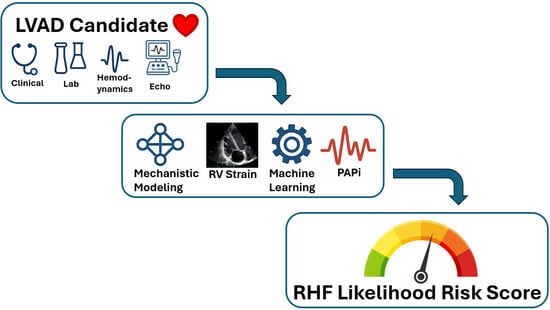
Prediction of Right Heart Failure in LVAD Candidates: Current Approaches and Future Directions
Abstract
1. Introduction
2. LVADs and Post-LVAD Right Heart Failure
2.1. Left Ventricular Assist Devices (LVADs)
2.2. Definitions of Right Heart Failure
2.3. Limitations of Comparing Models
2.4. Clinical Impact of Accurate RHF Prediction in LVAD Patient Care
3. The Right Ventricle Anatomy and Pathophysiology
3.1. Anatomy of the Right Ventricle
3.2. Pathophysiology of Post-LVAD Right Heart Failure
3.3. Risk Factors That Correlate with RHF
4. Predictive Models of Post-LVAD Right Heart Failure
4.1. Majority PF Patient Population Models
4.2. Bridge Models–The CRITT and Kormos et al. Scores
4.3. Majority CF Patient Population Models
5. Modern Approaches to Post-LVAD Right Heart Failure Prediction
5.1. Imaging-Based Parameters
5.1.1. Longitudinal Strain
5.1.2. Fractional Area Change (FAC)
5.1.3. Cardiac MRI and 3-D Echocardiography
5.2. Hemodynamic Parameters
Pulmonary Artery Pulsatile Index (PAPi)
5.3. Bayesian and Machine Learning (ML) Models
5.4. Mechanistic Modeling
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| RHF | Right heart failure |
| LVAD | Left ventricular assist device |
| PF | Pulsatile-flow |
| CF | Continuous-flow |
| LV | Left ventricle |
| RV | Right ventricle |
| BNP | Brain natriuretic peptide |
| ECM | Extracellular matrix |
| RVSWI | Right ventricular stroke work index |
| AUC | Area under the curve |
| RVAD | Right ventricular assist device |
| HR | Heart rate |
| BUN | Blood Urea Nitrogen |
| WBC | White blood count |
| CI | Cardiac index |
| TR | Tricuspid regurgitation |
| GLS | Global longitudinal strain |
| fwLS | Free-wall longitudinal strain |
| FAC | Fractional area change |
| PAPi | Pulmonary artery pulsatile index |
| Post-NTP | Post-sodium nitroprusside administration |
| iNO | Inhaled nitric oxide |
| PASP | Pulmonary artery systolic pressure |
| S’ | Peak systolic velocity of the RV free wall at the tricuspid annulus |
| E | Peak early trans-tricuspid filling velocity |
| E’ | Early diastolic velocity of the RV free wall at the tricuspid annulus |
| PS-fwLS | Peak systolic free-wall longitudinal strain |
| Hb | Hemoglobin |
| PCT | Procalcitonin |
| RVSP | Right ventricular systolic pressure |
| Pre-CVP | Central venous pressure intraoperative before insertion of the heart–lung machine’s cannula |
| PCWP | Pulmonary capillary wedge pressure |
| MRI | Magnetic resonance imaging |
| 3-D | Three dimensional |
| RVEF | Right ventricular ejection fraction |
| RA/PCWP | Right atrial pressure to pulmonary capillary wedge pressure ratio |
| ML | Machine learning |
| LV-ESA | Left ventricular end-systolic area |
| RV-ESA | Right ventricular end-diastolic area |
| TAPSE | Tricuspid annular plane systolic excursion |
| MCS | Mechanical circulatory support |
| PSCOPE | Physiology simulation coupled experiment |
References
- Bozkurt, B.; Ahmad, T.; Alexander, K.M.; Baker, W.L.; Bosak, K.; Breathett, K.; Fonarow, G.C.; Heidenreich, P.; Ho, J.E.; Hsich, E.; et al. Heart Failure Epidemiology and Outcomes Statistics: A Report of the Heart Failure Society of America. J. Card. Fail. 2023, 29, 1412–1451. [Google Scholar] [CrossRef]
- 2024 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 2020–2024. Department of Health and Human Services. Available online: https://www.srtr.org/reports/optnsrtr-annual-data-report/ (accessed on 18 May 2025).
- Jorde, U.P.; Saeed, O.; Koehl, D.; Morris, A.A.; Wood, K.L.; Meyer, D.M.; Cantor, R.; Jacobs, J.P.; Kirklin, J.K.; Pagani, F.D.; et al. The Society of Thoracic Surgeons Intermacs 2023 Annual Report: Focus on Magnetically Levitated Devices. Ann. Thorac. Surg. 2024, 117, 33–44. [Google Scholar] [CrossRef]
- Prinzing, A.; Herold, U.; Berkefeld, A.; Krane, M.; Lange, R.; Voss, B. Left Ventricular Assist Devices—Current State and Perspectives. J. Thorac. Dis. 2016, 8, E660–E666. [Google Scholar] [CrossRef] [PubMed]
- Eisen, H.J. Left Ventricular Assist Devices (LVADS): History, Clinical Application and Complications. Korean Circ. J. 2019, 49, 568–585. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Kovacevic, M.; Aung Kyaw, P.; Anderson, R.; Verma, R.; Provos, J.; Brandmire, A.; Iudice, J. CARD14: Wireless Transcutaneous Energy Transfer and Control System for Powering a Fully-Implanted Left-Ventricular Assist System. ASAIO J. 2023, 69, 64. [Google Scholar] [CrossRef]
- Kato, T.S.; Chokshi, A.; Singh, P.; Khawaja, T.; Cheema, F.; Akashi, H.; Shahzad, K.; Iwata, S.; Homma, S.; Takayama, H.; et al. Effects of Continuous-Flow Versus Pulsatile-Flow Left Ventricular Assist Devices on Myocardial Unloading and Remodeling. Circ. Heart Fail. 2011, 4, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, C.R.; Giridharan, G.A.; Litwak, K.N.; Sobieski, M.; Prabhu, S.D.; Slaughter, M.S.; Koenig, S.C. Hemodynamic Responses to Continuous versus Pulsatile Mechanical Unloading of the Failing Left Ventricle. ASAIO J. 2010, 56, 410. [Google Scholar] [CrossRef]
- Garcia, S.; Kandar, F.; Boyle, A.; Colvin-Adams, M.; Lliao, K.; Joyce, L.; John, R. Effects of Pulsatile- and Continuous-Flow Left Ventricular Assist Devices on Left Ventricular Unloading. J. Heart Lung Transplant. 2008, 27, 261–267. [Google Scholar] [CrossRef]
- Drakos, S.G.; Janicki, L.; Horne, B.D.; Kfoury, A.G.; Reid, B.B.; Clayson, S.; Horton, K.; Haddad, F.; Li, D.Y.; Renlund, D.G.; et al. Risk Factors Predictive of Right Ventricular Failure After Left Ventricular Assist Device Implantation. Am. J. Cardiol. 2010, 105, 1030–1035. [Google Scholar] [CrossRef]
- Matthews, J.C.; Koelling, T.M.; Pagani, F.D.; Aaronson, K.D. The Right Ventricular Failure Risk Score. J. Am. Coll. Cardiol. 2008, 51, 2163–2172. [Google Scholar] [CrossRef]
- Fitzpatrick, J.R.; Frederick, J.R.; Hsu, V.M.; Kozin, E.D.; O’Hara, M.L.; Howell, E.; Dougherty, D.; McCormick, R.C.; Laporte, C.M.; Cohen, J.E.; et al. A Risk Score Derived from Preoperative Data Analysis Predicts the Need for Biventricular Mechanical Circulatory Support. J. Heart Lung Transpl. 2008, 27, 1286–1292. [Google Scholar] [CrossRef]
- Atluri, P.; Goldstone, A.B.; Fairman, A.S.; MacArthur, J.W.; Shudo, Y.; Cohen, J.E.; Acker, A.L.; Hiesinger, W.; Howard, J.L.; Acker, M.A.; et al. Predicting Right Ventricular Failure in the Modern, Continuous Flow Left Ventricular Assist Device Era. Ann. Thorac. Surg. 2013, 96, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Kormos, R.L.; Teuteberg, J.J.; Pagani, F.D.; Russell, S.D.; John, R.; Miller, L.W.; Massey, T.; Milano, C.A.; Moazami, N.; Sundareswaran, K.S.; et al. Right Ventricular Failure in Patients with the HeartMate II Continuous-Flow Left Ventricular Assist Device: Incidence, Risk Factors, and Effect on Outcomes. J. Thorac. Cardiovasc. Surg. 2010, 139, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar] [CrossRef]
- Hall, S.A.; Copeland, H.; Alam, A.; Joseph, S.M. The “Right” Definition for Post–Left Ventricular Assist Device Right Heart Failure: The More We Learn, the Less We Know. Front. Cardiovasc. Med. 2022, 9, 893327. [Google Scholar] [CrossRef]
- Sciaccaluga, C.; Procopio, M.C.; Potena, L.; Masetti, M.; Bernazzali, S.; Maccherini, M.; Landra, F.; Righini, F.M.; Cameli, M.; Valente, S. Right Ventricular Dysfunction in Left Ventricular Assist Device Candidates: Is It Time to Change Our Prospective? Heart Fail. Rev. 2024, 29, 559–569. [Google Scholar] [CrossRef]
- Maitz, T.; Shah, S.; Gupta, R.; Goel, A.; Sreenivasan, J.; Hajra, A.; Vyas, A.V.; Lavie, C.J.; Hawwa, N.; Lanier, G.M.; et al. Pathophysiology, Diagnosis and Management of Right Ventricular Failure: A State of the Art Review of Mechanical Support Devices. Prog. Cardiovasc. Dis. 2024, 85, 103–113. [Google Scholar] [CrossRef]
- Takeda, K.; Naka, Y.; Yang, J.A.; Uriel, N.; Colombo, P.C.; Jorde, U.P.; Takayama, H. Timing of Temporary Right Ventricular Assist Device Insertion for Severe Right Heart Failure After Left Ventricular Assist Device Implantation. ASAIO J. 2013, 59, 564. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Mork, C.; Gahl, B.; Appenzeller-Herzog, C.; von Segesser, L.K.; Eckstein, F.; Berdajs, D.A. Outcome of Right Ventricular Assist Device Implantation Following Left Ventricular Assist Device Implantation: Systematic Review and Meta-Analysis. Perfusion 2022, 37, 773–784. [Google Scholar] [CrossRef]
- Wang, J.M.H.; Rai, R.; Carrasco, M.; Sam-Odusina, T.; Salandy, S.; Gielecki, J.; Zurada, A.; Loukas, M. An Anatomical Review of the Right Ventricle. Transl. Res. Anat. 2019, 17, 100049. [Google Scholar] [CrossRef]
- Fan, L.; Choy, J.S.; Lee, S.; Campbell, K.S.; Wenk, J.F.; Kassab, G.S.; Burkhoff, D.; Lee, L.C. An in Silico Study of the Effects of Left Ventricular Assist Device on Right Ventricular Function and Inter-Ventricular Interaction. Artif. Organs 2023, 47, 1831–1847. [Google Scholar] [CrossRef]
- Maughan, W.L.; Sunagawa, K.; Sagawa, K. Ventricular Systolic Interdependence: Volume Elastance Model in Isolated Canine Hearts. Am. J. Physiol.-Heart Circ. Physiol. 1987, 253, H1381–H1390. [Google Scholar] [CrossRef]
- Taylor, R.; Covell, J.; Sonnenblick, E.; Ross, J. Dependence of Ventricular Distensibility on Filling of the Opposite Ventricle. Am. J. Physiol.-Leg. Content. 1967, 213, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, M.K. Imaging Right-Left Ventricular Interactions. JACC Cardiovasc. Imaging 2018, 11, 755–771. [Google Scholar] [CrossRef]
- Belenkie, I.; Dani, R.; Smith, E.R.; Tyberg, J.V. The Importance of Pericardial Constraint in Experimental Pulmonary Embolism and Volume Loading. Am. Heart J. 1992, 123, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Hahn, R.; Rodríguez-Zanella, H.; Araiza Garaygordobil, D.; Ochoa-Jimenez, R.C.; Muraru, D. Morphological Assessment of the Tricuspid Apparatus and Grading Regurgitation Severity in Patients with Functional Tricuspid Regurgitation: Thinking Outside the Box. JACC Cardiovasc. Imaging 2019, 12, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Bravo, C.A.; Navarro, A.G.; Dhaliwal, K.K.; Khorsandi, M.; Keenan, J.E.; Mudigonda, P.; O’Brien, K.D.; Mahr, C. Right Heart Failure after Left Ventricular Assist Device: From Mechanisms to Treatments. Front. Cardiovasc. Med. 2022, 9, 1023549. [Google Scholar] [CrossRef]
- Nemoto, N.; Lesser, J.R.; Pedersen, W.R.; Sorajja, P.; Spinner, E.; Garberich, R.F.; Vock, D.M.; Schwartz, R.S. Pathogenic Structural Heart Changes in Early Tricuspid Regurgitation. J. Thorac. Cardiovasc. Surg. 2015, 150, 323–330. [Google Scholar] [CrossRef]
- Spinner, E.M.; Shannon, P.; Buice, D.; Jimenez, J.H.; Veledar, E.; Del Nido, P.J.; Adams, D.H.; Yoganathan, A.P. In Vitro Characterization of the Mechanisms Responsible for Functional Tricuspid Regurgitation. Circulation 2011, 124, 920–929. [Google Scholar] [CrossRef]
- Burger, W.; Straube, M.; Behne, M.; Sarai, K.; Beyersdorf, F.; Eckel, L.; Dereser, A.; Satter, P.; Kaltenbach, M. Role of Pericardial Constraint for Right Ventricular Function in Humans. Chest 1995, 107, 46–49. [Google Scholar] [CrossRef]
- Zanobini, M.; Loardi, C.; Poggio, P.; Tamborini, G.; Veglia, F.; Di Minno, A.; Myasoedova, V.; Mammana, L.F.; Biondi, R.; Pepi, M.; et al. The Impact of Pericardial Approach and Myocardial Protection onto Postoperative Right Ventricle Function Reduction. J. Cardiothorac. Surg. 2018, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Bowen, D.J.; Strachinaru, M.; Caliskan, K. ‘Pseudo’-D-Shaped Septum Post-Left Ventricular Assist Device Implantation. Eur Heart J. Case Rep. 2020, 4, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Mouratoglou, S.A.; Kallifatidis, A.; Pitsiou, G.; Grosomanidis, V.; Kamperidis, V.; Chalikias, G.; Kristo, D.; Tziakas, D.; Konstantinides, S.; Hadjimiltiades, S.; et al. Duration of Interventricular Septal Shift toward the Left Ventricle Is Associated with Poor Clinical Outcome in Precapillary Pulmonary Hypertension: A Cardiac Magnetic Resonance Study. Hell. J. Cardiol. 2020, 61, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Rajapreyar, I.; Soliman, O.; Brailovsky, Y.; Tedford, R.J.; Gibson, G.; Mohacsi, P.; Hajduczok, A.G.; Tchantchaleishvili, V.; Wieselthaler, G.; Rame, J.E.; et al. Late Right Heart Failure After Left Ventricular Assist Device Implantation. JACC Heart Fail. 2023, 11, 865–878. [Google Scholar] [CrossRef]
- Kormos, R.L.; Antonides, C.F.J.; Goldstein, D.J.; Cowger, J.A.; Starling, R.C.; Kirklin, J.K.; Rame, J.E.; Rosenthal, D.; Mooney, M.L.; Caliskan, K.; et al. Updated Definitions of Adverse Events for Trials and Registries of Mechanical Circulatory Support: A Consensus Statement of the Mechanical Circulatory Support Academic Research Consortium. J. Heart Lung Transplant. 2020, 39, 735–750. [Google Scholar] [CrossRef]
- Fukamachi, K.; McCarthy, P.M.; Smedira, N.G.; Vargo, R.L.; Starling, R.C.; Young, J.B. Preoperative Risk Factors for Right Ventricular Failure after Implantable Left Ventricular Assist Device Insertion. Ann. Thorac. Surg. 1999, 68, 2181–2184. [Google Scholar] [CrossRef]
- Nakatani, S.; Thomas, J.D.; Savage, R.M.; Vargo, R.L.; Smedira, N.G.; McCarthy, P.M. Prediction of Right Ventricular Dysfunction after Left Ventricular Assist Device Implantation. Circulation 1996, 94, II216–II221. [Google Scholar]
- Ochiai, Y.; McCarthy, P.M.; Smedira, N.G.; Banbury, M.K.; Navia, J.L.; Feng, J.; Hsu, A.P.; Yeager, M.L.; Buda, T.; Hoercher, K.J.; et al. Predictors of Severe Right Ventricular Failure After Implantable Left Ventricular Assist Device Insertion: Analysis of 245 Patients. Circulation 2002, 106, I198–I202. [Google Scholar] [CrossRef]
- Loforte, A.; Montalto, A.; Musumeci, F.; Amarelli, C.; Mariani, C.; Polizzi, V.; Lilla Della Monica, P.; Grigioni, F.; Di Bartolomeo, R.; Marinelli, G. Calculation of the ALMA Risk of Right Ventricular Failure After Left Ventricular Assist Device Implantation. ASAIO J. 2018, 64, e140. [Google Scholar] [CrossRef]
- Tchantchaleishvili, V.; Maltais, S.; Sharma, S.; Haglund, N.A.; Davis, M.E.; Cowger, J.; Shah, P.; Desai, S.S.; Aaronson, K.D.; Pagani, F.D.; et al. A Novel, Highly Discriminatory Risk Model Predicting Acute Severe Right Ventricular Failure in Patients Undergoing Continuous-Flow Left Ventricular Assist Device Implant. Artif. Organs 2019, 43, 624–632. [Google Scholar] [CrossRef]
- Soliman, O.I.I.; Akin, S.; Muslem, R.; Boersma, E.; Manintveld, O.C.; Krabatsch, T.; Gummert, J.F.; de By, T.M.M.H.; Bogers, A.J.J.C.; Zijlstra, F.; et al. Derivation and Validation of a Novel Right-Sided Heart Failure Model After Implantation of Continuous Flow Left Ventricular Assist Devices. Circulation 2018, 137, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, D.; Iacovoni, A.; Scardulla, C.; Moja, L.; Pilato, M.; Kushwaha, S.S.; Senni, M.; Clemenza, F.; Agnese, V.; Falletta, C.; et al. Prediction of Right Ventricular Failure after Ventricular Assist Device Implant: Systematic Review and Meta-Analysis of Observational Studies. Eur. J. Heart Fail. 2017, 19, 926–946. [Google Scholar] [CrossRef]
- Wang, Y.; Simon, M.; Bonde, P.; Harris, B.U.; Teuteberg, J.J.; Kormos, R.L.; Antaki, J.F. DECISION TREE FOR ADJUVANT RIGHT VENTRICULAR SUPPORT IN PATIENTS RECEIVING A LEFT VENTRICULAR ASSIST DEVICE. J. Heart Lung Transpl. 2012, 31, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Isaza, N.; Gonzalez, M.; Saijo, Y.; Vega Brizneda, M.; Estep, J.; Starling, R.C.; Albert, C.; Soltesz, E.; Tong, M.Z.-Y.; Smedira, N.; et al. Incremental Value of Global Longitudinal Strain to Michigan Risk Score and Pulmonary Artery Pulsatility Index in Predicting Right Ventricular Failure Following Left Ventricular Assist Devices. Heart Lung Circ. 2022, 31, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.D.M.; Smedira, N.G.; Starling, R.C.; Marwick, T.H. Independent and Incremental Role of Quantitative Right Ventricular Evaluation for the Prediction of Right Ventricular Failure After Left Ventricular Assist Device Implantation. J. Am. Coll. Cardiol. 2012, 60, 521–528. [Google Scholar] [CrossRef]
- Varshney, A.S.; DeFilippis, E.M.; Cowger, J.A.; Netuka, I.; Pinney, S.P.; Givertz, M.M. Trends and Outcomes of Left Ventricular Assist Device Therapy. J. Am. Coll. Cardiol. 2022, 79, 1092–1107. [Google Scholar] [CrossRef]
- Farr, M.; Mitchell, J.; Lippel, M.; Kato, T.S.; Jin, Z.; Ippolito, P.; Dove, L.; Jorde, U.P.; Takayama, H.; Emond, J.; et al. Combination of Liver Biopsy with MELD-XI Scores for Post-Transplant Outcome Prediction in Patients with Advanced Heart Failure and Suspected Liver Dysfunction. J. Heart Lung Transpl. 2015, 34, 873–882. [Google Scholar] [CrossRef]
- Power, L.C.; O’Grady, G.L.; Hornung, T.S.; Jefferies, C.; Gusso, S.; Hofman, P.L. Imaging the Heart to Detect Cardiomyopathy in Duchenne Muscular Dystrophy: A Review. Neuromuscul. Disord. 2018, 28, 717–730. [Google Scholar] [CrossRef]
- Kato, T.S.; Jiang, J.; Schulze, P.C.; Jorde, U.; Uriel, N.; Kitada, S.; Takayama, H.; Naka, Y.; Mancini, D.; Gillam, L.; et al. Serial Echocardiography Using Tissue Doppler and Speckle Tracking Imaging to Monitor Right Ventricular Failure Before and After Left Ventricular Assist Device Surgery. JACC Heart Fail. 2013, 1, 216–222. [Google Scholar] [CrossRef]
- Liang, L.W.; Jamil, A.; Mazurek, J.A.; Urgo, K.A.; Wald, J.; Birati, E.Y.; Han, Y. Right ventricular global longitudinal strain as a predictor of acute and early right heart failure post left ventricular assist device implantation. ASAIO J. 2022, 68, 333–339. [Google Scholar] [CrossRef]
- Boegershausen, N.; Zayat, R.; Aljalloud, A.; Musetti, G.; Goetzenich, A.; Tewarie, L.; Moza, A.; Amerini, A.; Autschbach, R.; Hatam, N. Risk Factors for the Development of Right Ventricular Failure after Left Ventricular Assist Device Implantation—a Single-Centre Retrospective with Focus on Deformation Imaging†. Eur. J. Cardio-Thorac. Surg. 2017, 52, 1069–1076. [Google Scholar] [CrossRef]
- Stricagnoli, M.; Sciaccaluga, C.; Mandoli, G.E.; Rizzo, L.; Sisti, N.; Aboumarie, H.S.; Benfari, G.; Maritan, L.; Tsioulpas, C.; Bernazzali, S.; et al. Clinical, Echocardiographic and Hemodynamic Predictors of Right Heart Failure after LVAD Placement. Int. J. Cardiovasc. Imaging 2022, 38, 561–570. [Google Scholar] [CrossRef]
- Cameli, M.; Lisi, M.; Righini, F.M.; Tsioulpas, C.; Bernazzali, S.; Maccherini, M.; Sani, G.; Ballo, P.; Galderisi, M.; Mondillo, S. Right Ventricular Longitudinal Strain Correlates Well with Right Ventricular Stroke Work Index in Patients with Advanced Heart Failure Referred for Heart Transplantation. J. Card. Fail. 2012, 18, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Lisi, M.; Righini, F.M.; Focardi, M.; Lunghetti, S.; Bernazzali, S.; Marchetti, L.; Biagioli, B.; Galderisi, M.; Maccherini, M.; et al. Speckle Tracking Echocardiography as a New Technique to Evaluate Right Ventricular Function in Patients with Left Ventricular Assist Device Therapy. J. Heart Lung Transplant. 2013, 32, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Cacioli, G.; Polizzi, V.; Ciabatti, M.; Cristiano, E.; Pergolini, A.; Distefano, G.; Lilla Della Monica, P.; Comisso, M.; Piazza, V.; Sbaraglia, F.; et al. Prediction of Right Ventricular Failure after Left Ventricular Assist Device Implantation: Role of Vasodilator Challenge. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.; Seetha Rammohan, H.R.; Gertz, Z.M.; Rame, J.E.; Woo, Y.J.; Kirkpatrick, J.N. Postoperative Right Ventricular Failure after Left Ventricular Assist Device Placement Is Predicted by Preoperative Echocardiographic Structural, Hemodynamic, and Functional Parameters. J. Card Fail. 2013, 19, 16–24. [Google Scholar] [CrossRef]
- Sugeng, L.; Mor-Avi, V.; Weinert, L.; Niel, J.; Ebner, C.; Steringer-Mascherbauer, R.; Bartolles, R.; Baumann, R.; Schummers, G.; Lang, R.M.; et al. Multimodality Comparison of Quantitative Volumetric Analysis of the Right Ventricle. JACC Cardiovasc. Imaging 2010, 3, 10–18. [Google Scholar] [CrossRef]
- Shimada, Y.J.; Shiota, M.; Siegel, R.J.; Shiota, T. Accuracy of Right Ventricular Volumes and Function Determined by Three-Dimensional Echocardiography in Comparison with Magnetic Resonance Imaging: A Meta-Analysis Study. J. Am. Soc. Echocardiogr. 2010, 23, 943–953. [Google Scholar] [CrossRef]
- Nagata, Y.; Wu, V.C.-C.; Kado, Y.; Otani, K.; Lin, F.-C.; Otsuji, Y.; Negishi, K.; Takeuchi, M. Prognostic Value of Right Ventricular Ejection Fraction Assessed by Transthoracic 3D Echocardiography. Circ. Cardiovasc. Imaging 2017, 10, e005384. [Google Scholar] [CrossRef]
- Kang, G.; Ha, R.; Banerjee, D. Pulmonary Artery Pulsatility Index Predicts Right Ventricular Failure after Left Ventricular Assist Device Implantation. J. Heart Lung Transplant. 2016, 35, 67–73. [Google Scholar] [CrossRef]
- Lim, H.S.; Gustafsson, F. Pulmonary Artery Pulsatility Index: Physiological Basis and Clinical Application. Eur. J. Heart Fail. 2020, 22, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Shad, R.; Quach, N.; Fong, R.; Kasinpila, P.; Bowles, C.; Castro, M.; Guha, A.; Suarez, E.E.; Jovinge, S.; Lee, S.; et al. Predicting Post-Operative Right Ventricular Failure Using Video-Based Deep Learning. Nat. Commun. 2021, 12, 5192. [Google Scholar] [CrossRef] [PubMed]
- Taleb, I.; Kyriakopoulos, C.P.; Fong, R.; Ijaz, N.; Demertzis, Z.; Sideris, K.; Wever-Pinzon, O.; Koliopoulou, A.G.; Bonios, M.J.; Shad, R.; et al. Machine Learning Multicenter Risk Model to Predict Right Ventricular Failure After Mechanical Circulatory Support. JAMA Cardiol. 2024, 9, 272–282. [Google Scholar] [CrossRef]
- Loghmanpour, N.A.; Kormos, R.L.; Kanwar, M.K.; Teuteberg, J.J.; Murali, S.; Antaki, J.F. A Bayesian Model to Predict Right Ventricular Failure Following Left Ventricular Assist Device Therapy. JACC Heart Fail. 2016, 4, 711–721. [Google Scholar] [CrossRef]
- Pirozzi, I.; Kight, A.; Aranda-Michael, E.; Shad, R.; Zhu, Y.; Waldman, L.K.; Hiesinger, W.; Cutkosky, M. Cardiac Support for the Right Ventricle: Effects of Timing on Hemodynamics-Biomechanics Tradeoff. In Proceedings of the Functional Imaging and Modeling of the Heart; Ennis, D.B., Perotti, L.E., Wang, V.Y., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 385–395. [Google Scholar]
- Yuan, V.; De Gaetano, F.; Osouli, K.; Marsden, A.L.; Costantino, M.L. Investigating the Hemodynamics of Berlin Heart EXCOR Support in Norwood Patients across Diverse Clinical Scenarios with Computational Modeling. Artif. Organs 2023, 47, 1133–1150. [Google Scholar] [CrossRef]
- Yuan, V.; Verma, A.; Schiavone, N.K.; Rosenthal, D.N.; Marsden, A.L. A Mechanistic Lumped Parameter Model of the Berlin Heart EXCOR to Analyze Device Performance and Physiologic Interactions. Cardiovasc. Eng. Technol. 2022, 13, 603–623. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Zafar, F.; Lorts, A.; Kung, E. Hemodynamic Response to Device Titration in the Shunted Single Ventricle Circulation: A Patient Cohort Modeling Study. ASAIO J. 2022, 68, 268. [Google Scholar] [CrossRef]
- Farahmand, M.; Kavarana, M.N.; Kung, E.O. Risks and Benefits of Using a Commercially Available Ventricular Assist Device for Failing Fontan Cavopulmonary Support: A Modeling Investigation. IEEE Trans. Biomed. Eng. 2020, 67, 213–219. [Google Scholar] [CrossRef]
- Farahmand, M.; Kavarana, M.N.; Trusty, P.M.; Kung, E.O. Target Flow-Pressure Operating Range for Designing a Failing Fontan Cavopulmonary Support Device. IEEE Trans. Biomed. Eng. 2020, 67, 2925–2933. [Google Scholar] [CrossRef]
- Kung, E.; Corsini, C.; Marsden, A.; Vignon-Clementel, I.; Pennati, G.; Figliola, R.; Hsia, T.-Y.; Taylor, A.; Khambadkone, S.; Schievano, S.; et al. Multiscale Modeling of Superior Cavopulmonary Circulation: Hemi-Fontan and Bidirectional Glenn Are Equivalent. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 883–892. [Google Scholar] [CrossRef]
- Corsini, C.; Baker, C.; Kung, E.; Schievano, S.; Arbia, G.; Baretta, A.; Biglino, G.; Migliavacca, F.; Dubini, G.; Pennati, G.; et al. An Integrated Approach to Patient-Specific Predictive Modeling for Single Ventricle Heart Palliation. Comput. Methods Biomech. Biomed. Eng. 2014, 17, 1572–1589. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Rosenthal, D.; Reinhartz, O.; Riemer, K.; He, F.; Hsia, T.-Y.; Marsden, A.; Kung, E. Superior Performance of Continuous over Pulsatile Flow Ventricular Assist Devices in the Single Ventricle Circulation: A Computational Study. J. Biomech. 2017, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kung, E.; Farahmand, M.; Gupta, A. A Hybrid Experimental-Computational Modeling Framework for Cardiovascular Device Testing. J. Biomech. Eng. 2019, 141. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, E.; Farahmand, M.; Kung, E. An Algorithm for Coupling Multibranch in Vitro Experiment to Numerical Physiology Simulation for a Hybrid Cardiovascular Model. Int. J. Numer. Methods Biomed. Eng. 2020, 36, e3289. [Google Scholar] [CrossRef]
- Umo, A.; Kung, E.O. A Protocol for Coupling Volumetrically Dynamic In Vitro Experiments to Numerical Physiology Simulation for A Hybrid Cardiovascular Model. IEEE Trans. Biomed. Eng. 2023, 70, 1351–1358. [Google Scholar] [CrossRef]
- Abraham, E.U.; Brett, A.W.; Kilic, A.; Ethan, O.K. Clinical Validation of the PSCOPE Hybrid Model Prediction of Left Ventricular Assist Device Implantation Hemodynamics: Three Patient-Specific Cases. Medrxiv 2025, 25323688. [Google Scholar] [CrossRef]
| Type of Finding | Cutoff |
|---|---|
| Ascites | Yes/No |
| Functionally limiting peripheral edema | >2+ |
| Elevated estimated jugular venous pressure at least halfway up the neck in an upright patient | Yes/No |
| Elevated measured central venous pressure or right atrial pressure | ≥16 mm Hg |
| Type of Finding | Cutoff |
|---|---|
| Renal failure with serum creatinine | >2 × baseline values |
| Liver injury with an elevation | 2 × upper limit normal in aspartate aminotransferase/alanine aminotransferase, or total bilirubin > 2.0 |
| SVO2 | <50%. |
| Cardiac index | <2.2 L/min/m2 |
| Reduction in pump flow | >30% from the previous baseline in the absence of mechanical causes |
| Parameter Modality | Parameter | Cutoff | Risk Score |
|---|---|---|---|
| Clinical | LVAD as DT | Yes/No | ALMA |
| MELD-XI | >17 | ALMA | |
| MII | Yes/No | EUROMACS | |
| INTERMACS | ≥3 | EUROMACS | |
| Hemodynamic | PAPi | <2 | ALMA |
| RVSWI | <300 mmHg/mL/m2 | ALMA | |
| HR | CoE2 | MCSRN | |
| CI | CoE2 | MCSRN | |
| RA/PCWP | ≥0.54 | EUROMACS | |
| Laboratory | Albumin | CoE2 | MCSRN |
| Creatinine WBC Hb | CoE2 CoE2 ≤10 g/dL | MCSRN MCSRN EUROMACS | |
| Echcardiographic | RV/LV ratio | >0.75 | ALMA |
| TR Severity | CoE2 | MCSRN | |
| RVD | Yes/No | EUROMACS |
| Study | Patient Population | Parameter | AUC |
|---|---|---|---|
| Isaza et al. [45] | 246 | PAPi, GLS, Michigan score | 0.87 |
| Liang et al. [51] | 55 | GLS | 0.85 |
| Cacioli et al. [56] | 54 | FAC, post-NTP PAPi, PASP | 0.949 |
| post-NTP PAPi | 0.75 | ||
| Kato et al. [50] | 24 | GLS, S′, RV E/E′ | 0.8475 |
| GLS | 0.745 | ||
| Grant et al. [46] | 117 | GLS, Michigan score | 0.77 |
| Boegerhausen et al. [52] | 46 | PS-fwLS, Hb, PCT, RVSP, Pre-CVP | 0.92 |
| PS-fwLS | 0.71 | ||
| Kang et al. [55] | 85 | PAPi | 0.77 |
| Stricagnoli et al. [53] | 38 | PAPi | 0.85 |
| fwLS | 0.93 |
| Study | Type of ML Architecture | Patient Population | AUC |
|---|---|---|---|
| STOP-RVF score [64] | Supervised machine learning using multiple imputations of chain equations imputed model coefficients | Derivation = 798 | AUC = 0.729 |
| Validation = 327 | |||
| Shad et al. [63] | Two-stream fusion 152-layer 3D residual network with bottlenecks incorporated within the residual block | Training = 467 | AUC = 0.749 |
| Testing = 121 | |||
| Validation = 135 | |||
| Loghmanpour et al. [65] | Tree-augmented naïve Bayesian architecture | Training = 9818Testing = 1091 | Acute RHF = 0.903 |
| Early RHF = 0.835 | |||
| Late RHF = 0.883 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogel, F.; Sollie, Z.W.; Kilic, A.; Kung, E. Prediction of Right Heart Failure in LVAD Candidates: Current Approaches and Future Directions. J. Cardiovasc. Dev. Dis. 2025, 12, 240. https://doi.org/10.3390/jcdd12070240
Vogel F, Sollie ZW, Kilic A, Kung E. Prediction of Right Heart Failure in LVAD Candidates: Current Approaches and Future Directions. Journal of Cardiovascular Development and Disease. 2025; 12(7):240. https://doi.org/10.3390/jcdd12070240
Chicago/Turabian StyleVogel, Frederick, Zachary W. Sollie, Arman Kilic, and Ethan Kung. 2025. "Prediction of Right Heart Failure in LVAD Candidates: Current Approaches and Future Directions" Journal of Cardiovascular Development and Disease 12, no. 7: 240. https://doi.org/10.3390/jcdd12070240
APA StyleVogel, F., Sollie, Z. W., Kilic, A., & Kung, E. (2025). Prediction of Right Heart Failure in LVAD Candidates: Current Approaches and Future Directions. Journal of Cardiovascular Development and Disease, 12(7), 240. https://doi.org/10.3390/jcdd12070240