4 | ADMIRE (Siemens)—strength 4 |
| Sample (n) | 65 |
| Age (years, mean ± SD) | 51.2 ± 11.5 |
| Male (n, %)—Female (n, %) | 44 (67.7)—21 (32.3) |
| Weight (mean ± SD) | 64.8 ± 9.8 |
| Height (mean ± SD) | 170.1 ± 13.2 |
| Risk factors | |
| Hyperlipidemia (n, %) | 28 (43.1) |
| Hypertension (n, %) | 40 (61.5) |
| Smoking (n, %) | 19 (29.2) |
| Diabetes (n, %) | 16 (24.6) |
| Clinical presentations | |
| Stable angina (n, %) | 38 (58.5) |
| Atypical chest pain (n, %) | 24 (36.9) |
| ECG abnormalities suggestive for possible ischemia (n, %) | 22 (33.8) |
| CAC score | |
| Low (0–99 Agatston units) | 26 (40.0%) |
| Moderate (100–399 Agatston units) | 23 (35.4%) |
| Severe (≥400 Agatston units) | 16 (24.6%) |
| Coronary artery stenosis distribution | |
| Single-vessel stenosis | 42 (64.6%) |
| Two-vessel stenosis | 18 (27.7%) |
| Three-vessel stenosis | 5 (7.7%) |
| Coronary artery stenosis | |
| LAD (n, %) | 52 (55.9%) |
| CX (n, %) | 17 (18.3%) |
| RCA (n, %) | 24 (25.8%) |
| CAD-RADS | |
| Plaque burden (P1, P2, P3, P4) | 15 (23.1%), 18 (27.8%), 22 (33.%), 10 (15.4%) |
| High-risk plaque (n, %) | 25 (38.5%) |
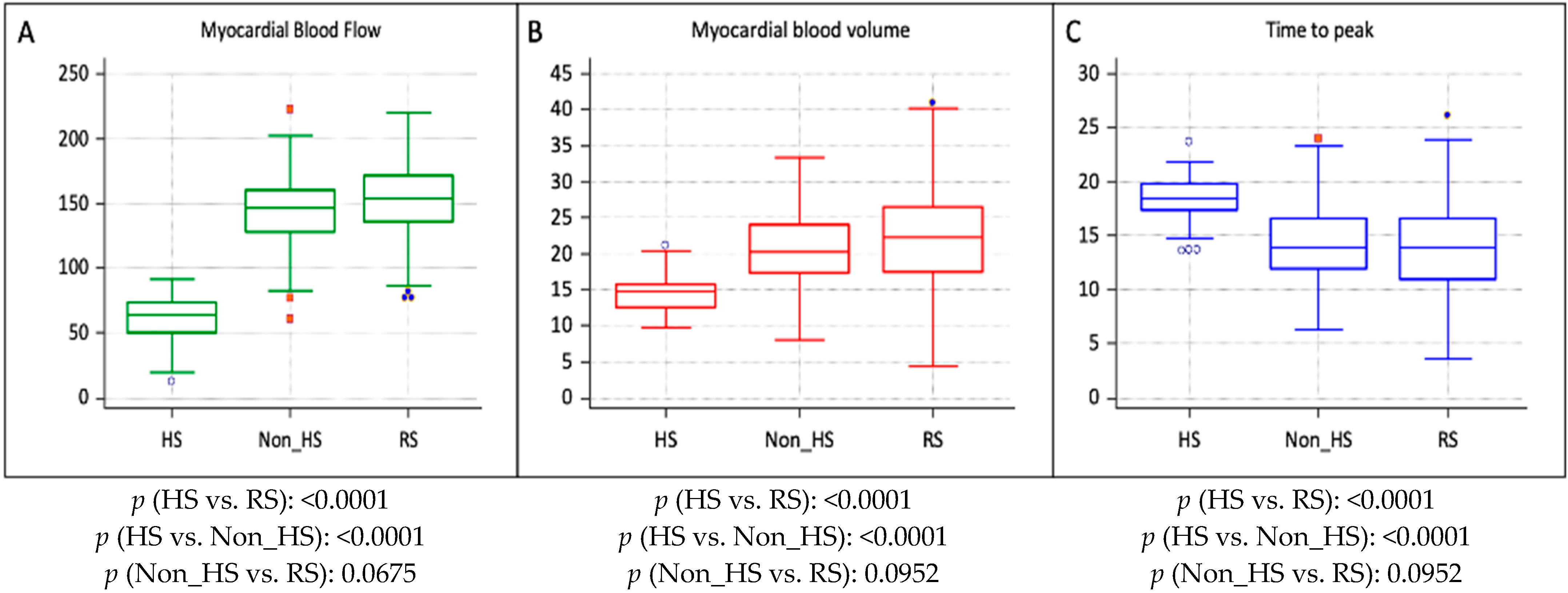
| Ratios | Hypoperfused CAD Segments (n = 62) | Non-Hypoperfused CAD Segments (n = 208) | p |
|---|---|---|---|
| MBFCADsegments/MBFRS | 0.50 ± 0.08 | 0.95 ± 0.05 | <0.0001 |
| MBVCADsegments/MBVRS | 0.65 ± 0.13 | 0.93 ± 0.11 | <0.0001 |
| TTPCADsegments/TTPRS | 1.10 ± 0.22 | 1.05 ± 0.11 | 0.1150 |
| Parameters | HS (n = 62) | Non_HS (n = 208) | RS (n = 770) | p (HS vs. RS) | p (HS vs. Non_HS) | p (Non_HS vs. RS) |
|---|---|---|---|---|---|---|
| MBF (mL/100 mL/min) | 65.1 ± 19.8 | 147.5 ± 28.4 | 155.2 ± 24.1 | <0.0001 | <0.0001 | 0.0675 |
| MBV (mL/100 mL) | 14.5 ± 2.7 | 21.1 ± 5.9 | 22.0 ± 6.9 | <0.0001 | <0.0001 | 0.0952 |
| TTP (s) | 18.5 ± 3.5 | 13.7 ± 3.3 | 14.0 ± 3.6 | 0.0012 | 0.0014 | 0.0870 |
| Modality | Effective Dose (mSv) | Chest X-Rays Equivalent | Cancer Risk Estimate (BEIR VII Phase 2 Model: 0.01%/mSv) | Cumulative Risk Over 10 Years | Estimated Cost (USD) |
|---|---|---|---|---|---|
| Cardiac CT | 4–8 | 200–400 | 0.04–0.08% (1 in 2500–1250) | ~0.1% (2 studies) | $400–$500 |
| Cardiac CT + CT Perfusion | 6–10 | 300–500 | 0.06–0.1% (1 in 1667–1000) | ~0.2% (2 scans) | $700–$800 |
| SPECT (Sestamibi) | 9–10 | 450–500 | 0.09–0.1% (1 in 1111–1000) | ~0.2% (2 scans) | $1000–$1500 |
| PET | 8–9 | 400–450 | 0.08–0.09% (1 in 2500–1111) | ~0.1% (2 scans) | $2000–$3000 |
| Cardiac MRI | 0 | 0 | 0% | 0% | $700–$900 |
| Stress Echo (TTE) | 0 | 0 | 0% | 0% | $300–$600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fogante, M.; Paolini, E.; Cela, F.; Esposto Pirani, P.; Balardi, L.; Perna, G.P.; Schicchi, N. Feasibility, Added Value, and Radiation Dose of Combined Coronary CT Angiography and Stress Dynamic CT Myocardial Perfusion Imaging in Moderate Coronary Artery Disease: A Real-World Study. J. Cardiovasc. Dev. Dis. 2025, 12, 241. https://doi.org/10.3390/jcdd12070241
Fogante M, Paolini E, Cela F, Esposto Pirani P, Balardi L, Perna GP, Schicchi N. Feasibility, Added Value, and Radiation Dose of Combined Coronary CT Angiography and Stress Dynamic CT Myocardial Perfusion Imaging in Moderate Coronary Artery Disease: A Real-World Study. Journal of Cardiovascular Development and Disease. 2025; 12(7):241. https://doi.org/10.3390/jcdd12070241
Chicago/Turabian StyleFogante, Marco, Enrico Paolini, Fatjon Cela, Paolo Esposto Pirani, Liliana Balardi, Gian Piero Perna, and Nicolò Schicchi. 2025. "Feasibility, Added Value, and Radiation Dose of Combined Coronary CT Angiography and Stress Dynamic CT Myocardial Perfusion Imaging in Moderate Coronary Artery Disease: A Real-World Study" Journal of Cardiovascular Development and Disease 12, no. 7: 241. https://doi.org/10.3390/jcdd12070241
APA StyleFogante, M., Paolini, E., Cela, F., Esposto Pirani, P., Balardi, L., Perna, G. P., & Schicchi, N. (2025). Feasibility, Added Value, and Radiation Dose of Combined Coronary CT Angiography and Stress Dynamic CT Myocardial Perfusion Imaging in Moderate Coronary Artery Disease: A Real-World Study. Journal of Cardiovascular Development and Disease, 12(7), 241. https://doi.org/10.3390/jcdd12070241






