Effect of DAPAgliflozin on Myocardial Fibrosis and Ventricular Function in Patients with ST-Segment Elevation Myocardial Infarction—DAPA-STEMI Trial
Abstract
1. Introduction
2. Methods
2.1. Trial Organization and Sources of Funding
2.2. Protocol Amendment
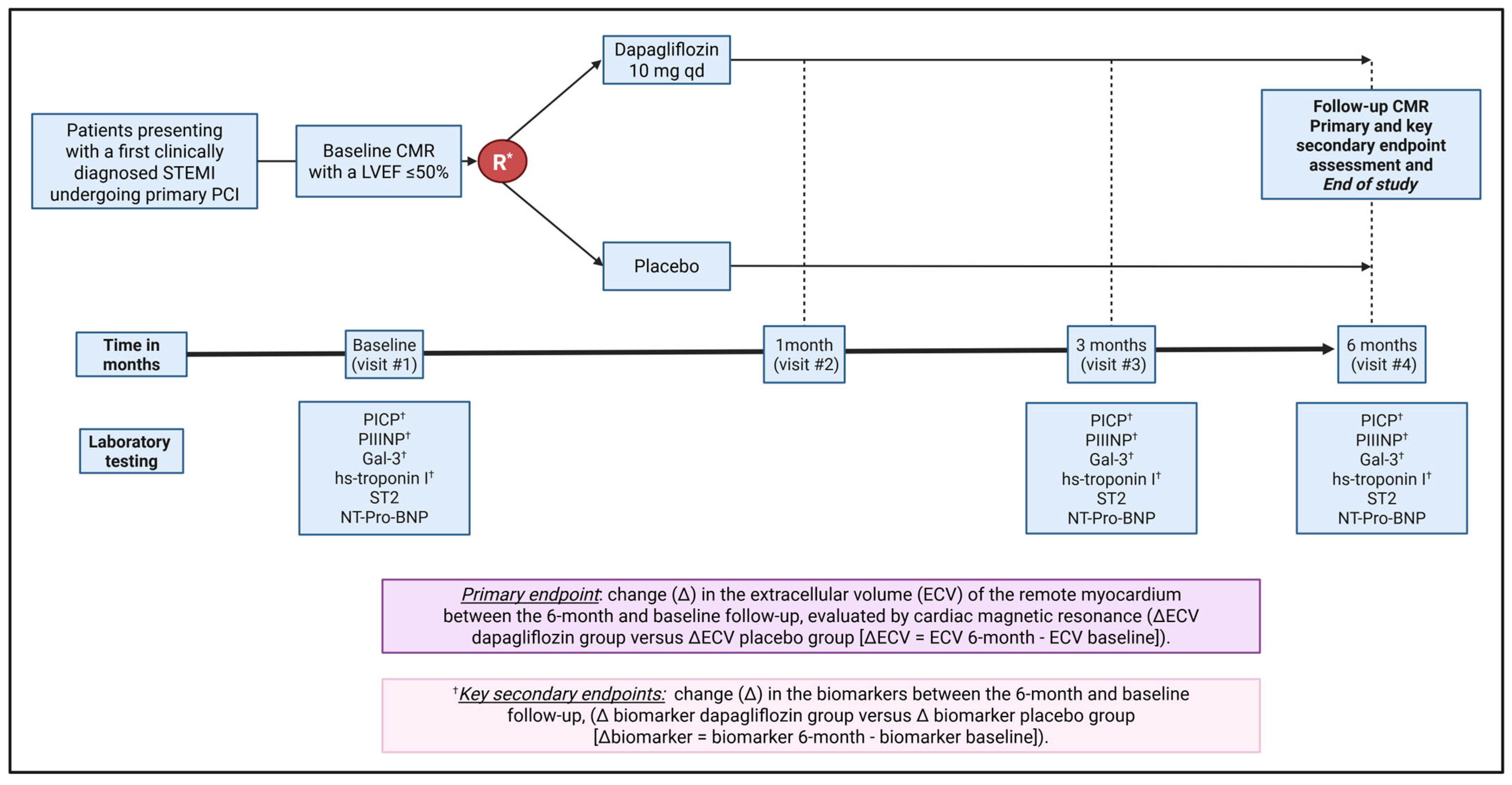
2.3. Patient Eligibility
2.3.1. Inclusion Criteria
- Patients between 30 and 85 years of age.
- Patients with a first myocardial infarction with ST-segment elevation, documented in an ambulance or a cardiac catheterization laboratory (ST-segment elevation ≥2 mm in at least two contiguous leads) <12 h after the onset of symptoms that last ≥20 min, undergoing primary percutaneous cardiac intervention. The target lesion must be a de novo lesion located in a native vessel.
- The patient understands and accepts clinical monitoring and CMR.
- The patient must be hemodynamically stable (Killip classification 1) at the time of the initial CMR.
- A left ventricular ejection fraction ≤50% at baseline echocardiogram.
2.3.2. Exclusion Criteria
- Pregnancy or lactation in women.
- Type 1 diabetes mellitus.
- Previous treatment with SGLT2is.
- Severe liver disease (Child-Pugh C).
- Kidney disease defined as stage III or worse (eGFR < 45 mL/min).
- Systolic blood pressure <90 mmHg at the screening visit.
- Malignancy (receiving active treatment) or other life-threatening diseases.
- Any contraindication to CMR (e.g., claustrophobia; cerebrovascular implants; metal implants; penetrating eye injury; exposure to metal fragments in the eye that require medical attention; or hemodynamic or electrical instability).
- Previous complicated urinary tract infection in men or repeated urinary infection in women.
- Treatment with fibrinolytic therapy.
2.4. Screening
2.5. Trial Procedures
2.5.1. Randomization and Blinding
2.5.2. Data Collection Plan
2.5.3. Follow-Up Visits
2.5.4. Cardiac Magnetic Resonance Imaging
Native T1 and ECV Value Derivation
2.5.5. Biomarkers
2.5.6. Drug Discontinuation During the Trial
2.6. Trial Outcomes
- C-terminal propeptide of type I procollagen;
- N-terminal propeptide of type III procollagen;
- Galectin-3;
- High-sensitivity troponin I.
- Left ventricular end-diastolic and end-systolic volumes;
- Left ventricular mass;
- Left ventricular ejection fraction.
- N-terminal pro-B-type natriuretic peptide.
- Suppression of Tumorigenicity 2.
- Rate of major adverse cardiovascular events (MACE), including cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, and heart failure hospitalization. All these endpoints will be adjudicated independently by a clinical event committee (CEC) (Supplementary Materials).
- Adverse events (AEs) and serious AEs were actively collected at follow-up visits and through spontaneous reporting by participants during the study. AEs of interest were represented by severe hypoglycemia, acute kidney injury, acute liver function profile impairment, severe dehydration, symptomatic and sustained hypotension, complicated genitourinary infections, diabetic ketoacidosis, and diabetic foot complications (in diabetic patients). AEs were recorded using a standardized form and assessed for severity, causality, and expectedness. All adverse events were communicated to the DSMB.
- Change in body weight from baseline to six months.
3. Statistical Considerations
3.1. Monitoring
3.2. Dissemination Plans
4. Discussion
4.1. Anti-Myocardial Fibrosis and Post-MI Effects of SGLT2i
4.2. Clinical Evidence for SGLT2is in Post-MI Patients
4.3. Key Aspects of the Design of the DAPA-STEMI Trial
4.4. Protocol Amendments and Early Termination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAD | Coronary Artery Disease |
| CMR | Cardiac Magnetic Resonance |
| DAPA-HF | Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure |
| DAPA-MI | Dapagliflozin Effects on Cardiometabolic Outcomes in Patients With an Acute Heart Attack |
| DAPA-STEMI | effect of DAPAgliflozin on myocardial fibrosis and ventricular function in patients with STEMI |
| DSMB | Data Safety Monitoring Board |
| ECV | Extracellular Volume |
| GAL3 | Galectin-3 |
| HbA1c | Glycated Hemoglobin |
| IDIBAPS | Institut d’Investigacions Biomèdiques August Pi i Sunyer |
| LVEF | Left Ventricular Ejection Fraction |
| MACE | Major Adverse Cardiovascular Events |
| MI | Myocardial Infarction |
| PCI | Percutaneous Coronary Intervention |
| RCT | Randomized Controlled Trial |
| REDCap | Research Electronic Data Capture |
| SGLT2i | Sodium–Glucose Cotransporter 2 Inhibitors |
| STEMI | ST-Segment Elevation Myocardial Infarction |
| T2DM | Type 2 Diabetes Mellitus |
References
- Del Monte-Nieto, G.; Fischer, J.W.; Gorski, D.J.; Harvey, R.P.; Kovacic, J.C. Basic Biology of Extracellular Matrix in the Cardiovascular System, Part 1/4: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 2169–2188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perea, R.J.; Morales-Ruiz, M.; Ortiz-Perez, J.T.; Bosch, X.; Andreu, D.; Borras, R.; Acosta, J.; Penela, D.; Prat-Gonzalez, S.; de Caralt, T.M.; et al. Utility of galectin-3 in predicting post-infarct remodeling after acute myocardial infarction based on extracellular volume fraction mapping. Int. J. Cardiol. 2016, 223, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G.; Kovacic, J.C. Extracellular Matrix in Ischemic Heart Disease, Part 4/4: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 2219–2235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ortega-Paz, L.; Cristobal, H.; Ortiz-Perez, J.T.; Garcia de Frutos, P.; Mendieta, G.; Sandoval, E.; Rodriguez, J.J.; Ortega, E.; Garcia-Alvarez, A.; Brugaletta, S.; et al. Direct actions of dapagliflozin and interactions with LCZ696 and spironolactone on cardiac fibroblasts of patients with heart failure and reduced ejection fraction. ESC Heart Fail. 2023, 10, 453–464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mason, T.; Coelho-Filho, O.R.; Verma, S.; Chowdhury, B.; Zuo, F.; Quan, A.; Thorpe, K.E.; Bonneau, C.; Teoh, H.; Gilbert, R.E.; et al. Empagliflozin Reduces Myocardial Extracellular Volume in Patients with Type 2 Diabetes and Coronary Artery Disease. JACC Cardiovasc. Imaging 2021, 14, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Erlinge, D.; Storey, R.F.; McGuire, D.K.; de Belder, M.; Eriksson, N.; Andersen, K.; Austin, D.; Arefalk, G.; Carrick, D.; et al. Dapagliflozin in Myocardial Infarction without Diabetes or Heart Failure. NEJM Evid. 2024, 3, EVIDoa2300286. [Google Scholar] [CrossRef] [PubMed]
- Bouchardy, B.; Majno, G. Histopathology of early myocardial infarcts. A new approach. Am. J. Pathol. 1974, 74, 301–330. [Google Scholar] [PubMed] [PubMed Central]
- Abraham, N.S.; Barkun, A.N.; Sauer, B.G.; Douketis, J.; Laine, L.; Noseworthy, P.A.; Telford, J.J.; Leontiadis, G.I. American College of Gastroenterology-Canadian Association of Gastroenterology Clinical Practice Guideline: Management of Anticoagulants and Antiplatelets During Acute Gastrointestinal Bleeding and the Periendoscopic Period. Am. J. Gastroenterol. 2022, 117, 542–558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Hundley, W.G.; Bluemke, D.A.; Bogaert, J.; Flamm, S.D.; Fontana, M.; Friedrich, M.G.; Grosse-Wortmann, L.; Karamitsos, T.D.; Kramer, C.M.; Kwong, R.Y.; et al. Society for Cardiovascular Magnetic Resonance (SCMR) guidelines for reporting cardiovascular magnetic resonance examinations. J. Cardiovasc. Magn. Reson. 2022, 24, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghazal, R.; Wang, M.; Liu, D.; Tschumperlin, D.J.; Pereira, N.L. Cardiac Fibrosis in the Multi-Omics Era: Implications for Heart Failure. Circ. Res. 2025, 136, 773–802. [Google Scholar] [CrossRef]
- Chaffin, M.; Papangeli, I.; Simonson, B.; Akkad, A.-D.; Hill, M.C.; Arduini, A.; Fleming, S.J.; Melanson, M.; Hayat, S.; Kost-Alimova, M.; et al. Single-nucleus profiling of human dilated and hypertrophic cardiomyopathy. Nature 2022, 608, 174–180. [Google Scholar] [CrossRef]
- Sharma, U.C.; Pokharel, S.; van Brakel, T.J.; van Berlo, J.H.; Cleutjens, J.P.M.; Schroen, B.; André, S.; Crijns, H.J.G.M.; Gabius, H.-J.; Maessen, J.; et al. Galectin-3 Marks Activated Macrophages in Failure-Prone Hypertrophied Hearts and Contributes to Cardiac Dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Manzano-Fernández, S.; Boronat, M.; Casas, T.; Garrido, I.P.; Bonaque, J.C.; Pastor-Perez, F.; Valdés, M.; Januzzi, J.L. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: Complementary role for risk stratification in acutely decompensated heart failure. Eur. J. Heart Fail. 2011, 13, 718–725. [Google Scholar] [CrossRef]
- Armillotta, M.; Angeli, F.; Paolisso, P.; Belmonte, M.; Raschi, E.; Di Dalmazi, G.; Amicone, S.; Canton, L.; Fedele, D.; Suma, N.; et al. Cardiovascular therapeutic targets of sodium-glucose co-transporter 2 (SGLT2) inhibitors beyond heart failure. Pharmacol. Ther. 2025, 270, 108861. [Google Scholar] [CrossRef]
- Wu, Q.; Yao, Q.; Hu, T.; Yu, J.; Jiang, K.; Wan, Y.; Tang, Q. Dapagliflozin protects against chronic heart failure in mice by inhibiting macrophage-mediated inflammation, independent of SGLT2. Cell Rep. Med. 2023, 4, 101334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- von Lewinski, D.; Kolesnik, E.; Tripolt, N.J.; Pferschy, P.N.; Benedikt, M.; Wallner, M.; Alber, H.; Berger, R.; Lichtenauer, M.; Saely, C.H.; et al. Empagliflozin in acute myocardial infarction: The EMMY trial. Eur. Heart J. 2022, 43, 4421–4432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santos-Gallego, C.G.; Vargas-Delgado, A.P.; Requena-Ibanez, J.A.; Garcia-Ropero, A.; Mancini, D.; Pinney, S.; Macaluso, F.; Sartori, S.; Roque, M.; Sabatel-Perez, F.; et al. Randomized Trial of Empagliflozin in Nondiabetic Patients with Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.Y.; Brooksbank, K.J.M.; Wetherall, K.; Mangion, K.; Roditi, G.; Campbell, R.T.; Berry, C.; Chong, V.; Coyle, L.; Docherty, K.F.; et al. Effect of Empagliflozin on Left Ventricular Volumes in Patients with Type 2 Diabetes, or Prediabetes, and Heart Failure with Reduced Ejection Fraction (SUGAR-DM-HF). Circulation 2021, 143, 516–525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santos-Gallego, C.G.; Requena-Ibanez, J.A.; San Antonio, R.; Ishikawa, K.; Watanabe, S.; Picatoste, B.; Flores, E.; Garcia-Ropero, A.; Sanz, J.; Hajjar, R.J.; et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J. Am. Coll. Cardiol. 2019, 73, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Xu, Y.; Wang, D.; Chen, F.; Tu, Z.; Qian, J.; Xu, S.; Xu, Y.; Hwa, J.; Li, J.; et al. Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis. Protein Cell 2022, 13, 336–359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Selvaraj, S.; Vaduganathan, M.; Claggett, B.L.; Miao, Z.M.; Fang, J.C.; Vardeny, O.; Desai, A.S.; Shah, S.J.; Lam, C.S.P.; Martinez, F.A.; et al. Blood Pressure and Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: DELIVER. JACC Heart Fail. 2023, 11, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.C.; Lin, Y.K.; Chen, Y.C.; Kao, Y.H.; Yeh, Y.H.; Trang, N.N.; Chen, Y.J. Empagliflozin suppressed cardiac fibrogenesis through sodium-hydrogen exchanger inhibition and modulation of the calcium homeostasis. Cardiovasc. Diabetol. 2023, 22, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carberry, J.; Marquis-Gravel, G.; O’Meara, E.; Docherty, K.F. Where Are We with Treatment and Prevention of Heart Failure in Patients Post-Myocardial Infarction? JACC Heart Fail. 2024, 12, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Jones, W.S.; Udell, J.A.; Anker, S.D.; Petrie, M.C.; Harrington, J.; Mattheus, M.; Zwiener, I.; Amir, O.; Bahit, M.C.; et al. Empagliflozin after Acute Myocardial Infarction. N. Engl. J. Med. 2024, 390, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.D.; Weinberg, R.; Kapoor, S.; Ostovaneh, M.R.; Kato, Y.; Liu, C.Y.; Shea, S.; McClelland, R.L.; Post, W.S.; Bluemke, D.A.; et al. Myocardial fibrosis by T1 mapping magnetic resonance imaging predicts incident cardiovascular events and all-cause mortality: The Multi-Ethnic Study of Atherosclerosis. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1407–1416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, A.W.; Tetzlaff, J.M.; Gøtzsche, P.C.; Altman, D.G.; Mann, H.; Berlin, J.A.; Dickersin, K.; Hróbjartsson, A.; Schulz, K.F.; Parulekar, W.R.; et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ 2013, 346, e7586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Study Period | |||||
|---|---|---|---|---|---|
| Enrollment | Allocation | Post-Allocation | Close-Out | ||
| Timepoint | 0–15 Days after Index Myocardial Infarction | 0 | 1 Month | 3 Months | 6 Months |
| Enrollment: | |||||
| Eligibility screen | X | ||||
| Informed consent | X | ||||
| Baseline cardiac magnetic resonance | X | ||||
| Allocation | X | ||||
| Interventions: | |||||
| Dapagliflozin (10 mg) | Start administration | Daily administration | Daily administration | Daily administration | |
| Placebo | Start administration | Daily administration | Daily administration | Daily administration | |
| Assessments: | |||||
| Physical exam | X | X | X | X | |
| Follow-up cardiac magnetic resonance | X | X | |||
| Circulating biomarkers | X | X | X | ||
| Adverse events | X | X | X | ||
| Pill count | X | X | X | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Paz, L.; Laudani, C.; Sionis, A.; Vidal-Cales, P.; Arevalos, V.; Andrea, R.; Morr, C.I.; De Diego, O.; Ortega, E.; Jimenez-Trinidad, F.-R.; et al. Effect of DAPAgliflozin on Myocardial Fibrosis and Ventricular Function in Patients with ST-Segment Elevation Myocardial Infarction—DAPA-STEMI Trial. J. Cardiovasc. Dev. Dis. 2025, 12, 220. https://doi.org/10.3390/jcdd12060220
Ortega-Paz L, Laudani C, Sionis A, Vidal-Cales P, Arevalos V, Andrea R, Morr CI, De Diego O, Ortega E, Jimenez-Trinidad F-R, et al. Effect of DAPAgliflozin on Myocardial Fibrosis and Ventricular Function in Patients with ST-Segment Elevation Myocardial Infarction—DAPA-STEMI Trial. Journal of Cardiovascular Development and Disease. 2025; 12(6):220. https://doi.org/10.3390/jcdd12060220
Chicago/Turabian StyleOrtega-Paz, Luis, Claudio Laudani, Alessandro Sionis, Pablo Vidal-Cales, Victor Arevalos, Rut Andrea, Carlos Igor Morr, Oriol De Diego, Emilio Ortega, Francisco-Rafael Jimenez-Trinidad, and et al. 2025. "Effect of DAPAgliflozin on Myocardial Fibrosis and Ventricular Function in Patients with ST-Segment Elevation Myocardial Infarction—DAPA-STEMI Trial" Journal of Cardiovascular Development and Disease 12, no. 6: 220. https://doi.org/10.3390/jcdd12060220
APA StyleOrtega-Paz, L., Laudani, C., Sionis, A., Vidal-Cales, P., Arevalos, V., Andrea, R., Morr, C. I., De Diego, O., Ortega, E., Jimenez-Trinidad, F.-R., Dantas, A. P., Angiolillo, D. J., Sabaté, M., Ortiz-Pérez, J. T., & Brugaletta, S. (2025). Effect of DAPAgliflozin on Myocardial Fibrosis and Ventricular Function in Patients with ST-Segment Elevation Myocardial Infarction—DAPA-STEMI Trial. Journal of Cardiovascular Development and Disease, 12(6), 220. https://doi.org/10.3390/jcdd12060220







