The Efficacy and Safety of Outpatient Exercise Training for Patients with Chronic Thromboembolic Pulmonary Hypertension After Balloon Pulmonary Angioplasty
Abstract
1. Introduction
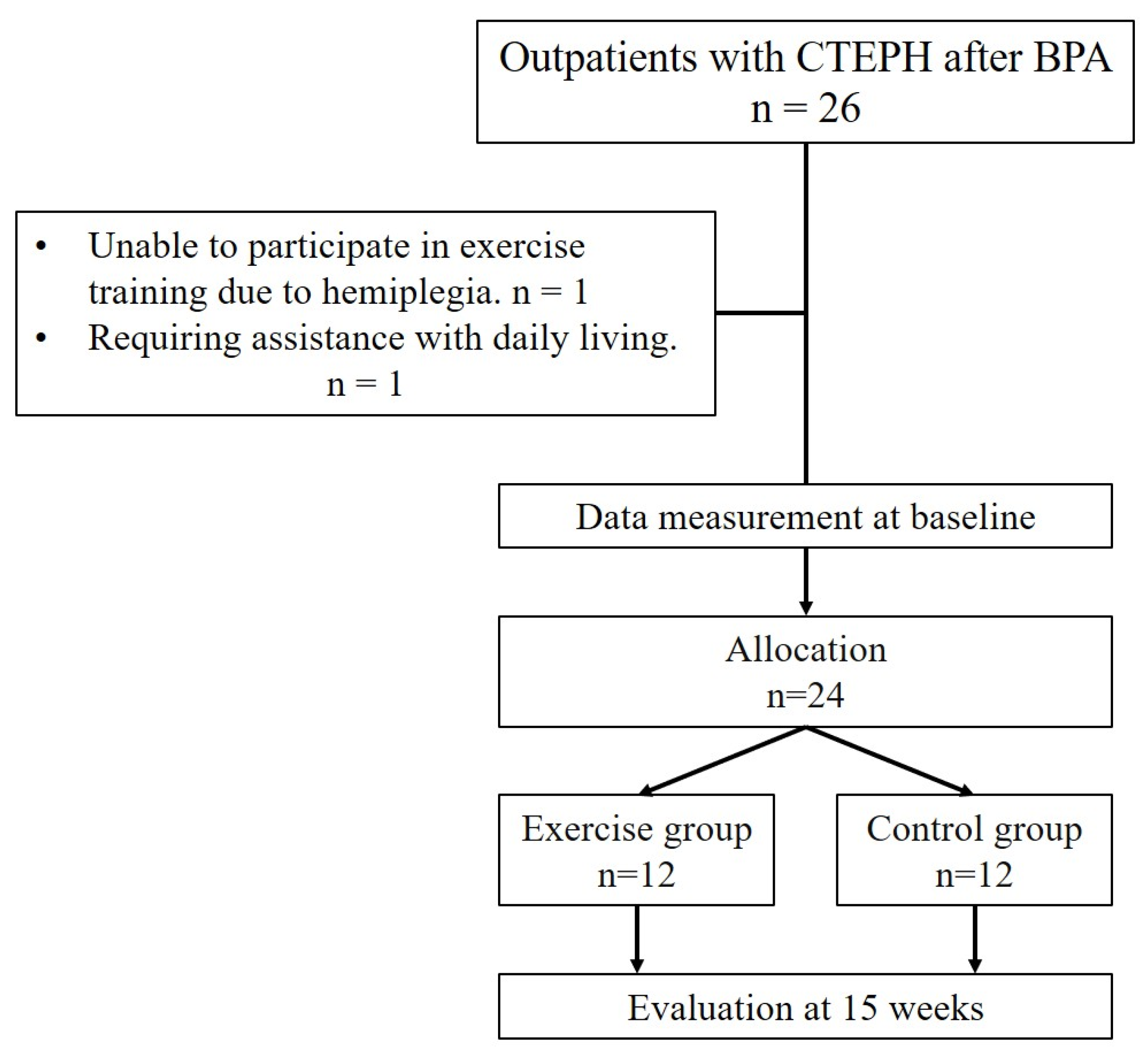
2. Materials and Methods
2.1. Study Protocol
2.2. BPA Procedure
2.3. Echocardiography, Right Heart Catheterization, and Skeletal Muscle Magnetic Resonance Imaging
2.4. Primary Endpoint and Secondary Endpoint
2.5. Exercise Training Program
2.6. CPET
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Primary Endpoint
3.3. Findings of Skeletal Muscle Magnetic Resonance Imaging and Echocardiography
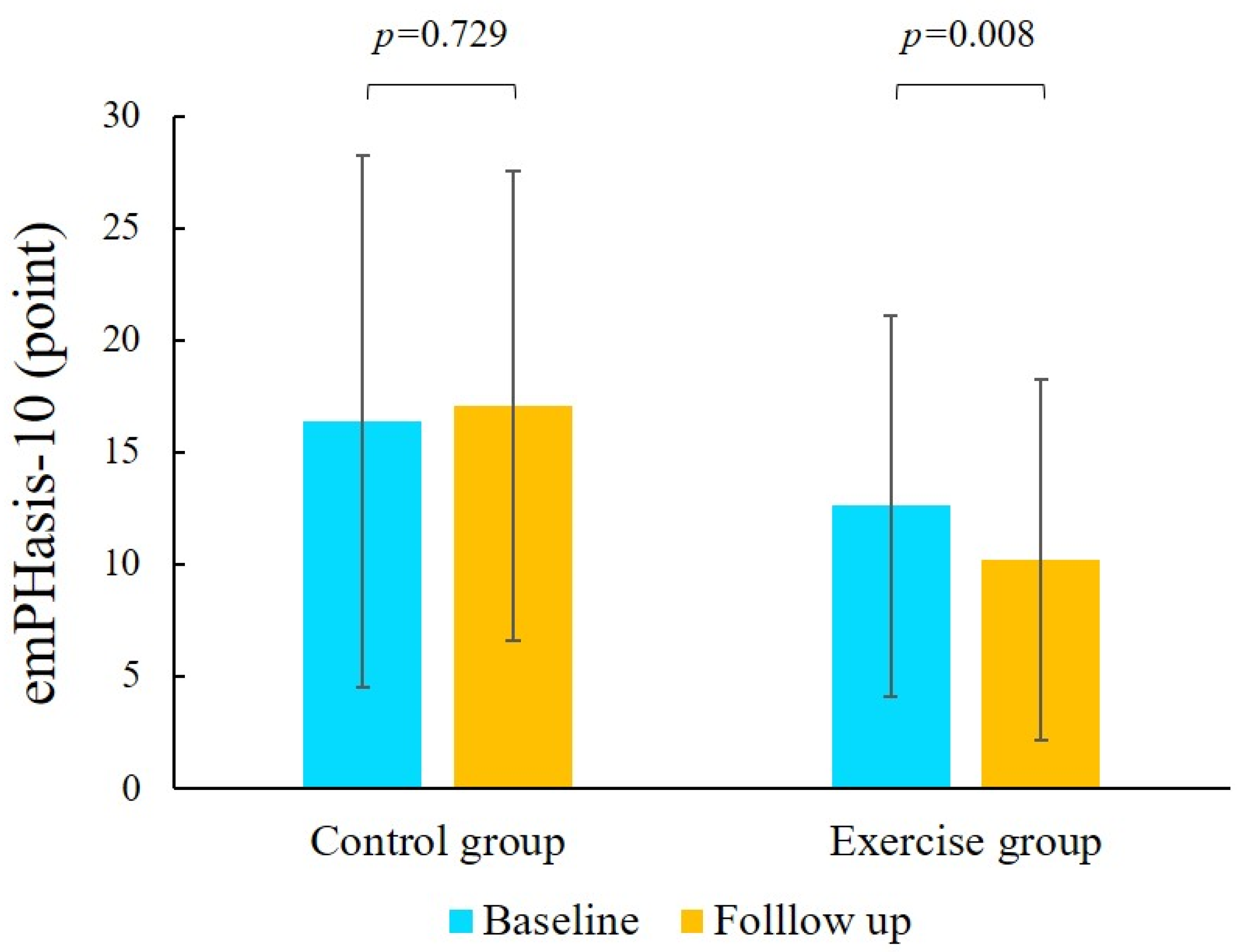
3.4. Comparison Between the Baseline and Follow-Up Periods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTEPH | Chronic thromboembolic pulmonary hypertension |
| BPA | Balloon pulmonary angioplasty |
| PAH | Pulmonary arterial hypertension |
| QOL | Quality of life |
| 6MWD | 6-min walk distance |
| COVID-19 | Coronavirus disease 2019 |
| RHC | Right heart catheterization |
| PVR | Pulmonary vascular resistance |
| mPAP | Mean pulmonary artery pressure |
| AT | Anaerobic threshold |
| VO2 | Oxygen uptake |
| WR | Work rate |
| VE/VCO2 | Ventilatory equivalent for carbon dioxide |
| WHO-FC | World Health Organization-Functional Class |
| CPET | Cardiopulmonary exercise testing |
| SpO2 | Oxygen saturation |
| ECG | Electrocardiogram |
| SD | Standard deviation |
| NT-proBNP | N-terminal prohormone of brain natriuretic peptide |
Appendix A
| Overall Patients, n = 24 | |
|---|---|
| Age, years | 58.8 ± 15.8 |
| Female, n (%) | 21 (87.5) |
| Body mass index, kg/m2 | 23.8 ± 4.3 |
| Comorbidities | |
| Hypertension, n (%) | 5 (20.8) |
| Dyslipidemia, n (%) | 8 (33.3) |
| Diabetes, n (%) | 1 (4.2) |
| Chronic kidney disease, n (%) | 4 (16.7) |
| Cerebrovascular disease, n (%) | 2 (8.3) |
| Coronary artery disease, n (%) | 1 (4.2) |
| History of acute pulmonary embolism, n (%) | 3 (12.5) |
| Echocardiography | |
| LVDd, mm | 41.1 ± 5.6 |
| LVDs, mm | 25.6 ± 4.0 |
| LVEF, % | 67.3 ± 6.1 |
| TRPG, mmHg | 54.8 ± 31.9 |
| TAPSE, mm | 9.6 ± 8.2 |
| Pulmonary function testing parameters | |
| FVC, L | 2.8 ± 1.0 |
| FEV1, L | 2.0 ± 0.7 |
| TLC, L | 4.4 ± 1.0 |
| DLCO | 14.3 ± 5.8 |
| Overall Patients, n = 24 | |||
|---|---|---|---|
| Before BPA | After BPA | p-Value | |
| Blood test | |||
| NT-proBNP, pg/mL | 178 [88.5, 318.5] | 65.5 [43.5, 100.5] | <0.001 |
| eGFR, mL/min/1.73 m2 | 66.9 ± 17.2 | 67.4 ± 16.9 | 0.823 |
| Echocardiography | |||
| LVDd, mm | 41.1 ± 7.1 | 41.6 ± 4.5 | 0.664 |
| LVDs, mm | 25.3 ± 4.6 | 25.6 ± 3.9 | 0.587 |
| LVEF, % | 67.5 ± 5.3 | 67.8 ± 5.3 | 0.853 |
| TRPG, mmHg | 61.6 ± 36.2 | 28.6 ± 13.3 | 0.005 |
| TAPSE, mm | 8.9 ± 8.6 | 11.7 ± 8.9 | 0.466 |
| Right heart catheterization parameters | |||
| mean PAP, mmHg | 37.5 ± 10.5 | 23.9 ± 4.5 | <0.001 |
| mean RAP, mmHg | 7.9 ± 4.9 | 3.3 ± 3.0 | 0.010 |
| mean PAWP, mmHg | 9.8 ± 4.3 | 7.8 ± 3.4 | 0.192 |
| PVR, wood u | 6.6 ± 4.2 | 3.2 ± 1.4 | <0.001 |
| CO, L/min | 5.5 ± 2.0 | 5.7 ± 1.1 | 0.569 |
| CI, L/min/m2 | 3.4 ± 1.0 | 3.6 ± 0.6 | 0.494 |
| Pulmonary function testing parameters | |||
| FVC, L | 2.8 ± 0.9 | 2.9 ± 0.8 | 0.027 |
| FEV1, L | 2.2 ± 0.8 | 2.3 ± 0.7 | 0.043 |
| TLC, L | 4.3 ± 0.9 | 4.5 ± 0.9 | 0.002 |
| DLCO, mL/min/mmHg | 14.2 ± 5.4 | 14.7 ± 5.5 | 0.144 |
| Medications | |||
| Soluble guanylate cyclase-stimulator, n (%) | 24 (100) | 24 (100) | 1.000 |
| Selective prostacyclin receptor agonist, n (%) | 1 (4.2) | 3 (12.5) | 0.500 |
| Prostacyclin analog, n (%) | 2 (8.3) | 2 (8.3) | 1.000 |
| Warfarin, n (%) | 6 (25.0) | 5 (20.8) | 1.000 |
| DOAC, n (%) | 18 (75.0) | 19 (79.1) | 1.000 |
| Cardiopulmonary exercise testing | |||
| peak VO2, mL/kg/min | 14.1 [12.4, 16.1] | 15.6 [13.5, 18.4] | 0.006 |
| VO2 AT, mL/kg/min | 8.9 [7.7, 9.4] | 9.1 [7.7, 9.8] | 0.948 |
| WR peak, watt | 67.0 [52.5, 80.0] | 76.5 [58.3, 88.8] | 0.011 |
| VE peak, L/min | 50.1 [36.0, 59.1] | 48.1 [38.2, 59.2] | 0.794 |
| VE AT, L/min | 23.2 [18.2, 26.2] | 19.3 [16.9, 24.1] | 0.231 |
| OUES | 1105.0 [884.0, 1233.0] | 1111.0 [995.5, 1451.5] | 0.049 |
| VE/VCO2 slope | 45.6 [39.2, 52.6] | 36.9 [32.3, 39.9] | <0.001 |
| Lowest VE/VCO2 | 42.6 [39.8, 48.7] | 37.7 [32.9, 42.7] | <0.001 |
| VO2/WR slope | 8.9 [8.0, 10.3] | 9.6 [8.3, 10.5] | 0.407 |
| Lowest SpO2, % | 88.5 [85.8, 92.3] | 91.0 [89.3, 93.8] | 0.005 |
| Quality of life | |||
| emPHasis-10 | 22.5 ± 9.5 | 14.5 ± 10.4 | 0.009 |
| Exercise Group (n = 12) | Control Group (n = 12) | Group Difference; p-Value | Group Difference; Adjusted p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | Baseline | Follow-Up | Baseline | Follow-Up | |
| Right heart catheterization | ||||||||
| mean PAP, mmHg | 24.1 ± 5.6 | 24.9 ± 7.4 | 23.8 ± 3.3 | 23.0 ± 4.6 | 0.860 | 0.455 | 0.991 | 0.948 |
| mean RAP, mmHg | 2.57 ± 2.4 | 4.8 ± 1.8 | 3.9 ± 3.4 | 4.1 ± 2.9 | 0.403 | 0.492 | 0.991 | 0.948 |
| mean PAWP, mmHg | 6.3 ± 2.8 | 10.3 ± 6.0 | 8.9 ± 3.6 | 9.5 ± 5.6 | 0.132 | 0.729 | 0.717 | 0.948 |
| PVR, wood u | 4.0 ± 1.9 | 3.1 ± 1.8 | 2.7 ± 0.9 | 3.0 ± 0.7 | 0.109 | 0.804 | 0.716 | 0.948 |
| CO, L/min | 5.8 ± 1.1 | 5.1 ± 1.5 | 5.6 ± 1.1 | 4.6 ± 0.9 | 0.743 | 0.296 | 0.991 | 0.948 |
| CI, L/min/m2 | 3.6 ± 0.3 | 3.2 ± 0.7 | 3.6 ± 0.7 | 3.0 ± 0.5 | 0.933 | 0.381 | 0.991 | 0.948 |
| Echocardiographic findings | ||||||||
| LVDd, mm | 38.5 ± 3.4 | 43.6 ± 4.6 | 43.3 ± 4.1 | 44.5 ± 6.2 | 0.045 | 0.727 | 0.716 | 0.948 |
| LVDs, mm | 23.4 ± 4.0 | 27.1 ± 3.9 | 26.8 ± 3.4 | 27.5 ± 4.6 | 0.113 | 0.845 | 0.716 | 0.948 |
| LVEF, % | 71.9 ± 4.2 | 68.3 ± 4.5 | 65.5 ± 4.6 | 67.9 ± 4.7 | 0.025 | 0.847 | 0.716 | 0.948 |
| TRPG, mmHg | 30.0 ± 16.2 | 25.7 ± 14.6 | 26.5 ± 12.4 | 27.2 ± 8.6 | 0.684 | 0.790 | 0.991 | 0.948 |
| TAPSE, mm | 9.1 ± 10.1 | 14.3 ± 9.3 | 13.5 ± 7.7 | 13.5 ± 7.6 | 0.375 | 0.819 | 0.991 | 0.948 |
| Muscle strength | ||||||||
| Knee extension Rt, Nm | 31.4 ± 9.8 | 37.6 ± 10.6 | 34.3 ± 14.3 | 35.1 ± 17.7 | 0.585 | 0.701 | 0.991 | 0.948 |
| Knee extension Lt, Nm | 31.0 ± 10.6 | 36.5 ± 9.1 | 33.3 ± 16.5 | 35.5 ± 17.5 | 0.703 | 0.875 | 0.991 | 0.948 |
| Hand grip Rt, kg | 25.5 ± 6.1 | 27.9 ± 5.1 | 25.8 ± 7.2 | 27.7 ± 7.3 | 0.919 | 0.933 | 0.991 | 0.958 |
| Hand grip Lt, kg | 23.0 ± 5.5 | 25.6 ± 5.5 | 22.8 ± 5.6 | 25.1 ± 6.8 | 0.939 | 0.849 | 0.991 | 0.948 |
| Body composition | ||||||||
| Skeletal muscle index, kg/m2 | 6.20 [5.85, 7.03] | 6.25 [5.60, 7.03] | 6.30 [5.73, 7.40] | 6.20 [5.50, 7.25] | 0.692 | 0.902 | 0.991 | 0.951 |
| Skeletal muscle mass, kg | 20.70 [18.85, 22.48] | 20.55 [18.95, 23.52] | 19.05 [16.75, 23.30] | 17.80 [16.45, 24.50] | 0.575 | 0.498 | 0.991 | 0.948 |
| Body fat mass, kg | 17.90 [15.68, 20.33] | 18.30 [16.23, 19.35] | 26.40 [15.30, 29.50] | 20.60 [14.35, 26.05] | 0.477 | 0.805 | 0.991 | 0.948 |
| Percent body fat, % | 32.20 [30.68, 34.05] | 32.10 [30.45, 34.97] | 35.10 [29.58, 40.80] | 32.90 [29.15, 39.30] | 0.468 | 0.689 | 0.991 | 0.948 |
| Magnetic resonance imaging of the skeletal muscle | ||||||||
| IMCL, mmol/kg | 13.30 [6.18, 16.74] | 9.86 [7.84, 13.51] | 6.88 [1.34, 10.84] | 5.67 [3.73, 14.24] | 0.205 | 0.481 | 0.866 | 0.948 |
| EMCL, mmol/kg | 16.56 [12.30, 19.94] | 14.72 [9.66, 24.15] | 23.47 [8.67, 27.24] | 26.57 [11.16, 34.59] | 0.841 | 0.181 | 0.991 | 0.882 |
References
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports. 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Mordi, I.R.; Bridges, C.; Sagar, V.A.; Davies, E.J.; Coats, A.J.; Dalal, H.; Rees, K.; Singh, S.J.; Taylor, R.S. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst. Rev. 2019, 1, CD003331. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; von Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Channick, R.N.; Frantz, R.P.; Grünig, E.; Jing, Z.C.; Moiseeva, O.; Preston, I.R.; Pulido, T.; Safdar, Z.; Tamura, Y.; et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801889. [Google Scholar] [CrossRef] [PubMed]
- Nagel, C.; Prange, F.; Guth, S.; Herb, J.; Ehlken, N.; Fischer, C.; Reichenberger, F.; Rosenkranz, S.; Seyfarth, H.-J.; Mayer, E.; et al. Exercise training improves exercise capacity and quality of life in patients with inoperable or residual chronic thromboembolic pulmonary hypertension. PLoS ONE 2012, 7, e41603. [Google Scholar] [CrossRef] [PubMed]
- Grünig, E.; Eichstaedt, C.; Barberà, J.A.; Benjamin, N.; Blanco, I.; Bossone, E.; Cittadini, A.; Coghlan, G.; Corris, P.; D’Alto, M.; et al. ERS statement on exercise training and rehabilitation in patients with severe chronic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1800332. [Google Scholar] [CrossRef] [PubMed]
- González-Saiz, L.; Fiuza-Luces, C.; Sanchis-Gomar, F.; Santos-Lozano, A.; Quezada-Loaiza, C.A.; Flox-Camacho, A.; Munguía-Izquierdo, D.; Ara, I.; Santalla, A.; Morán, M.; et al. Benefits of skeletal-muscle exercise training in pulmonary arterial hypertension: The WHOLEi+12 trial. Int. J. Cardio 2017, 231, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Ehlken, N.; Lichtblau, M.; Klose, H.; Weidenhammer, J.; Fischer, C.; Nechwatal, R.; Uiker, S.; Halank, M.; Olsson, K.; Seeger, W.; et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: A prospective, randomized, controlled trial. Eur. Heart J. 2016, 37, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Morris, N.R.; Kermeen, F.D.; Jones, A.W.; Lee, J.Y.; Holland, A.E. Exercise-based rehabilitation programmes for pulmonary hypertension. Cochrane Database Syst. Rev. 2023, 3, CD011285. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.M.; Wiedenroth, C.B.; Kamp, J.C.; Breithecker, A.; Fuge, J.; Krombach, G.A.; Haas, M.; Hamm, C.; Kramm, T.; Guth, S.; et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: The initial German experience. Eur. Respir. J. 2017, 49, 1602409. [Google Scholar] [CrossRef] [PubMed]
- Brenot, P.; Jaïs, X.; Taniguchi, Y.; Garcia Alonso, C.; Gerardin, B.; Mussot, S.; Mercier, O.; Fabre, D.; Parent, F.; Jevnikar, M.; et al. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1802095. [Google Scholar] [CrossRef] [PubMed]
- Darocha, S.; Pietura, R.; Pietrasik, A.; Norwa, J.; Dobosiewicz, A.; Piłka, M.; Florczyk, M.; Biederman, A.; Torbicki, A.; Kurzyna, M. Improvement in Quality of Life and Hemodynamics in Chronic Thromboembolic Pulmonary Hypertension Treated with Balloon Pulmonary Angioplasty. Circ. J. 2017, 81, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Ogo, T.; Morita, Y.; Tsuji, A.; Tateishi, E.; Ozaki, K.; Sanda, Y.; Fukuda, T.; Yasuda, S.; Ogawa, H.; et al. Right ventricular reverse remodelling after balloon pulmonary angioplasty. Eur. Respir. J. 2014, 43, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Tsugu, T.; Murata, M.; Kawakami, T.; Yasuda, R.; Tokuda, H.; Minakata, Y.; Tamura, Y.; Kataoka, M.; Hayashida, K.; Tsuruta, H.; et al. Significance of echocardiographic assessment for right ventricular function after balloon pulmonary angioplasty in patients with chronic thromboembolic induced pulmonary hypertension. Am. J. Cardiol. 2015, 115, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Ogo, T.; Goto, Y.; Ueda, J.; Tsuji, A.; Sanda, Y.; Kumasaka, R.; Arakawa, T.; Nakanishi, M.; Fukuda, T.; et al. Exercise intolerance and ventilatory inefficiency improve early after balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Int. J. Cardiol 2015, 180, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Anghel, R.; Adam, C.A.; Marcu, D.T.M.; Mitu, O.; Mitu, F. Cardiac Rehabilitation in Patients with Peripheral Artery Disease-A Literature Review in COVID-19 Era. J. Clin. Med. 2022, 11, 416. [Google Scholar] [CrossRef] [PubMed]
- Passantino, A.; Dalla Vecchia, L.A.; Corrà, U.; Scalvini, S.; Pistono, M.; Bussotti, M.; Gambarin, F.I.; Scrutinio, D.; La Rovere, M.T. The Future of Exercise-Based Cardiac Rehabilitation for Patients with Heart Failure. Front Cardiovasc. Med. 2021, 8, 709898. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Kohno, T.; Kawakami, T.; Kataoka, M.; Tsugu, T.; Akita, K.; Isobe, S.; Itabashi, Y.; Maekawa, Y.; Murata, M.; et al. Midterm Effect of Balloon Pulmonary Angioplasty on Hemodynamics and Subclinical Myocardial Damage in Chronic Thromboembolic Pulmonary Hypertension. Can. J. Cardiol. 2017, 33, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Saitoh, T.; Iguchi, K.; Suwa, K.; Ohtani, H.; Saotome, M.; Maekawa, Y. Electrocardiogram Electrode Positioning on the Back During Echocardiography: An Exploratory Cross-Sectional Study. Cureus 2024, 16, e57967. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Kataoka, M.; Kawakami, T.; Fukuoka, R.; Tamura, Y.; Kimura, M.; Takei, M.; Maekawa, Y.; Sano, M.; Fukuda, K. Moderate-to-severe obstructive sleep apnea is associated with subclinical myocardial injury and impaired hemodynamics in pulmonary hypertension patients. Sleep Med. 2017, 30, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Huscher, D.; Vonk-Noordegraaf, A.; Ewert, R.; Lange, T.J.; Klose, H.; Dumitrescu, D.; Halank, M.; Held, M.; Gall, H.; et al. The 6MWT as a prognostic tool in pulmonary arterial hypertension: Results from the COMPERA registry. Clin. Res. Cardiol. 2018, 107, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Mathai, S.C.; Puhan, M.A.; Lam, D.; Wise, R.A. The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Satake, S.; Arai, H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr. Gerontol. Int. 2020, 20, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Yorke, J.; Corris, P.; Gaine, S.; Gibbs, J.S.; Kiely, D.G.; Harries, C.; Pollock, V.; Armstrong, I. emPHasis-10: Development of a health-related quality of life measure in pulmonary hypertension. Eur. Respir. J. 2014, 43, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M.; Andreassen, A.K.; Andersen, A.; Bouvaist, H.; Coghlan, G.; Escribano-Subias, P.; Jansa, P.; Kopec, G.; Kurzyna, M.; Matsubara, H.; et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: A clinical consensus statement of the ESC working group on pulmonary circulation and right ventricular function. Eur. Heart J. 2023, 44, 2659–2671. [Google Scholar] [CrossRef] [PubMed]
- Grünig, E.; MacKenzie, A.; Peacock, A.J.; Eichstaedt, C.A.; Benjamin, N.; Nechwatal, R.; Ulrich, S.; Saxer, S.; Bussotti, M.; Sommaruga, M.; et al. Standardized exercise training is feasible, safe, and effective in pulmonary arterial and chronic thromboembolic pulmonary hypertension: Results from a large European multicentre randomized controlled trial. Eur. Heart J. 2021, 42, 2284–2295. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Zhao, Z.; Liu, Z.; Ma, X.; Luo, Q.; Liu, W. The lowest VE/VCO2 ratio best identifies chronic thromboembolic pulmonary hypertension. Thromb. Res. 2014, 134, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Ogo, T.; Takaki, H.; Ueda, J.; Tsuji, A.; Morita, Y.; Kumasaka, R.; Arakawa, T.; Nakanishi, M.; Fukuda, T.; et al. Efficacy of cardiac rehabilitation after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Heart 2016, 102, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
| Exercise Group (n = 12) | Control Group (n = 12) | p-Value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, years | 54.5 ± 15.6 | 63.2 ± 15.5 | 0.186 |
| Female, n (%) | 11 (91.7) | 10 (83.3) | 0.500 |
| Body mass index, kg/m2 | 22.8 ± 3.6 | 24.8 ± 4.9 | 0.261 |
| WHO-FC II or III or IV n (%) | 6 (50.0) | 8 (66.7) | 0.408 |
| Comorbidities | |||
| Hypertension | 0 (0.0) | 5 (41.6) | 0.019 |
| Dyslipidemia | 3 (25.0) | 5 (41.6) | 0.333 |
| Diabetes | 0 (0.0) | 1 (8.3) | 0.500 |
| Chronic kidney disease | 1 (8.3) | 3 (25.0) | 0.295 |
| Cerebrovascular disease | 1 (8.3) | 1 (8.3) | 0.761 |
| Coronary artery disease | 0 (0.0) | 1 (8.3) | 0.500 |
| History of acute pulmonary embolism | 1 (8.3) | 2 (16.7) | 0.500 |
| Blood test | |||
| NT-proBNP, pg/mL | 80.5 [50.3, 113.3] | 58.5 [43.5, 67.3] | 0.347 |
| Soluble fibrin, μg/mL | 1.5 ± 1.7 | 2.5 ± 1.2 | 0.132 |
| eGFR, mL/min/1.73 m2 | 72.2 ± 17.7 | 62.6 ± 15.3 | 0.169 |
| Medications | |||
| Soluble guanylate cyclase-stimulator, n (%) | 12 (100) | 12 (100) | 1.000 |
| Selective prostacyclin receptor agonist, n (%) | 3 (25.0) | 0 (0.0) | 0.109 |
| Prostacyclin analog, n (%) | 1 (8.3) | 1 (8.3) | 0.761 |
| Warfarin, n (%) | 2 (16.7) | 4 (33.3) | 0.158 |
| DOAC, n (%) | 10 (83.3) | 8 (66.7) | 0.320 |
| Diuretic, n (%) | 6 (50.0) | 6 (50.0) | 0.658 |
| Exercise Group (n = 12) | Control Group (n = 12) | Group Difference; p-Value | Group Difference; Adjusted p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | Baseline | Follow-Up | Baseline | Follow-Up | |
| Walking test | ||||||||
| 6MWD, m | 440.0 [401.3, 510.0] | 510.0 [467.5, 595.0] | 430.0 [348.8, 471.3] | 425.0 [395.0, 465.0] | 0.574 | 0.002 | 0.991 | 0.020 |
| 10 m walk test, s | 7.3 [6.9, 8.3] | 6.4 [5.9, 7.5] | 8.1 [7.4, 9.3] | 8.9 [8.1, 9.1] | 0.076 | 0.001 | 0.716 | 0.020 |
| Gait speed, m/s | 1.4 [1.2, 1.5] | 1.6 [1.3, 1.7] | 1.2 [1.1, 1.4] | 1.1 [1.1, 1.2] | 0.076 | 0.001 | 0.716 | 0.020 |
| Change in 6MWD | 75.0 [42.5, 101.3] | −10.0 [−20.0, 13.8] | 0.002 | 0.020 | ||||
| Cardiopulmonary exercise testing | ||||||||
| peak VO2, mL/kg/min | 17.24 [14.62, 19.10] | 16.83 [14.94, 19.09] | 14.16 [13.04, 15.56] | 14.36 [13.87, 16.88] | 0.165 | 0.102 | 0.784 | 0.568 |
| AT, mL/kg/min | 8.71 [7.70, 10.01] | 9.53 [9.25, 11.34] | 9.24 [7.65, 9.83] | 9.66 [8.41, 9.93] | 1.000 | 0.310 | 1.000 | 0.948 |
| WR peak, watt | 82.50 [64.25, 90.00] | 84.00 [66.00, 100.00] | 67.00 [57.75, 85.00] | 67.00 [60.00, 82.00] | 0.487 | 0.331 | 0.991 | 0.948 |
| VE peak, L/min | 49.00 [41.55, 69.28 | 53.50 [38.30, 75.40] | 47.25 [35.95, 55.45] | 46.90 [37.10, 56.80] | 0.375 | 0.310 | 0.991 | 0.948 |
| VE AT, L/min | 18.30 [15.80, 22.27] | 23.30 [20.30, 26.10] | 20.25 [18.98, 24.08] | 21.00 [18.50, 24.90] | 0.280 | 0.757 | 0.991 | 0.948 |
| OUES | 1111.00 [1003.00, 1338.00] | 1084.00 [980.00, 1351.00] | 1192.00 [943.75, 1474.75] | 1171.00 [1060.75, 1550.50] | 0.934 | 0.637 | 0.991 | 0.948 |
| VE/VCO2 slope | 39.00 [32.95, 40.60] | 37.50 [30.20, 42.80] | 34.80 [32.80, 37.10] | 36.20 [33.00, 38.00] | 0.355 | 0.724 | 0.991 | 0.948 |
| Lowest VE/VCO2 | 40.75 [31.27, 43.88] | 37.30 [32.10, 42.30] | 35.75 [34.28, 39.15] | 38.80 [36.20, 41.10] | 0.563 | 0.965 | 0.991 | 0.965 |
| VO2/WR slope | 9.78 [9.18, 10.50] | 9.83 [8.47, 9.89] | 8.55 [8.10, 9.83] | 9.18 [8.59, 10.10] | 0.297 | 0.859 | 0.991 | 0.948 |
| Lowest SpO2, % | 91.00 [88.00, 94.00] | 91.50 [88.75, 93.50] | 91.00 [89.50, 93.00] | 92.50 [90.25, 94.00] | 0.928 | 0.516 | 0.991 | 0.948 |
| peak RER | 1.21 [1.17, 1.37] | 1.21 [1.17, 1.24] | 1.25 [1.18, 1.27] | 1.24 [1.16, 1.24] | 0.938 | 0.824 | 0.991 | 0.948 |
| peak VO2/HR | 7.11 [5.37, 7.43] | 7.33 [5.36, 7.60] | 5.68 [5.05, 7.10] | 5.80 [5.60, 6.80] | 0.700 | 0.508 | 0.991 | 0.948 |
| J-CHS categorization | ||||||||
| Pre-Frail or Frail, n (%) | 9 (75.0) | 1 (8.3) | 8 (66.7) | 9 (75.0) | 1.000 | 0.003 | 1.000 | 0.023 |
| Quality of life | ||||||||
| emPHasis-10 | 15.8 ± 11.0 | 10.2 ± 8.0 | 16.4 ± 11.9 | 17.7 ± 10.1 | 0.891 | 0.008 | 0.991 | 0.052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuda, T.; Akita, K.; Sato, R.; Ikoma, T.; Mizuno, Y.; Satoh, T.; Takao, M.; Suwa, K.; Shimizu, M.; Odagiri, K.; et al. The Efficacy and Safety of Outpatient Exercise Training for Patients with Chronic Thromboembolic Pulmonary Hypertension After Balloon Pulmonary Angioplasty. J. Cardiovasc. Dev. Dis. 2025, 12, 216. https://doi.org/10.3390/jcdd12060216
Masuda T, Akita K, Sato R, Ikoma T, Mizuno Y, Satoh T, Takao M, Suwa K, Shimizu M, Odagiri K, et al. The Efficacy and Safety of Outpatient Exercise Training for Patients with Chronic Thromboembolic Pulmonary Hypertension After Balloon Pulmonary Angioplasty. Journal of Cardiovascular Development and Disease. 2025; 12(6):216. https://doi.org/10.3390/jcdd12060216
Chicago/Turabian StyleMasuda, Takayuki, Keitaro Akita, Ryota Sato, Takenori Ikoma, Yusuke Mizuno, Terumori Satoh, Masashi Takao, Kenichiro Suwa, Mikihiro Shimizu, Keiichi Odagiri, and et al. 2025. "The Efficacy and Safety of Outpatient Exercise Training for Patients with Chronic Thromboembolic Pulmonary Hypertension After Balloon Pulmonary Angioplasty" Journal of Cardiovascular Development and Disease 12, no. 6: 216. https://doi.org/10.3390/jcdd12060216
APA StyleMasuda, T., Akita, K., Sato, R., Ikoma, T., Mizuno, Y., Satoh, T., Takao, M., Suwa, K., Shimizu, M., Odagiri, K., Yamauchi, K., & Maekawa, Y. (2025). The Efficacy and Safety of Outpatient Exercise Training for Patients with Chronic Thromboembolic Pulmonary Hypertension After Balloon Pulmonary Angioplasty. Journal of Cardiovascular Development and Disease, 12(6), 216. https://doi.org/10.3390/jcdd12060216







