Outcomes and Cost-Effectiveness of Transcatheter Versus Surgical Aortic Valve Replacement in Patients with and Without Coronary Artery Disease
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carabello, B.A.; Paulus, W.J. Aortic stenosis. Lancet 2009, 373, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Moat, N.E.; Ludman, P.; de, B.M.A.; Bridgewater, B.; Cunningham, A.D.; Young, C.P.; Thomas, M.; Kovac, J.; Spyt, T.; MacCarthy, P.A.; et al. Long-Term Outcomes After Transcatheter Aortic Valve Implantation in High-Risk Patients With Severe Aortic Stenosis. JACC 2011, 58, 2130–2138. [Google Scholar] [CrossRef] [PubMed]
- Thyregod, H.G.H.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Faroux, L.; Villecourt, A.; Metz, D. The Management of Coronary Artery Disease in TAVR Patients. J. Clin. Med. 2023, 12, 7126. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes: Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, ehae177. [Google Scholar] [CrossRef]
- Alperi, A.; Mohammadi, S.; Campelo-Parada, F.; Munoz-Garcia, E.; Nombela-Franco, L.; Faroux, L.; Veiga, G.; Serra, V.; Fischer, Q.; Pascual, I.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Complex Coronary Artery Disease. JACC Cardiovasc. Interv. 2021, 14, 2490–2499. [Google Scholar] [CrossRef] [PubMed]
- Ullah, W.; Sarvepalli, D.; Kumar, A.; Zahid, S.; Saleem, S.; Muhammadzai, H.Z.U.; Khattak, F.; Block, P.C.; Jaber, W.A.; Shishehbor, M.H.; et al. Trends and outcomes of combined percutaneous (TAVI+PCI) and surgical approach (SAVR+CABG) for patients with aortic valve and coronary artery disease: A National Readmission Database (NRD) analysis. Catheter. Cardiovasc. Interv. 2023, 102, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Lemor, A.; Villablanca, P.; Hernandez, G.; Dyal, M.; Jain, T.; Frisoli, T.M.; Wang, D.D.; Eng, M.H.; O’Neill, W. Comparison of Outcomes of Transcatheter Versus Surgical Aortic Valve Replacement in Patients ≥80 Years of Age. Am. J. Cardiol. 2019, 123. [Google Scholar] [CrossRef] [PubMed]
- Talanas, G.; Laconi, A.; Kereiakes, D.J.; Merella, P.; Reardon, M.J.; Spano, A.; Petretto, G.; Lauriola, F.; Casula, M.; Micheluzzi, V.; et al. Long-Term Outcomes of Transcatheter vs Surgical Aortic Valve Replacement: Meta-analysis of Randomized Trials. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3. [Google Scholar] [CrossRef] [PubMed]
- Amat-Santos, I.J.; García-Gómez, M.; Avanzas, P.; Jiménez-Diaz, V.; Alonso-Briales, J.H.; de la Torre Hernández, J.M.; Sanz-Sánchez, J.; Diarte-de Miguel, J.A.; Sánchez-Recalde, Á.; Nombela-Franco, L.; et al. Surgical vs Transcatheter Treatment in Patients With Coronary Artery Disease and Severe Aortic Stenosis. JACC Cardiovasc. Interv. 2024, 17, 2472–2485. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, W.; Wu, H. Percutaneous versus surgical approach to aortic valve replacement with coronary revascularization: A systematic review andmeta-analysis. Perfusion 2024, 39, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Călburean, P.-A.; Grebenișan, P.; Nistor, I.-A.; Șulea, I.P.; Scurtu, A.-C.; Brinzaniuc, K.; Suciu, H.; Harpa, M.; Dobreanu, D.; Hadadi, L. High long-term mortality in ischaemic heart disease accentuated among ethnic minorities in Eastern Europe: Findings from a prospective all-comers percutaneous coronary intervention registry in Romania. J. Epidemiol. Community Health 2025, 79, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Călburean, P.-A.; Grebenișan, P.; Nistor, I.-A.; Pal, K.; Vacariu, V.; Drincal, R.-K.; Ion, A.A.; Adorján, I.; Oltean, T.; Hadadi, L. Addition of eptifibatide and manual thrombus aspiration to ticagrelor does not improve long-term survival after STEMI treated with primary PCI. Front. Pharmacol. 2024, 15, 1415025. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, P.; Belmonte, M.; Gallinoro, E.; Scarsini, R.; Bergamaschi, L.; Portolan, L.; Armillotta, M.; Esposito, G.; Moscarella, E.; Benfari, G.; et al. SGLT2-inhibitors in diabetic patients with severe aortic stenosis and cardiac damage undergoing transcatheter aortic valve implantation (TAVI). Cardiovasc. Diabetol. 2024, 23, 420. [Google Scholar] [CrossRef] [PubMed]
- Armillotta, M.; Angeli, F.; Paolisso, P.; Belmonte, M.; Raschi, E.; Di Dalmazi, G.; Amicone, S.; Canton, L.; Fedele, D.; Suma, N.; et al. Cardiovascular therapeutic targets of sodium-glucose co-transporter 2 (SGLT2) inhibitors beyond heart failure. Pharmacol. Ther. 2025, 270, 108861. [Google Scholar] [CrossRef] [PubMed]
- Tam, D.Y.; Hughes, A.; Fremes, S.E.; Youn, S.; Hancock-Howard, R.L.; Coyte, P.C.; Wijeysundera, H.C. A cost-utility analysis of transcatheter versus surgical aortic valve replacement for the treatment of aortic stenosis in the population with intermediate surgical risk. J. Thorac. Cardiovasc. Surg. 2018, 155, 1978–1988.e1. [Google Scholar] [CrossRef] [PubMed]
- Geisler, B.P.; Jørgensen, T.H.; Thyregod, H.G.H.; Pietzsch, J.B.; Søndergaard, L. Cost-effectiveness of transcatheter versus surgical aortic valve replacement in patients at lower surgical risk: Results from the NOTION trial. EuroIntervention 2019, 15, e959–e967. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.J.; Wang, K.; House, J.A.; Magnuson, E.A.; Reynolds, M.R.; Makkar, R.; Herrmann, H.C.; Kodali, S.; Thourani, V.H.; Kapadia, S.; et al. Cost-Effectiveness of Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Intermediate Risk. Circulation 2019, 139, 877–888. [Google Scholar] [CrossRef] [PubMed]
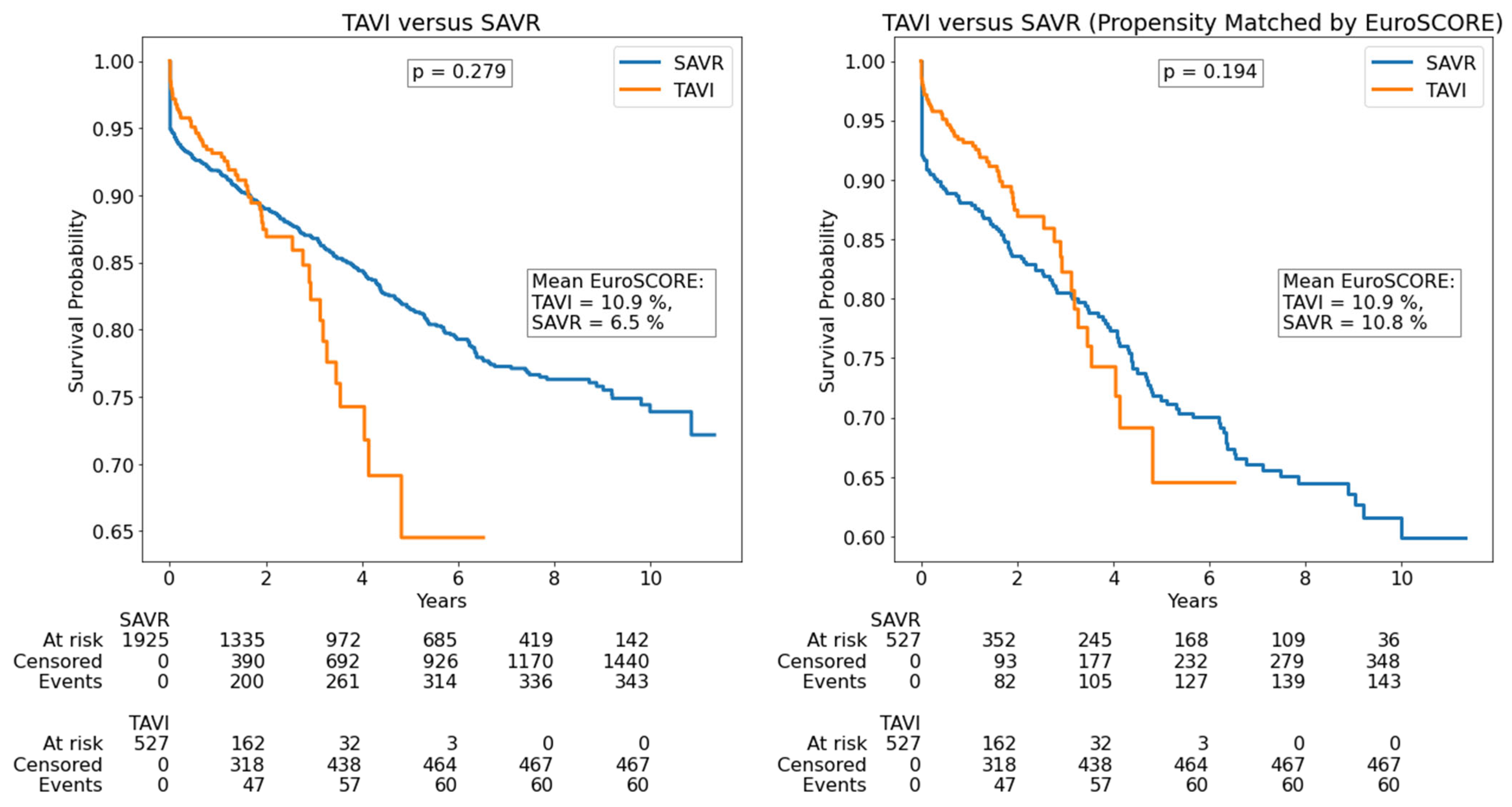
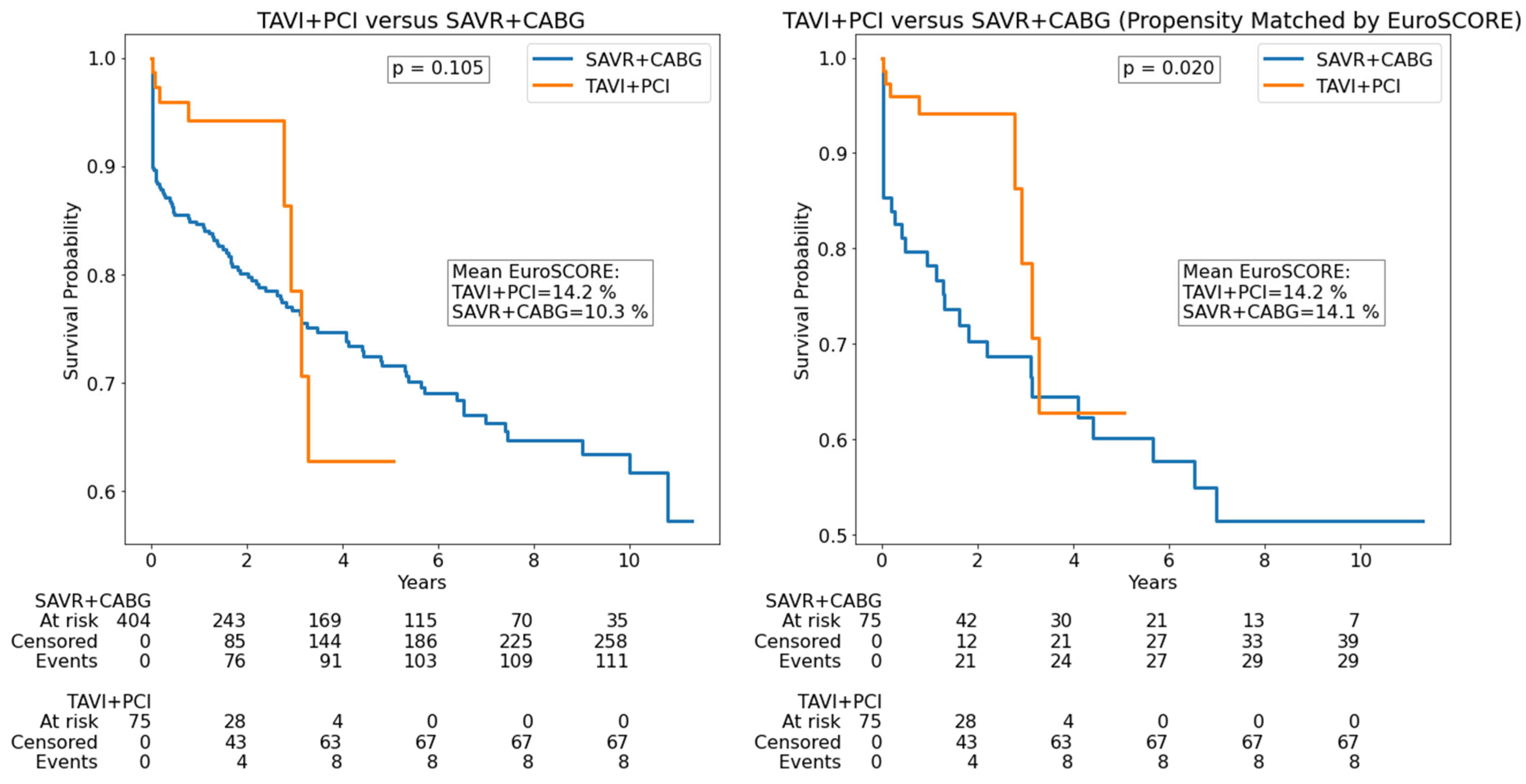
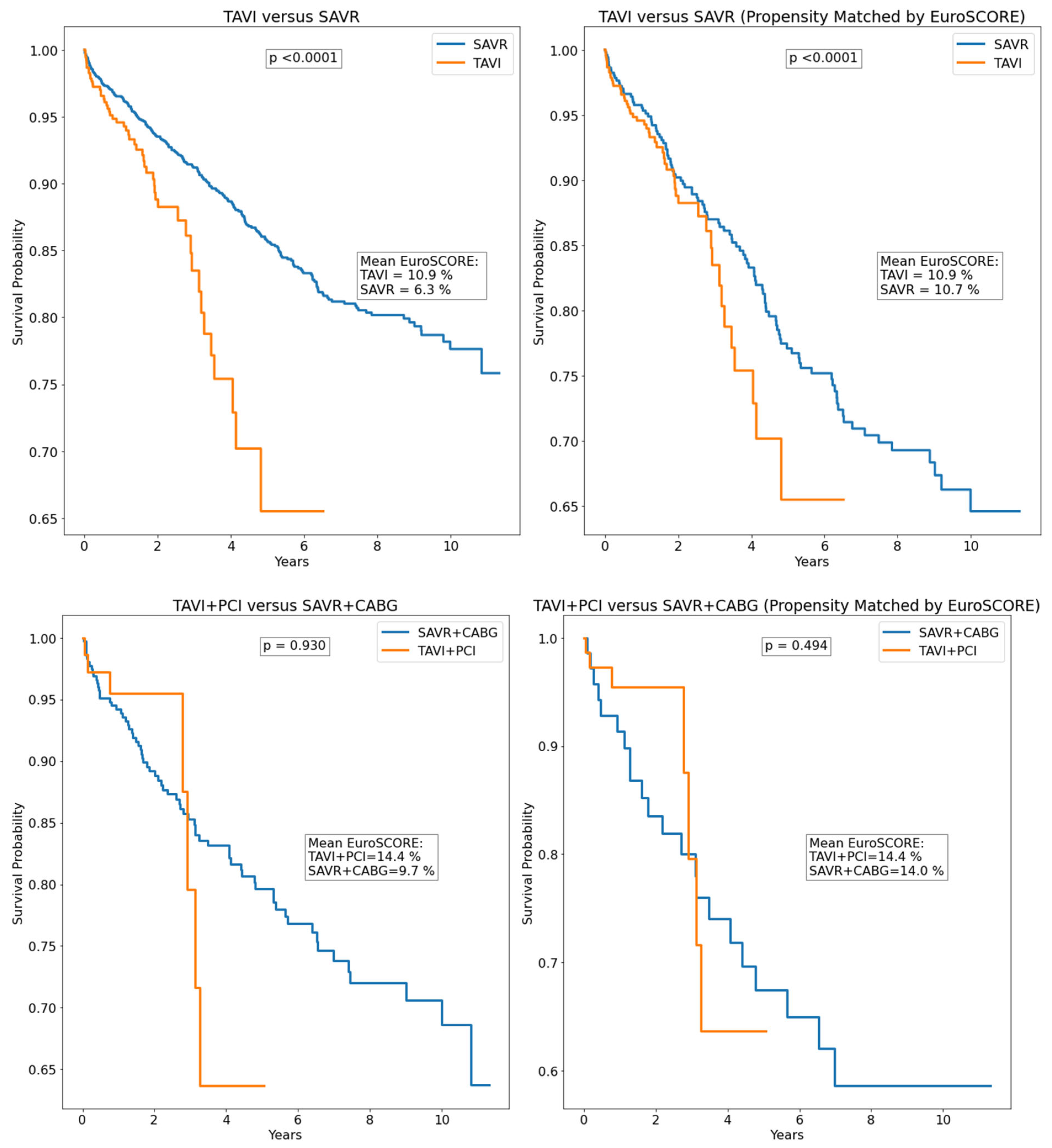
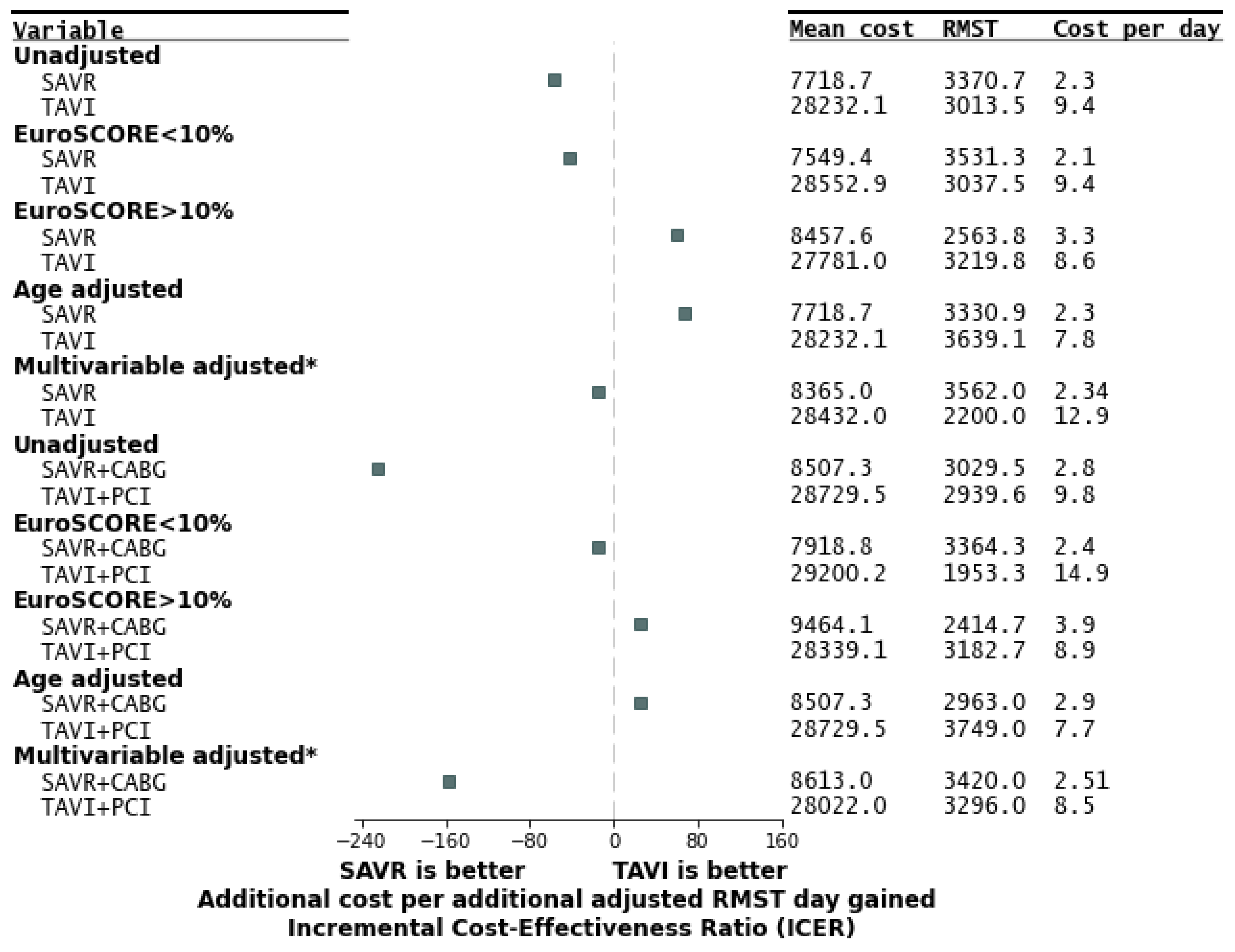
| Parameter | All Patients (n = 2452) | SAVR (n = 1925) | TAVI (n = 527) | p |
|---|---|---|---|---|
| Age (years) | 68.5 (61.6–73.8) | 66.4(59.1–71.1) | 77.3(73.3–81.1) | <0.0001 |
| Male sex | 1423 (58.03%) | 1134 (58.91%) | 289 (54.84%) | 0.10 |
| Cost (EUR ×1000) | 8.42(4.68–15.54) | 6.64(4.18–9.69) | 28.00(26.17–29.93) | <0.0001 |
| Hospitalization length (days) | 10 (8–14) | 11 (9–14) | 7 (5–10) | <0.0001 |
| Hypercholesterolemia | 1021 (41.64%) | 761 (39.53%) | 260 (49.34%) | 0.0005 |
| Diabetes mellitus | 757 (30.87%) | 557 (28.94%) | 200 (37.95%) | 0.001 |
| HBP | 1923 (78.43%) | 1464 (76.05%) | 459 (87.1%) | 0.0005 |
| Atrial fibrillation | 707 (28.83%) | 509 (26.44%) | 198 (37.57%) | 0.0005 |
| CKD | 135 (5.51%) | 55 (2.86%) | 80 (15.18%) | 0.0005 |
| History of stroke | 110 (4.49%) | 70 (3.64%) | 40 (7.59%) | 0.001 |
| History of MI | 344 (14.03%) | 237 (12.31%) | 107 (20.3%) | 0.0005 |
| LBBB | 141 (5.75%) | 51 (2.65%) | 90 (17.08%) | 0.0005 |
| Smoker | 143 (5.83%) | 126 (6.55%) | 17 (3.23%) | 0.0025 |
| COPD | 122 (4.98%) | 83 (4.31%) | 39 (7.4%) | 0.005 |
| DCM | 115 (4.69%) | 75 (3.9%) | 40 (7.59%) | 0.0005 |
| CAD | 537 (21.9%) | 415 (21.5%) | 122 (23.1%) | 0.73 |
| PAD | 167 (6.8%) | 103 (5.3%) | 64 (12.1%) | <0.0001 |
| Creatinine (mg/dL) | 1.01(0.82–1.27) | 1 (0.8–1.25) | 1.05 (0.85–1.31) | 0.02 |
| Hemoglobin (×103/µL) | 13.2(12–14.2) | 13.3 (12.1–14.28) | 12.77 ± 1.68 | <0.0001 |
| Platelets (×103/µL) | 206 (172–247) | 206 (173–247) | 205 (170- 246) | 0.60 |
| Leucocytes (×103/µL) | 7.13 (5.99–8.42) | 7.19 (6–8.45) | 6.98 (5.89–8.26) | 0.24 |
| LVEF (%) | 55 (50–60) | 55 (50–60) | 50 (45–50) | 0.0001 |
| Mean AV gradient (mmHg) | 46.2 (39.9–53.2) | 48.5 (42.8–57) | 44.1 (39.7–52.9) | 0.11 |
| Maximum AV velocity (m/s) | 4.31 (3.84–5.12) | 4.49 (4–5.33) | 4.27 (3.8–5.07) | 0.09 |
| Mean AVA (cm2) | 0.79 (0.71–0.99) | 0.75 (0.67–0.86) | 0.82 (0.72–0.92) | 0.04 |
| Post-procedure mean AV gradient (mmHg) | 11.9 (10.4–13.9) | 12.2 (10.4–13.9) | 11.5 (9.8–13.1) | 0.75 |
| Low-flow low-gradient severe AS | 111 (4.5%) | 86 (1.1%) | 25 (4.1%) | 0.87 |
| EuroSCORE (%) | 8.72 ± 7.82 | 6.44 ± 5.82 | 10.94 ± 8.12 | <0.0001 |
| EuroSCORE > 10% | 799 (32.5%) | 360 (18.7%) | 439 (83.3%) | <0.0001 |
| Complication | SAVR (n = 1925) | TAVI (n = 527) | p |
| Stroke | 9 (0.6%) | 2 (0.3%) | 0.78 |
| Tamponade | 7 (0.3%) | 2 (0.3%) | 0.95 |
| Acute kidney injury | 77 (4.0%) | 8 (1.5%) | 0.008 |
| Dialysis | 27 (1.4%) | 2 (0.3%) | 0.08 |
| Bleeding requiring transfusion * | 266 (13.8%) | 26 (4.9%) | <0.0001 |
| Pacemaker implantation ** | 40 (2.0%) | 55 (10.4%) | <0.0001 |
| In-hospital mortality | 93 (4.8%) | 8 (1.5%) | 0.001 |
| Complication | SAVR + CABG (n = 327) | TAVI + PCI (n = 75) | p |
| Stroke | 8 (2.1%) | 1 (1.3%) | 0.99 |
| Tamponade | 3 (0.9%) | 1 (1.3%) | 0.56 |
| Acute kidney injury | 52 (15.9%) | 4 (5.3%) | 0.01 |
| Dialysis | 5 (1.5%) | 1 (1.3%) | 0.99 |
| Bleeding requiring transfusion * | 67 (20.4%) | 3 (4.0%) | 0.0003 |
| Pacemaker implantation ** | 9 (2.7%) | 9 (12.0%) | 0.002 |
| In-hospital mortality | 26 (7.9%) | 1 (1.3%) | 0.03 |
| Parameter | Univariate Cox Regression | Stepwise Multivariate Cox Regression |
|---|---|---|
| Age (years) | p < 0.001, HR = 1.05 (1.03–1.06) | p < 0.001, HR = 1.04 (1.03–1.06) |
| Male sex | p = NS | - |
| Hypercholesterolemia | p = 0.05, HR = 0.81 (0.66–1.00) | - |
| Diabetes mellitus | p < 0.001, HR = 1.63 (1.33–1.99) | - |
| HBP | p = 0.02, HR = 1.33 (1.04–1.70) | - |
| Atrial fibrillation | p < 0.001, HR = 1.64 (1.34–2.02) | p = 0.01, HR = 1.45 (1.09–1.93) |
| CKD | p < 0.001, HR = 2.28 (1.59–3.29) | - |
| History of stroke | p < 0.001, HR = 1.90 (1.28–2.83) | p < 0.001, HR = 2.35 (1.47–3.77) |
| History of MI | p = 0.04, HR = 1.34 (1.01–1.78) | - |
| LBBB | p = NS | - |
| Smoker | p = NS | - |
| COPD | p = 0.02, HR = 1.57 (1.06–2.32) | p = 0.02, HR = 1.74 (1.1–2.75) |
| DCM | p = NS | - |
| Number of CAD | p = 0.05, HR = 1.17 (1.00–1.37) | - |
| LVEF (%) | p < 0.001, HR = 0.97 (0.96–0.99) | p = 0.02, HR = 0.98 (0.97–1.00) |
| Creatinine (mg/dL) | p < 0.001, HR = 1.52 (1.28–1.81) | p < 0.001, HR = 1.05 (1.04–1.08) |
| Hemoglobin (×103/µL) | p = 0.01, HR = 1.08 (1.02–1.14) | p = 0.02, HR = 1.08 (1.01–1.16) |
| Platelets (×103/µL) | p = NS | - |
| Leucocytes (×103/µL) | p = NS | - |
| TAVI performed * | p = 0.03, HR = 0.89 (0.82–0.97) | - |
| PCI performed | p < 0.001, HR = 2.89 (1.43–5.84) | - |
| CABG performed | p < 0.001, HR = 1.63 (1.28–2.07) | - |
| EuroSCORE (%) | p < 0.001, HR = 1.056 (1.046–1.067) | - ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suciu, H.; Elkahlout, A.; Nicolae, V.; Tomșa, F.; Stan, A.; Al-Hussein, H.; Călburean, P.-A.; Scurtu, A.-C.; Aniței, D.E.; Hadadi, L.; et al. Outcomes and Cost-Effectiveness of Transcatheter Versus Surgical Aortic Valve Replacement in Patients with and Without Coronary Artery Disease. J. Cardiovasc. Dev. Dis. 2025, 12, 217. https://doi.org/10.3390/jcdd12060217
Suciu H, Elkahlout A, Nicolae V, Tomșa F, Stan A, Al-Hussein H, Călburean P-A, Scurtu A-C, Aniței DE, Hadadi L, et al. Outcomes and Cost-Effectiveness of Transcatheter Versus Surgical Aortic Valve Replacement in Patients with and Without Coronary Artery Disease. Journal of Cardiovascular Development and Disease. 2025; 12(6):217. https://doi.org/10.3390/jcdd12060217
Chicago/Turabian StyleSuciu, Horațiu, Ayman Elkahlout, Viorel Nicolae, Flavius Tomșa, Alexandru Stan, Hussam Al-Hussein, Paul-Adrian Călburean, Anda-Cristina Scurtu, David Emanuel Aniței, László Hadadi, and et al. 2025. "Outcomes and Cost-Effectiveness of Transcatheter Versus Surgical Aortic Valve Replacement in Patients with and Without Coronary Artery Disease" Journal of Cardiovascular Development and Disease 12, no. 6: 217. https://doi.org/10.3390/jcdd12060217
APA StyleSuciu, H., Elkahlout, A., Nicolae, V., Tomșa, F., Stan, A., Al-Hussein, H., Călburean, P.-A., Scurtu, A.-C., Aniței, D. E., Hadadi, L., Brînzaniuc, K., & Harpa, M. M. (2025). Outcomes and Cost-Effectiveness of Transcatheter Versus Surgical Aortic Valve Replacement in Patients with and Without Coronary Artery Disease. Journal of Cardiovascular Development and Disease, 12(6), 217. https://doi.org/10.3390/jcdd12060217







