An Analysis of Arterial Pulse Wave Time Features and Pulse Wave Velocity Calculations Based on Radial Electrical Bioimpedance Waveforms in Patients Scheduled for Coronary Catheterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection and Characteristics of Patients
2.2. Patient’s Characteristics
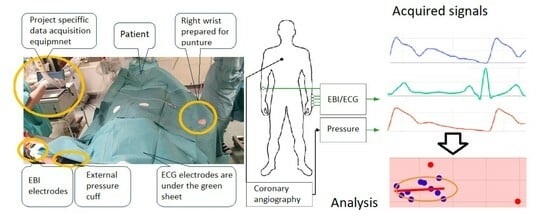
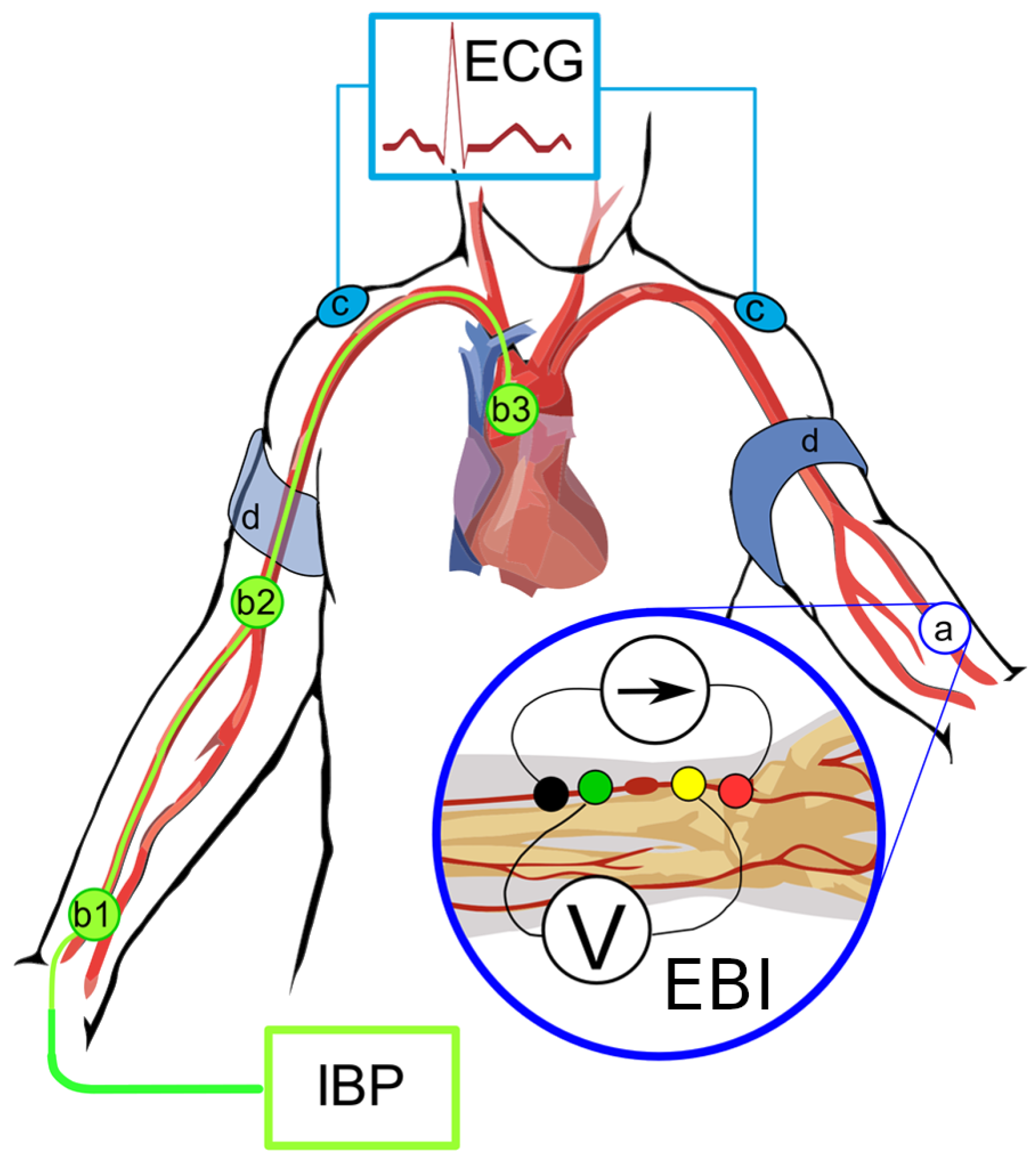
2.3. Coronary Catheterization and Measurement of the Invasive Hemodynamic Data and EBI
- 0:
- Normal coronaries;
- 1:
- Minimal (≤25)% decrease in vessel diameter;
- 2:
- Moderate (26–50)% decrease in vessel diameter;
- 3:
- Medium (51–75)% decrease in vessel diameter;
- 4:
- Severe (76–90)% decrease in vessel diameter;
- 5:
- Preocclusion (91–99)%;
- 6:
- Occlusion (100)%.
2.4. Data Analysis
- Normal coronaries;
- Minimal/moderate (≤50)% decrease in vessel diameter;
- Medium (51–75)% decrease in vessel diameter, stenoses that are non-revascularization objects, and 1–2 vessel disease;
- Significant (51–100)% decrease in vessel diameter, stenoses that are objects of revascularization, and three-vessel disease.
2.5. Signal Preprocessing
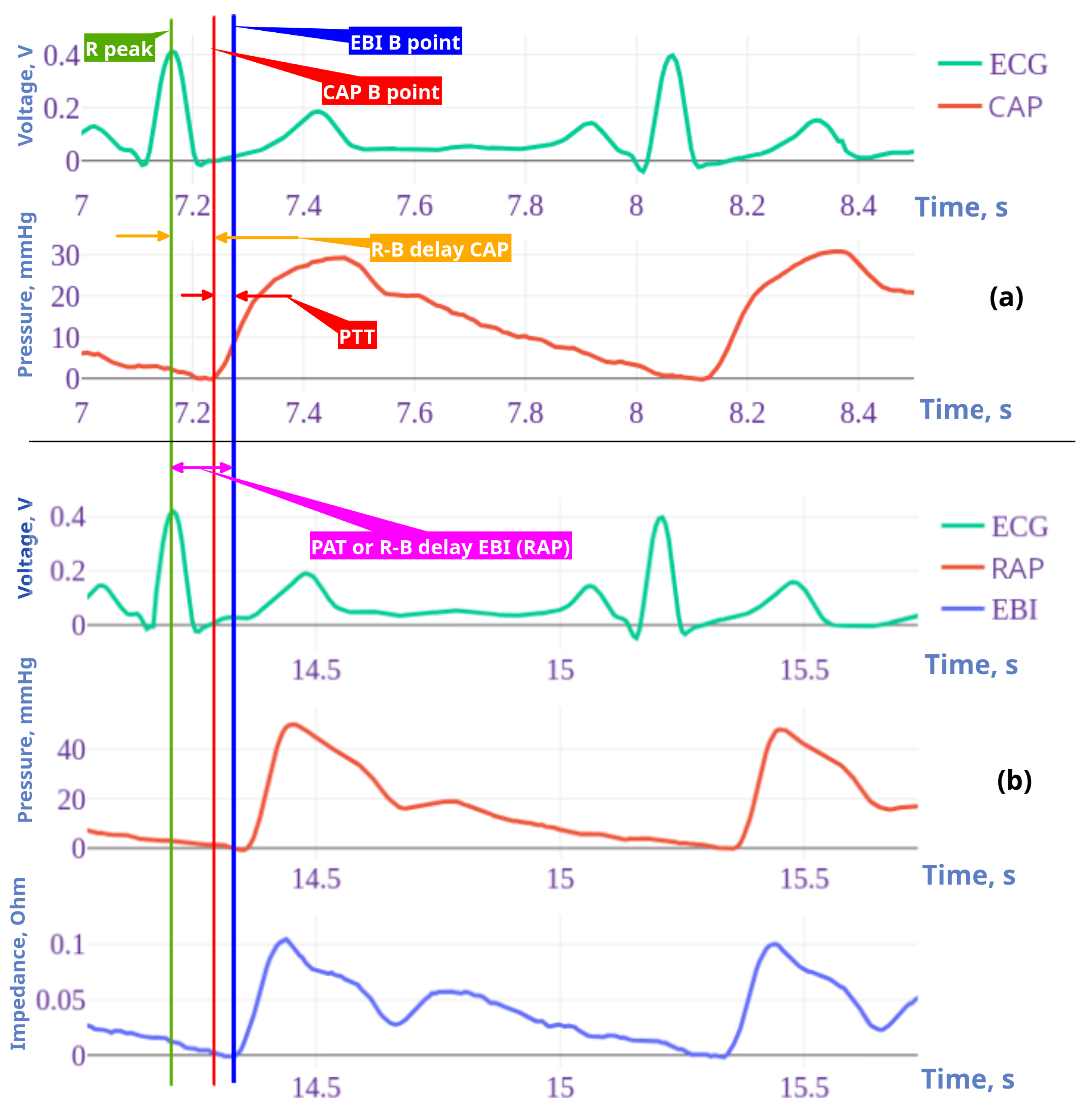
2.6. Fiducial Points Overview
2.6.1. EBI/RAP Fiducial Point Estimation
- B point:In cardiac impedance waveform analysis, the B point marks the beginning of the peripheral impedance curve initiated by cardiac systolic ejection. The B point detection algorithm uses a straightforward approach by identifying the first point of the signal. This initial point represents the onset of the arterial systolic curve before the rapid upstroke phase of the impedance curve. Unlike more complex feature detection methods, B point identification is based on temporal positioning rather than derivative analysis or peak detection.
- C point:The C point detection algorithm identifies the maximum slope in the cardiac impedance waveform, representing the rapid ventricular ejection phase. This critical point is mathematically determined by finding the maximum value of the first derivative using equation . The C point occurs as a result of ventricular systole, usually appearing between the B point (cycle onset) and the D point (peak impedance) and serves as a clinically significant marker for the assessment of contractility.
- D point:The D point detection algorithm identifies the peak amplitude in the cardiac EBI waveform, representing the maximum blood flow during ventricular ejection. This critical cardiac feature is computationally determined by finding the global maximum value within the signal.
- F point:The F point detection algorithm identifies a critical inflection point in the descending phase of the cardiac impedance waveform, marking the beginning of reduced ejection velocity after peak systolic function. This point is computationally determined by analyzing the third derivative of the impedance signal, specifically at the first transition from positive to negative slope after point D, as represented by the equation . Point F serves as a significant marker for the onset of the cardiac relaxation phase, located between the peak ejection (point D) and the beginning of diastole.
- G point:The G point detection algorithm identifies the onset of the dicrotic notch in the cardiac impedance waveform, marking the beginning of isovolumic relaxation. This inflection point is determined by analyzing the third derivative of the impedance signal, specifically finding the most significant negative excursion after the F point according to , where and is a limiting factor. The G point serves as a critical marker for the onset of the ventricular relaxation phase, positioned during the early descending limb of the impedance curve after peak ejection.
2.6.2. CAP Fiducial Point Estimation
- B point:This estimates the location of point B in the CAP waveform. Point B represents the onset of the systolic upstroke in the CAP signal, corresponding to the opening of the aortic valve and the beginning of ventricular ejection. This implementation simply identifies the first point in the signal as point B, which serves as a reference point for subsequent cardiac cycle analysis.
- C point:This estimates the C point in the CAP waveform, which represents the maximum rate of pressure increase during systole. The C point is identified as the maximum of the first derivative of the pressure signal, corresponding to the steepest ascending slope of the pressure curve. Physiologically, this point reflects the rapid ejection phase and provides information about ventricular–arterial coupling. The time of occurrence and the amplitude of the C point are important markers for assessing left ventricular contractility and arterial compliance.
- D point:This estimates the D point in the central aortic pressure (CAP) waveform, which represents the completion of the deceleration of the ventricular ejection. The method analyzes the third derivative of pressure to identify the first positive segment after point C, locating D in the first quarter of this segment. Mathematically, the location of the D point (time instance) is defined as follows: in the interval , where is the time at point C, and is the time at point F. This approach captures the maximum rate of change in pressure acceleration during late ventricular ejection, with the search window limited between points C and F to ensure physiological relevance.
- F point:This estimates the F point in the CAP waveform. The F point represents the maximum systolic pressure in the CAP signal, occurring during the ventricular ejection phase. This method identifies F by locating the maximum amplitude value in the CAP signal .
- G point:This estimates the G point in the CAP waveform, which represents the onset of left ventricular ejection. The algorithm identifies G by locating the minimum of the first derivative and then finding the beginning of the first negative segment in the third derivative within a defined interval. This interval spans from the minimum of the first derivative to halfway between this minimum and the end of the signal, providing a focused search region for detecting inflection changes. This approach exploits the inflection characteristics in which the third derivative becomes negative, indicating the acceleration change at the onset of the systolic ejection phase in the CAP signal.
2.7. Period Ensemble Processing
2.7.1. Period Ensemble Synchronization
2.7.2. EBI and CAP Period Selection
2.7.3. ECG Period Selection
2.7.4. Detection of Period Outliers
- Filtering of periods by their lengths, with very long and very short periods excluded;
- A PCA-based outlier detector from the pyOD Python library was used [27];
2.7.5. Period Normalization
2.8. Feature Selection
2.8.1. Fiducial Point Features
2.8.2. Time Intervals as Features
2.8.3. PAT and PTT as Features
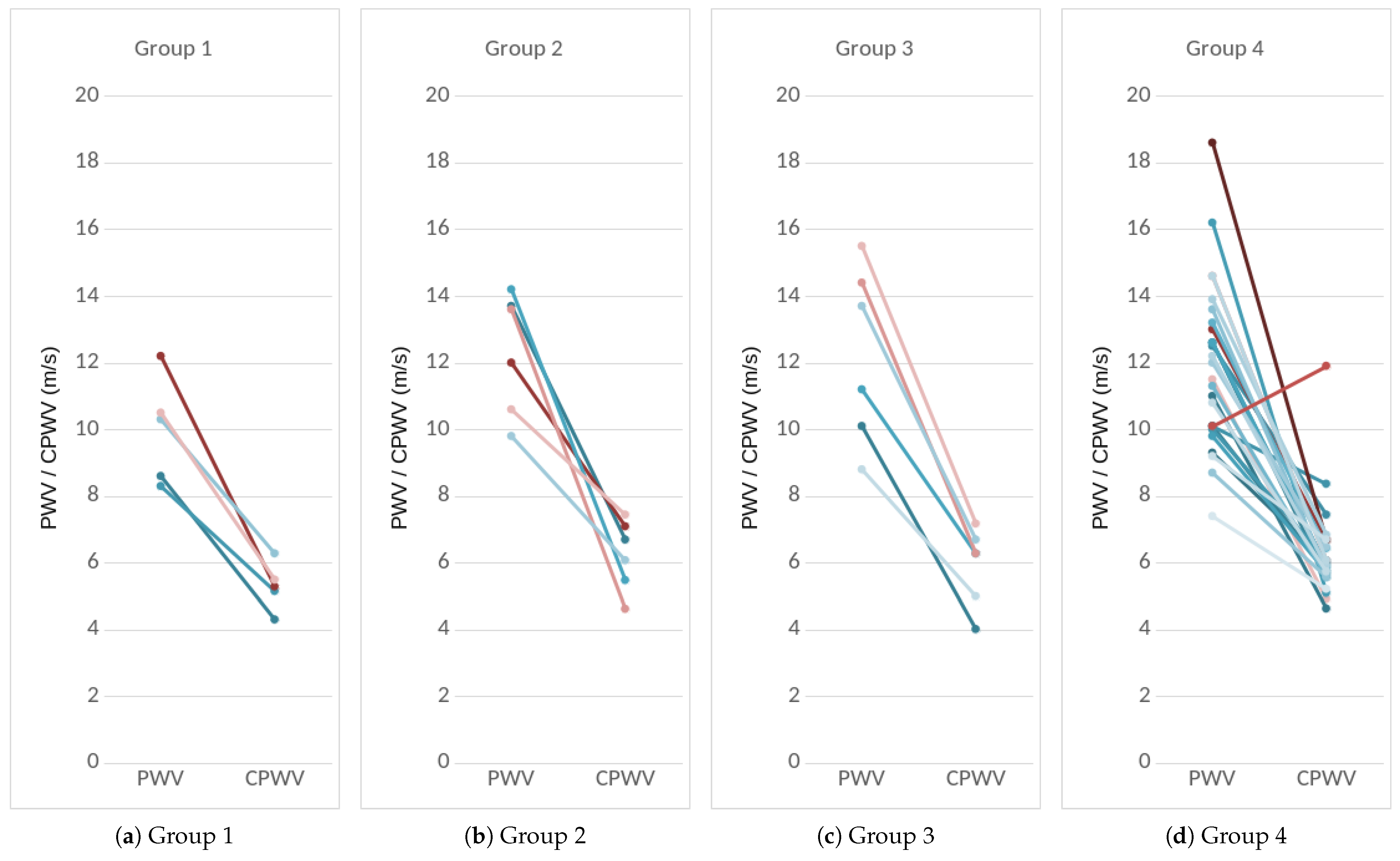
2.8.4. PWV as a Feature
2.8.5. CPWV as a Feature
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Atrial Fibrillation |
| AP | Arterial Pressure |
| BMI | Body Mass Index |
| BP | Blood Pressure (non-invasive) |
| CABG | Coronary Artery Bypass Grafting |
| CAD | Coronary Artery Disease |
| CAP | Central Aortic Pressure (invasive) |
| CFR | Coronary Flow Reserve |
| COPOD | Copula-Based Outlier Detection |
| CPWV | Complimentary Pulse Wave Velocity |
| CV | Cardiovascular |
| EBI | Electrical Bio-Impedance |
| ECG | Electrocardiogram |
| ED | Ejection Duration |
| FFR | Fractional Flow Reserve |
| IBP | Invasive Blood Pressure |
| IMR | Index of Microvascular Resistance |
| IVUS | Intravascular Ultrasound |
| LDL | Low-Density Lipoprotein (cholesterol) |
| LVEF | Left Ventricular Ejection Fraction |
| MFLI | Multi Frequency Lock-In |
| NIRS | Near-Infrared Spectroscopy |
| NaCl | chemical formula of Sodium chloride |
| PAT | Pulse Arrival Time |
| PCA | Principal Component Analysis |
| PCI | Percutaneous Coronary Intervention |
| PEP | Pre-Ejection Period |
| PPG | Photoplethysmography |
| PTT | Pulse Transit Time |
| PWV | Pulse Wave Velocity |
| RAP | Radial Artery Pressure (invasive) |
| TEVAR | Thoracic Endovascular Aortic Repair |
| eGFR | Estimated Glomerular Filtration Rate |
| pyOD | Python Outlier Detection (Python language library) |
References
- Volpe, M.; Gallo, G.; Tocci, G. Is Early and Fast Blood Pressure Control Important in Hypertension Management? Int. J. Cardiol. 2018, 254, 328–332. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the Management of Elevated Blood Pressure and Hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef] [PubMed]
- Avolio, A.P.; Butlin, M.; Walsh, A. Arterial Blood Pressure Measurement and Pulse Wave Analysis—Their Role in Enhancing Cardiovascular Assessment. Physiol. Meas. 2009, 31, R1. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Silva, B.V.; Sousa, C.; Caldeira, D.; Abreu, A.; Pinto, F.J. Management of Arterial Hypertension: Challenges and Opportunities. Clin. Cardiol. 2022, 45, 1094–1099. [Google Scholar] [CrossRef]
- Carey, R.M.; Muntner, P.; Bosworth, H.B.; Whelton, P.K. Prevention and Control of Hypertension: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1278–1293. [Google Scholar] [CrossRef]
- GBD 2019 Risk Factors Collaborators. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- O’Shea, P.; Griffin, T.; Fitzgibbon, M. Hypertension: The Role of Biochemistry in the Diagnosis and Management. Clin. Chim. Acta 2017, 465, 131–143. [Google Scholar] [CrossRef]
- Ogedegbe, G.; Pickering, T. Principles and Techniques of Blood Pressure Measurement. Cardiol. Clin. 2010, 28, 571–586. [Google Scholar] [CrossRef]
- Picone, D.S.; Schultz, M.G.; Otahal, P.; Aakhus, S.; Al, J.A.M.; Black, J.A.; Bos, W.J.; Chambers, J.B.; Chen, C.H.; Cheng, H.M.; et al. Accuracy of Cuff-Measured Blood Pressure. J. Am. Coll. Cardiol. 2017, 70, 572–586. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Safar, M.E. Relationship between Aortic Stiffening and Microvascular Disease in Brain and Kidney: Cause and Logic of Therapy. Hypertension 2005, 46, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Avolio, A.P.; Van Bortel, L.M.; Boutouyrie, P.; Cockcroft, J.R.; McEniery, C.M.; Protogerou, A.D.; Roman, M.J.; Safar, M.E.; Segers, P.; Smulyan, H. Role of Pulse Pressure Amplification in Arterial Hypertension: Experts’ Opinion and Review of the Data. Hypertension 2009, 54, 375–383. [Google Scholar] [CrossRef] [PubMed]
- McEniery, C.M.; Cockcroft, J.R.; Roman, M.J.; Franklin, S.S.; Wilkinson, I.B. Central Blood Pressure: Current Evidence and Clinical Importance. Eur. Heart J. 2014, 35, 1719–1725. [Google Scholar] [CrossRef]
- Dart, A.M.; Kingwell, B.A. Pulse Pressure—A Review of Mechanisms and Clinical Relevance. J. Am. Coll. Cardiol. 2001, 37, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef]
- Safar, M.E.; Levy, B.I.; Struijker-Boudier, H. Current Perspectives on Arterial Stiffness and Pulse Pressure in Hypertension and Cardiovascular Diseases. Circulation 2003, 107, 2864–2869. [Google Scholar] [CrossRef] [PubMed]
- Karunathilake, S.P.; Ganegoda, G.U. Secondary Prevention of Cardiovascular Diseases and Application of Technology for Early Diagnosis. BioMed Res. Int. 2018, 2018, 5767864. [Google Scholar] [CrossRef]
- Javaid, A.; Zghyer, F.; Kim, C.; Spaulding, E.M.; Isakadze, N.; Ding, J.; Kargillis, D.; Gao, Y.; Rahman, F.; Brown, D.E.; et al. Medicine 2032: The Future of Cardiovascular Disease Prevention with Machine Learning and Digital Health Technology. Am. J. Prev. Cardiol. 2022, 12, 100379. [Google Scholar] [CrossRef]
- Williams, G.J.; Al-Baraikan, A.; Rademakers, F.E.; Ciravegna, F.; van de Vosse, F.N.; Lawrie, A.; Rothman, A.; Ashley, E.A.; Wilkins, M.R.; Lawford, P.V.; et al. Wearable Technology and the Cardiovascular System: The Future of Patient Assessment. Lancet Digit. Health 2023, 5, e467–e476. [Google Scholar] [CrossRef]
- Park, J.B.; Sharman, J.E.; Li, Y.; Munakata, M.; Shirai, K.; Chen, C.H.; Jae, S.Y.; Tomiyama, H.; Kosuge, H.; Bruno, R.M.; et al. Expert Consensus on the Clinical Use of Pulse Wave Velocity in Asia. Pulse 2022, 10, 1–18. [Google Scholar] [CrossRef]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.S.; Protogerou, A.D.; et al. Expert Consensus Document on the Measurement of Aortic Stiffness in Daily Practice Using Carotid-Femoral Pulse Wave Velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef]
- Spronck, B.; Terentes-Printzios, D.; Avolio, A.P.; Boutouyrie, P.; Guala, A.; Jerončić, A.; Laurent, S.; Barbosa, E.C.; Baulmann, J.; Chen, C.H.; et al. 2024 Recommendations for Validation of Noninvasive Arterial Pulse Wave Velocity Measurement Devices. Hypertension 2024, 81, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Sel, K.; Mohammadi, A.; Pettigrew, R.I.; Jafari, R. Physics-Informed Neural Networks for Modeling Physiological Time Series for Cuffless Blood Pressure Estimation. NPJ Digit. Med. 2023, 6, 110. [Google Scholar] [CrossRef]
- Mansouri, S.; Alharbi, Y.; Alshrouf, A.; Alqahtani, A. Cardiovascular Diseases Diagnosis by Impedance Cardiography. J. Electr. Bioimpedance 2022, 13, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S. Determination of Arterial Compliance Using Electrical Peripheral Bioimpedance. IEEJ Trans. Electr. Electron. Eng. 2023, 18, 436–441. [Google Scholar] [CrossRef]
- Krivošei, A.; Min, M.; Annus, P.; Märtens, O.; Metshein, M.; Lotamõis, K.; Rist, M. Hankel Matrix Based algorithm for Cardiac Pulse Wave Base and Peak Lines Correction. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy, 22–24 June 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Zhao, Y.; Nasrullah, Z.; Li, Z. PyOD: A Python Toolbox for Scalable Outlier Detection. arXiv 2019, arXiv:1901.01588. [Google Scholar]
- Li, Z.; Zhao, Y.; Botta, N.; Ionescu, C.; Hu, X. COPOD: Copula-Based Outlier Detection. In Proceedings of the 2020 IEEE International Conference on Data Mining (ICDM), Sorrento, Italy, 17–20 November 2020; pp. 1118–1123. [Google Scholar] [CrossRef]
- Lona, G.; Hauser, C.; Köchli, S.; Infanger, D.; Endes, K.; Faude, O.; Hanssen, H. Blood Pressure Increase and Microvascular Dysfunction Accelerate Arterial Stiffening in Children: Modulation by Physical Activity. Front. Physiol. 2020, 11, 613003. [Google Scholar] [CrossRef]
- Rizzoni, D.; Agabiti-Rosei, C. Relationships between Blood Pressure Variability and Indices of Large Artery Stiffness: Does the Microvasculature Play a Role? Hypertens. Res. 2017, 40, 348–350. [Google Scholar] [CrossRef]
- Lantelme, P.; Mestre, C.; Lievre, M.; Gressard, A.; Milon, H. Heart Rate. Hypertension 2002, 39, 1083–1087. [Google Scholar] [CrossRef]
- Izzard, A.S.; Heagerty, A.M.; Leenen, F.H.H. The Amplifier Hypothesis: Permission to Dissent? J. Hypertens. 1999, 17, 1667. [Google Scholar] [CrossRef]
- Liang, Y.; Abbott, D.; Howard, N.; Lim, K.; Ward, R.; Elgendi, M. How Effective Is Pulse Arrival Time for Evaluating Blood Pressure? Challenges and Recommendations from a Study Using the MIMIC Database. J. Clin. Med. 2019, 8, 337. [Google Scholar] [CrossRef] [PubMed]
- Kamieńska, A.; Danieluk, A.; Niwińska, M.M.; Chlabicz, S. Arterial Stiffness and Ankle-Brachial Index—Cross-Sectional Study of 259 Primary Care Patients ge50 Year-Old. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2024, 30, e942718. [Google Scholar] [CrossRef]
- Lee, M.; Defall, B.; John, A. Enhanced Patient Comfort: A Wireless Monitoring System. 2024. Available online: https://www.researchgate.net/publication/387075206_Enhanced_Patient_Comfort_A_Wireless_Monitoring_System (accessed on 14 June 2025).
- Pavithra, L.S.; Khurdi, S.; Priyanka, T.G.; Patrisia Mary, S. Impact of Remote Patient Monitoring Systems on Nursing Time, Healthcare Providers, and Patient Satisfaction in General Wards. Cureus 2024, 16, e61646. [Google Scholar] [CrossRef]
- Weir-McCall, J.R.; Brown, L.; Summersgill, J.; Talarczyk, P.; Bonnici-Mallia, M.; Chin, S.C.; Khan, F.; Struthers, A.D.; Sullivan, F.; Colhoun, H.M.; et al. Development and Validation of a Path Length Calculation for Carotid—Femoral Pulse Wave Velocity Measurement. Hypertension 2018, 71, 937–945. [Google Scholar] [CrossRef]
- Muñoz-Torrero, J.F.S.; Calderón-García, J.F.; Nicolás-Jiménez, J.M.D.; García-Ortiz, L.; Rodilla-Salas, E.; Gómez-Marcos, M.A.; Suárez-Fernandez, C.; Cordovilla-Guardia, S.; Rico-Martín, S. Automatic or Manual Arterial Path for the Ankle-Brachial Differences Pulse Wave Velocity. PLoS ONE 2018, 13, e0206434. [Google Scholar] [CrossRef]
- Fred, H.L. Accurate Blood Pressure Measurements and the Other Arm. Tex. Heart Inst. J. 2013, 40, 217–219. [Google Scholar]
- Kortekaas, M.C.; Niehof, S.P.; van Velzen, M.H.N.; Galvin, E.M.; Stolker, R.J.; Huygen, F.J.P.M. Comparison of Bilateral Pulse Arrival Time before and after Induced Vasodilation by Axillary Block. Physiol. Meas. 2012, 33, 1993. [Google Scholar] [CrossRef]
- Mazzolai, L.; Teixido-Tura, G.; Lanzi, S.; Boc, V.; Bossone, E.; Brodmann, M.; Bura-Rivière, A.; De Backer, J.; Deglise, S.; Della Corte, A.; et al. 2024 ESC Guidelines for the Management of Peripheral Arterial and Aortic Diseases. Eur. Heart J. 2024, 45, 3538–3700. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, M.; Amiri, Z.; Haghjoo, E.; Afrashteh, S.; Dastmanesh, S.; Moghaddam, M.T.; Dehghan, A.; Tabibzadeh, H.A.S.; Mouseli, A. Association between Inter-Arm Blood Pressure Difference and Cardiovascular Disease: Result from Baseline Fasa Adults Cohort Study. Sci. Rep. 2023, 13, 9648. [Google Scholar] [CrossRef]
- Vanreusel, I.; De Wolf, D.; Van der Zijden, T.; Van Berendoncks, A. Interarm Blood Pressure Difference Revealing a Right-Sided Aortic Arch and Occluded Aberrant Left Subclavian Artery. JACC Case Rep. 2024, 29, 102246. [Google Scholar] [CrossRef]
- Shankar Kikkeri, N.; Nagalli, S. Subclavian Steal Syndrome. In StatPearls; StatPearls Publishing: Petersburg, FL, USA, 2025. [Google Scholar]
- Vennin, S.; Mayer, A.; Li, Y.; Fok, H.; Clapp, B.; Alastruey, J.; Chowienczyk, P. Noninvasive Calculation of the Aortic Blood Pressure Waveform from the Flow Velocity Waveform: A Proof of Concept. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H969–H976. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Seo, J.B.; Chung, W.Y.; Kim, S.H.; Kim, M.A.; Zo, J.H. Association Between Invasively Measured Central Aortic Pressure and Left Ventricular Diastolic Function in Patients Undergoing Coronary Angiography. Am. J. Hypertens. 2015, 28, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.W.; O’Rourke, M.; Vlachopoulos, C.; Edelman, E.R. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles, 7th ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Pereira, T.; Correia, C.; Cardoso, J. Novel Methods for Pulse Wave Velocity Measurement. J. Med. Biol. Eng. 2015, 35, 555–565. [Google Scholar] [CrossRef]
- Deshmukh, C.A.; Jindal, G.D.; Bagal, U.R.; Nagare, G.D. Pulse Arrival Time: Measurement and Clinical Applications. MGM J. Med. Sci. 2022, 9, 103. [Google Scholar] [CrossRef]
- Finnegan, E.; Davidson, S.; Harford, M.; Jorge, J.; Watkinson, P.; Young, D.; Tarassenko, L.; Villarroel, M. Pulse Arrival Time as a Surrogate of Blood Pressure. Sci. Rep. 2021, 11, 22767. [Google Scholar] [CrossRef]
- Díaz, A.; Galli, C.; Tringler, M.; Ramírez, A.; Cabrera Fischer, E.I. Reference Values of Pulse Wave Velocity in Healthy People from an Urban and Rural Argentinean Population. Int. J. Hypertens. 2014, 2014, 653239. [Google Scholar] [CrossRef]
- Blacher, J.; Asmar, R.; Djane, S.; London, G.M.; Safar, M.E. Aortic Pulse Wave Velocity as a Marker of Cardiovascular Risk in Hypertensive Patients. Hypertension 1999, 33, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From Vulnerable Plaque to Vulnerable Patient. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef]
- Park, S.J.; Kang, S.J.; Ahn, J.M.; Shim, E.B.; Kim, Y.T.; Yun, S.C.; Song, H.; Lee, J.Y.; Kim, W.J.; Park, D.W.; et al. Visual-Functional Mismatch Between Coronary Angiography and Fractional Flow Reserve. JACC Cardiovasc. Interv. 2012, 5, 1029–1036. [Google Scholar] [CrossRef]
- Brugaletta, S.; Garcia-Garcia, H.M.; Serruys, P.W.; de Boer, S.; Ligthart, J.; Gomez-Lara, J.; Witberg, K.; Diletti, R.; Wykrzykowska, J.; van Geuns, R.J.; et al. NIRS and IVUS for Characterization of Atherosclerosis in Patients Undergoing Coronary Angiography. JACC Cardiovasc. Imaging 2011, 4, 647–655. [Google Scholar] [CrossRef]
- Lee, J.M.; Jung, J.H.; Hwang, D.; Park, J.; Fan, Y.; Na, S.H.; Doh, J.H.; Nam, C.W.; Shin, E.S.; Koo, B.K. Coronary Flow Reserve and Microcirculatory Resistance in Patients With Intermediate Coronary Stenosis. JACC 2016, 67, 1158–1169. [Google Scholar] [CrossRef] [PubMed]



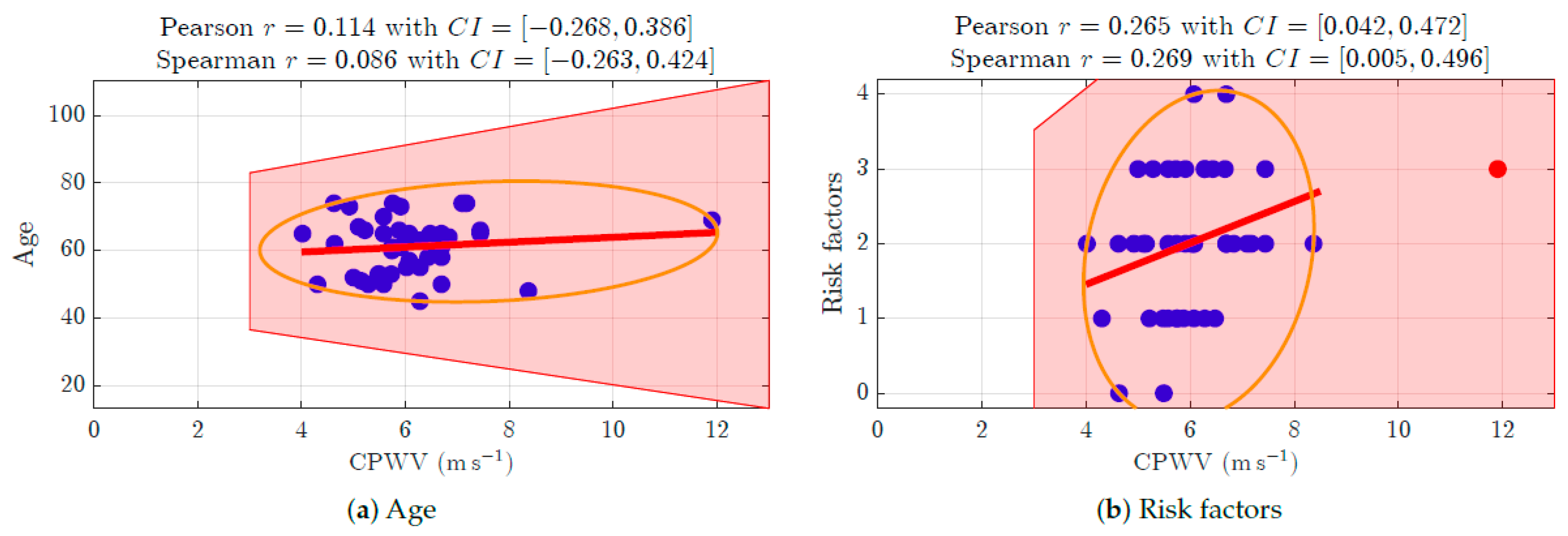
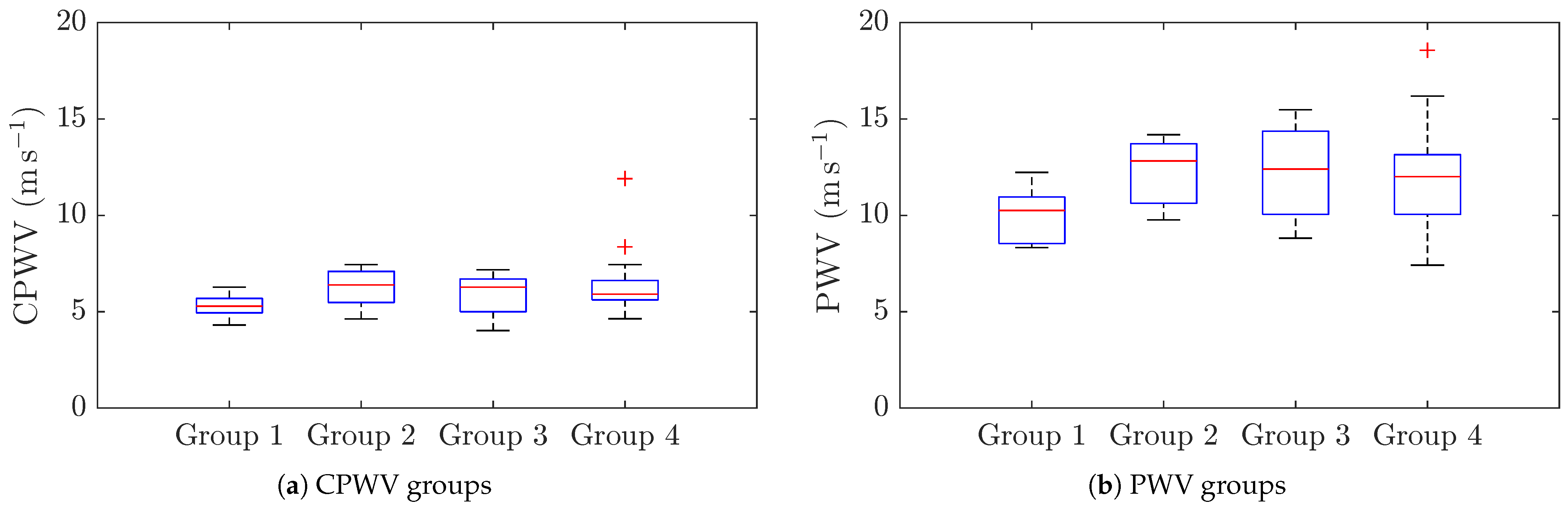
| Number | % | |
|---|---|---|
| Gender: | ||
| male | 32 | 72.7 |
| female | 12 | 27.3 |
| Age: | ||
| 45–54 | 11 | 25.0 |
| 55–64 | 15 | 34.1 |
| 65–74 | 18 | 40.9 |
| Risk factors: | ||
| hypertension | 41 | 93.2 |
| dyslipidemia with NO treatment | 8 | 18.2 |
| BMI | 4 | 9.1 |
| current/former smoking | 27 | 61.4 |
| diabetes | 8 | 18.2 |
| vascular pathology | 4 | 9.1 |
| Risks levels: | ||
| 0 risk factors | 2 | 4.5 |
| 1 risk factor | 10 | 22.7 |
| 2 risk factors | 19 | 43.2 |
| 3 risk factors | 11 | 25.0 |
| 4 risk factors | 2 | 4.5 |
| 5 risk factors | 0 | 0.0 |
| 6 risk factors | 0 | 0.0 |
| CAD stages: | ||
| 1: normal coronaries | 5 | 11.4 |
| 2: minimal/moderate (≤50%) decrease in vessel diameter | 6 | 13.6 |
| 3: medium (51–75)% decrease in vessel diameter 1 | 6 | 13.6 |
| 4: significant (51–100)% decrease in vessel diameter 2 | 27 | 61.4 |
| RAP D Time | EBI D Time | |
|---|---|---|
| RAP D time | ||
| EBI D time | 0.078 | |
| CAP D time | −0.3128 | −0.2191 |
| RAP F time | EBI F time | |
| RAP F time | ||
| EBI F time | 0.3002 | |
| CAP F time | 0.6249 | 0.0701 |
| RAP G time | EBI G time | |
| RAP G time | ||
| EBI G time | 0.10223 | |
| CAP G time | 0.8587 | 0.0981 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lotamõis, K.; Uuetoa, T.; Krivošei, A.; Annus, P.; Metshein, M.; Rist, M.; Margus, S.; Min, M.; Tamberg, G. An Analysis of Arterial Pulse Wave Time Features and Pulse Wave Velocity Calculations Based on Radial Electrical Bioimpedance Waveforms in Patients Scheduled for Coronary Catheterization. J. Cardiovasc. Dev. Dis. 2025, 12, 237. https://doi.org/10.3390/jcdd12070237
Lotamõis K, Uuetoa T, Krivošei A, Annus P, Metshein M, Rist M, Margus S, Min M, Tamberg G. An Analysis of Arterial Pulse Wave Time Features and Pulse Wave Velocity Calculations Based on Radial Electrical Bioimpedance Waveforms in Patients Scheduled for Coronary Catheterization. Journal of Cardiovascular Development and Disease. 2025; 12(7):237. https://doi.org/10.3390/jcdd12070237
Chicago/Turabian StyleLotamõis, Kristina, Tiina Uuetoa, Andrei Krivošei, Paul Annus, Margus Metshein, Marek Rist, Sulev Margus, Mart Min, and Gert Tamberg. 2025. "An Analysis of Arterial Pulse Wave Time Features and Pulse Wave Velocity Calculations Based on Radial Electrical Bioimpedance Waveforms in Patients Scheduled for Coronary Catheterization" Journal of Cardiovascular Development and Disease 12, no. 7: 237. https://doi.org/10.3390/jcdd12070237
APA StyleLotamõis, K., Uuetoa, T., Krivošei, A., Annus, P., Metshein, M., Rist, M., Margus, S., Min, M., & Tamberg, G. (2025). An Analysis of Arterial Pulse Wave Time Features and Pulse Wave Velocity Calculations Based on Radial Electrical Bioimpedance Waveforms in Patients Scheduled for Coronary Catheterization. Journal of Cardiovascular Development and Disease, 12(7), 237. https://doi.org/10.3390/jcdd12070237