Patients with Gastrointestinal Bleeding and Atrial Fibrillation: Potential Ideal Target for Epicardial Appendage Occlusion
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Selection
2.2. Etiology and Severity of Bleeding
2.3. Study Outcomes
2.4. Statistical Analysis
2.5. Follow-Up
2.6. Preoperative Workup
2.7. Procedure
2.8. Post-Procedure Antithrombotic Therapy
3. Results
3.1. Procedural Success
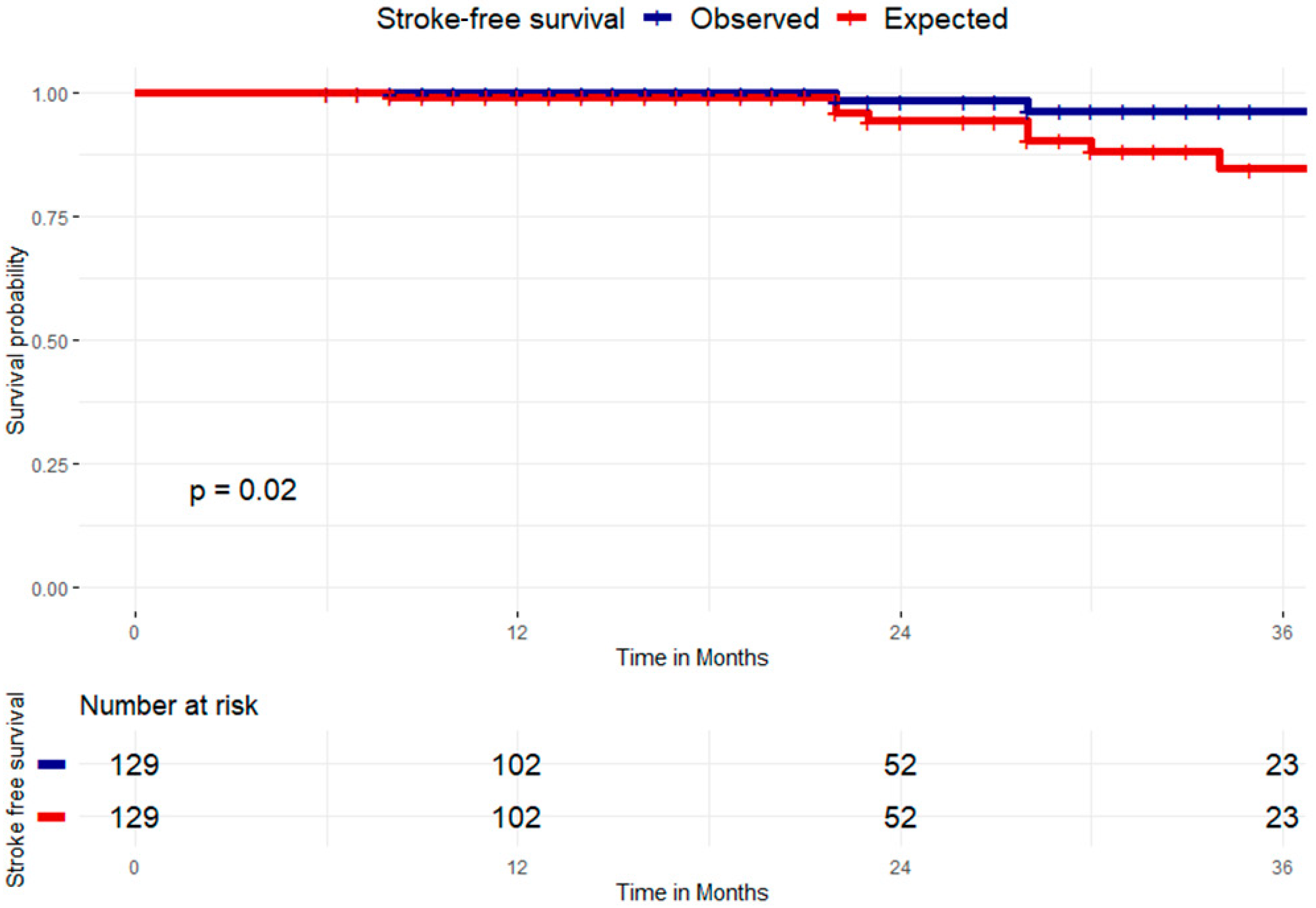
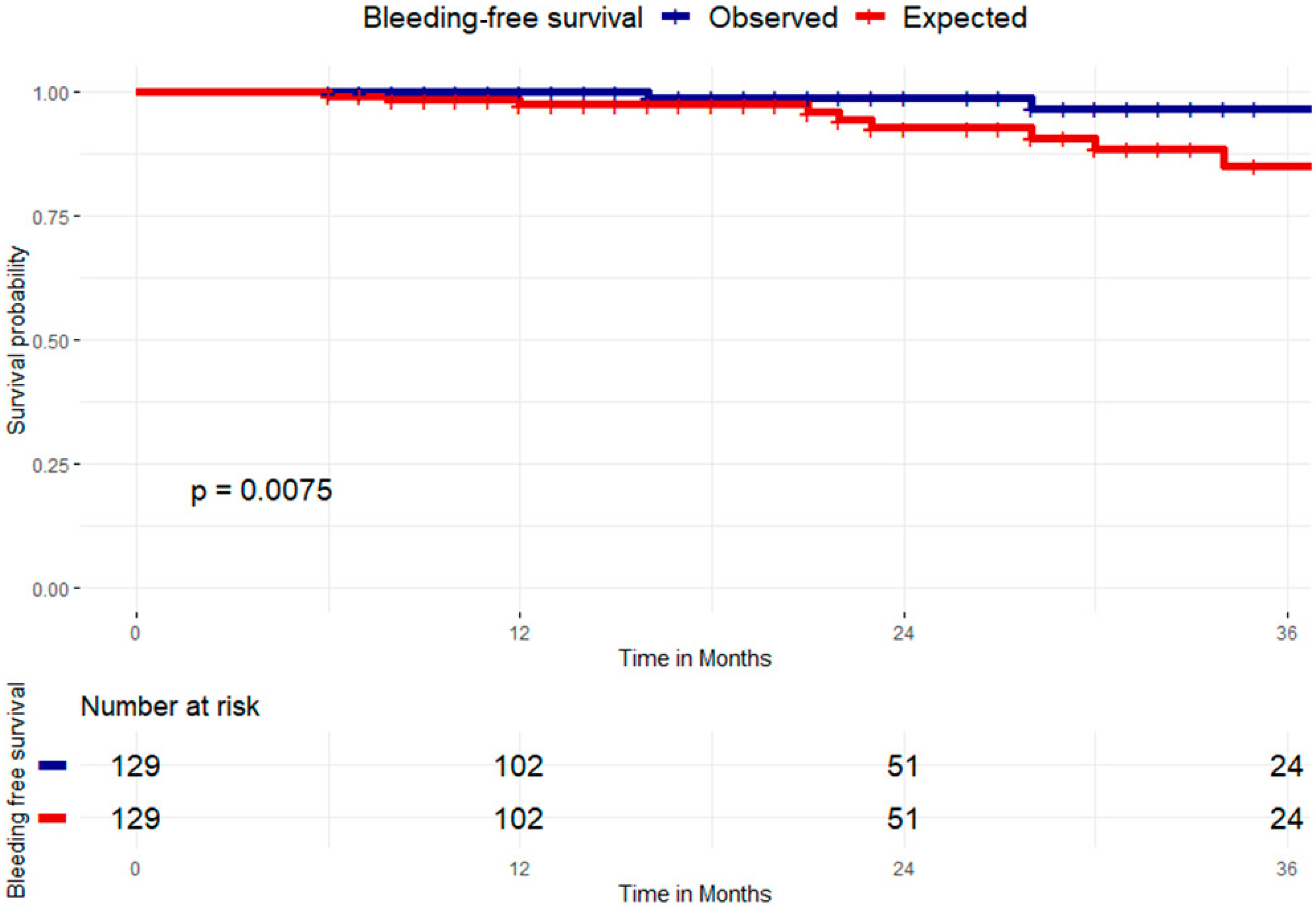
3.2. Clinical Success at Follow-Up
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| GIB | Gastrointestinal bleeding |
| LAA | Left atrial appendage |
| SAPT | Single antiplatelet therapy |
| NOACS | New oral anticoagulants |
| DAPT | Dual antiplatelet therapy |
| TOE | Transoesophageal echo |
| TTLAAO | Totally thoracoscopic appendage occlusion |
References
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef] [PubMed]
- Tzeis, S.; Gerstenfeld, E.; Kalman, J.; Saad, E.; Shamloo, A.; Andrade, J.; Barbhaiya, C.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 Eurpean heart Rhythm Association/Heart Rhythm Society/Asaia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statment on catheter and surgical ablation of atrial fibrillation. J. Interv. Card. Electrophysiol. 2024, 67, 921–1072. [Google Scholar] [PubMed]
- Ruff, C.; Giuliano, P.; Braunwald, E.; Hoffman, E.; Deenadayalu, N.; Ezekowitz, M.; Camm, J.; Weitz, J.; Lewis, B.; Parkhomewnko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A metanalysis of randomized trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-L.; Lopes, R.D.; Patel, M.R.; Büller, H.R.; Tan, D.S.-Y.; Chiang, C.-E.; Giugliano, R.P. Efficacy and safety of reduced-dose non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: A meta-analysis of randomized controlled trials. Eur. Heart J. 2018, 40, 1492–1500. [Google Scholar] [CrossRef]
- Bawa, D.; Darden, D.; Ahmed, A.; Garg, J.; Karst, E.; Kabra, R.; Pothineni, K.; Gopinathannair, R.; Mansour, M.; Winterfield, J.; et al. Lower–adherence direct oral anticoagulant use is associated with increased risk of thromboembolic events than warfarin. Int. J. Card. Electrophys. 2024, 67, 709–718. [Google Scholar] [CrossRef]
- Perino, A.; Kaiser, D.; Lee, R.; Fan, J.; Askari, M.; Schmitt, S.; Turakhia, M. Incidence outcomes of patients with atrial fibrillation major bleeding complications:from the TREAT-AFstudy. J. Interv. Card. Electr. 2021, 62, 133–142. [Google Scholar] [CrossRef]
- Gallo, F.; Ronco, F.; D’Amico, G.; Della Rocca, D.G.; Mazzone, P.; Bordignon, S.; Casu, G.; Giannini, F.; Berti, S.; Horton, R.P.; et al. Clinical outcomes of left atrial appendage occlusion in patients with previous intracranial or gastrointestinal bleeding: Insights from the LOGIC (Left atrial appendage Occlusion in patients with Gastrointestinal or IntraCranial bleeding) International Multicenter Registry. Catheter. Cardiovasc. Interv. 2023, 101, 1144–1153. [Google Scholar] [CrossRef]
- Lempereur, M.; Aminian, A.; Freixa, X.; Gafoor, S.; Shakir, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; et al. Left atrial appendage occlusion in patients with atrial fibrillation and previous major gastrointestinal bleeding (from the amplatzer cardiac plug multicenter registry). Am. J. Cardiol. 2017, 120, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Patti, G.; Nielsen-Kudsk, J.; Beri, S.; Korsholm, K. Left atrial appendage occlusion and post procedural antithrombotic management. J. Clin. Med. 2024, 13, 803. [Google Scholar] [CrossRef]
- Prasad, R.; Al-abcha, A.; Radwan, Y.; Srivastava, S.; Baloch, Z.; Elsayed, M.; Rayamajhi, S.; Abela, S. Metanalysis comparing antiplatelet therapy versus direct oral anticoagulation in percutaneous left atrial appendage closure. J. Am. Coll. Cardiol. 2021, 153, 144–146. [Google Scholar] [CrossRef]
- Mahady, S.; Margolis, K.; Chan, A.; Polekhina, G.; Woods, R.; Wolfe, R.; Nelson, M.; Lockery, J.; Wood, E.; Reid, C.; et al. Major GI bleeding in older persons using aspirin: Incidence and risk factors in the ASPREE randomized controlled trial. Gut 2021, 70, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Saperas, E.; Videla, S.; Dot, J.; Bayarri, C.; Lobo, B.; Abu-Suboh, M.; Armengol, J.; Malagelada, J. Risk factors for recurrence of acute gastrointestinal bleeding from angiodysplasia. Eur. J. Gastroenterol. Hepatol. 2009, 21, 1333–1339. [Google Scholar] [CrossRef]
- Triantafyllou, K.; Gkolfakis, P.; Gralnek, I.; Oakland, K.; Manes, G.; Radaelli, F.; Awadie, H.; Duboc, M.; Christodoulou, D.; Fedorov, E.; et al. Diagnosis and management of acute lower gastrointestinal bleeding: European Society of Gatrointestinal Endoscopy (ESGE) Guidelin. Endoscopy 2021, 53, 850–868. [Google Scholar]
- Gralnek, I.; Stanley, A.; Morris, A.; Camus, M.; Lau, J.; Lanas, A.; Laursen, S.; Radaelli, F.; Papanikolaou, I.S.; Dinis-Ribeiro, M.; et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscoy (ESGE) Guidelines-update. Endoscopy 2021, 53, 300–332. [Google Scholar]
- Mehran, R.; Rao, S.; Bhatt, D.L.; Gibson, M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.; Menon, V.; Nikolsky Eet, a.l. Standardized bleeding definitions for cardiovascular clinical trials. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Joglar, J.; Chung, M.; Armbruster, A.; Benjamin, E.; Chyou, J.; Cronin, E.; Deswal, A.; Eckhardt, L.; Goldberg, Z.; Gopinathannair, R.; et al. AHA Guidelines for diagnosis and management of atrial Fibrillation. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef]
- Jones, W.; Williams, L.; Meschia, J. Validating the Questionnaire for verifying stroke free status QSVS by neurological history and examination. Stroke 2001, 32, 2232–2236. [Google Scholar] [CrossRef]
- Branzoli, S.; Marini, M.; Guarracini, F.; Pederzolli, C.; D’onghia, G.; Centonze, M.; Pomarolli, C.; Graffigna, A.; La Meir, M. Stand alone totally thoracoscopic left appendage clipping: Safe simple standardized. Ann. Thor. Surg. 2021, 111, e61–e63. [Google Scholar] [CrossRef]
- Lanas, A.; Carrera-Lasfuentes, P.; Arguedas, Y.; Garcia, S.; Bujada, L.; Calvet, X.; Ponce, J.; Perez-Aisa, A.; Casdtro, M.; Munoz, M.; et al. Risk of upper and lower gastrointestinal bleeding in patients taking non steroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin. Gastroenterol. Hepatol. 2015, 13, 906–912. [Google Scholar] [CrossRef]
- Dave, M.; Kumar, A.; Majmundar, M.; Adalja, D.; Shariff, M.; Shah, P.; Desai, R.; Patel, K.; Jagirdhar, G.S.K.; Vallabhajosyula, S.; et al. Frequency, trend, predictors, and impact of gastrointestinal bleeding in atrial fibrillation hospitalizations. Am. J. Cardiol. 2021, 146, 29–35. [Google Scholar] [CrossRef]
- Qureshi, W.; Mittal, C.; Patsias, I.; Garikapati, K.; Kuchipudi, A.; Cheema, G.; Elbatta, M.; Alirhayim, Z.; Khalid, F. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am. J. Cardiol. 2014, 113, 662–668. [Google Scholar] [CrossRef]
- Witt, D.M.; Delate, T.; Garcia, D.A.; Clark, N.P.; Hylek, E.M.; Ageno, W.; Dentali, F.; Crowther, M.A. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch. Intern. Med. 2012, 172, 1484–1491. [Google Scholar] [CrossRef]
- Sengupta, N.; Feuerstein, J.D.; Patwardhan, V.R.; Tapper, E.B.; AKetwaroo, G.; Thaker, A.M.; ALeffler, D. The risks of thromboembolism vs. recurrent gastrointestinal bleeding after interruption of systemic anticoagulation in hospitalized inpatients with gastrointestinal bleeding: A prospective study. Am. J. Gastroenterol. 2015, 110, 328–335. [Google Scholar] [CrossRef]
- Abraham, N.; Barkun, A.; Saur, B.; Douketis, J.; Laine, L.; Noseworthy, P.; Telford, J.; Leontiadis, G. American College of Gastroenterology–Canadian Association of Gastroenterology practice Guideline: Management of anticoagulants and antiplatelet during acute gastrointestinal bleeding and the periendoscopic period. Am. J. Gastroenterol. 2022, 117, 542–558. [Google Scholar] [CrossRef]
- Veitch, A.; Radaelli, F.; Alikhan, R.; Dumonceau, J.; Eaton, D.; Jerrome, J.; Lester, W.; Nylander, D.; Thoufeeq, M.; Vanbiervliet, G.; et al. Endoscopy in patients on antiplatelet or anticoagulnat therapy: British Society of GAstroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) Guideline update. Gut 2021, 70, 1611–1628. [Google Scholar] [CrossRef]
- Sostres, C.; Marcen, B.; Laredo, V.; Alfaro, E.; Ruiz, L.; Camo, P.; CarreraLasfuentes, P.; Lanas, A. Risk of rebleeding, vascular and death after gastrointestinal bleeding in anticoagulant and/or antiplatelet users. Aliment. Pharmacol. Ther. 2019, 50, 919–929. [Google Scholar] [CrossRef]
- Barada, K.; Abdul-Baki, H.; El Hajj, I.I.; Hashash, J.G.; Green, P.H. Gastrointestinal bleeding in the setting of anticoagulation and antiplatelet therapy. J. Clin. Gastroenterol. 2009, 43, 5–12. [Google Scholar] [CrossRef]
- Généreux, P.; Giustino, G.; Witzenbichler, B.; Weisz, G.; Stuckey, T.D.; Rinaldi, M.J.; Neumann, F.-J.; Metzger, D.C.; Henry, T.D.; Cox, D.A.; et al. Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2015, 66, 1036–1045. [Google Scholar] [CrossRef]
- Abrignani, M.; Gatta, L.; Gabrielli, D.; MIlazzo, G.; De Rrancesco, V.; De Luca, L.; Francese, M.; Imazio, M.; Riccio, E.; Rossini, R.; et al. Gastroprotection in patients on antiplatelet and/or anticoagulant therapy: A position paperof national Association of Hospital cardiologist (ANMCO) and the Italian association of Hospital gastroenterologist and endoscopist (AIGO). Eur. J. Intern. Med. 2021, 85, 1–13. [Google Scholar] [CrossRef]
- Valgimigli, M.; Frigioli, E.; Heg, D.; Tijssen, J.; Juni, P.; Vranckx, P.; Ozaki, Y.; Morice, M.; Chevalier, B.; Onuma, Y.; et al. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N. Engl. J. Med. 2021, 385, 1643–1655. [Google Scholar] [CrossRef]
- Han, Y.; Liao, Z.; Li, Y.; Zhao, X.; Ma, S.; Bao, D.; Qiu, M.; Deng, J.; Wang, J.; Qu, P.; et al. Magnetically controlled capsule endoscopy for assessment of antiplatelet therapy-induced gastrointestinal injury. J. Am. Coll. Cardiol. 2022, 79, 116–128. [Google Scholar] [CrossRef]
- Patel, N.J.; Pau, D.; Nalluri, N.; Bhatt, P.; Thakkar, B.; Kanotra, R.; Agnihotri, K.; Ainani, N.; Patel, N.; Patel, N.; et al. Temporal trends, predictors, and outcomes of in-hospital gastrointestinal bleeding associated with percutaneous coronary intervention. Am. J. Cardiol. 2016, 118, 1150–1157. [Google Scholar] [CrossRef]
- Branzoli, S.; Guarracini, F.; Marini, M.; D’onghia, G.; Catanzariti, D.; Merola, E.; Annicchiarico, L.; Casagranda, G.; Stegagno, C.; Fantinel, M.; et al. Heart team for left appendage occlusion without the use of antithrombotic therapy: The epicardial perspective. J. Clin. Med. 2022, 11, 6492. [Google Scholar] [CrossRef]
- Tilz, R.; Fink, T.; Bartus, K.; Wong, T.; Vogler, J.; Nentwich, K.; Panniker, S.; Fang, Q.; Piokowski, C.; Liosis Set, a.l. A Collective European experience with left atrial appendage suture ligation using the LARIAT device. Europace 2020, 22, 924–931. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Nonaka, T.; Hisagi, M.; Ninomiya, M.; Masukawa, A.; Ota, T. Thoracoscopic stapler and loop technique for left atrial appendage closure in non valvular atrial fibrillation: Mid term outcomes in 201 patients. Heart Rhthm. 2018, 15, 1314–1320. [Google Scholar] [CrossRef]
- Hildick-Smith, D.; Landmesser, U.; Camm, J.; Diener, H.; Paul, V.; Schmidt, B.; Settergren, M.; Teiger, E.; Nielsen-Kudsk, J.; Tondo, C. Left atrial appendage occlusion with the Amplatzer Amulet device: Full results of the prospective global observational study. Eur. Heart J. 2020, 41, 2894–2901. [Google Scholar] [CrossRef]
- Tarantini, G.; D’amico, G.; Schmidt, B.; Mazzone, P.; Berti, S.; Fischer, S.; Lund, J.; Montorfano, M.; Della Bella, P.; Lam, S.C.C.; et al. The impact of CHA2DS2-VASc and HAS-BLED scores on clinical outcomes in the Amplatzer Amulet study. JACC Cardiovasc. Interv. 2020, 13, 2099–2108. [Google Scholar] [CrossRef]
- Boersma, L.V.; Ince, H.; Kische, S.; Pokushalov, E.; Schmitz, T.; Schmidt, B.; Gori, T.; Meincke, F.; Protopopov, A.V.; Betts, T.; et al. Evaluating real-world clinical outcomes in atrial fibrillation patients receiving the Watchman left atrial appendage closure technology. Circ. Arrhythmia Electrophysiol. 2019, 12, e006841. [Google Scholar] [CrossRef]
- Alkhouli, M.; Ellis, C.; Daniles, M.; Coylewright, M.; Nielsen-Kudsk, J.; Holmes, D. Left atrial appendage occlusion, current advances and remaining challenges. JACC Adv. 2022, 1, 100136. [Google Scholar] [CrossRef]
- Toale, C.; Fitzmaurice, G.; Eaton, D.; Lyne, J.; Redmond, K. Outcomes of left atrial appendage occlusion using the AtriClip device: A systematic review. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 655–662. [Google Scholar] [CrossRef]
- Cartledge, R.; Suwalski, G.; Witkowska, A.; Gottlieb, G.; Cioci, A.; Chidiac, G.; Ilsin, B.; Merrill, B.; Suwalski, P. Standalone epicardial left appendage exclusion for thromboemolism prevention in atrial fibrillation. Inter. Cardiovasc. Thor. Surg. 2021, 34, 548–555. [Google Scholar] [CrossRef] [PubMed]


| Variable | Value |
|---|---|
| Gender (M) (%) | 85 |
| Age (mean ± SD) | 76.6 ± 7.1 |
| CHA2DS2Vasc (mean ± SD) | 3.8 ± 1.5 |
| HAS BLED (mean ± SD) | 3.2 ± 1 |
| HASBLED ≥ 3 (%) | 78.3 |
| HASBLED < 3 (%) | 21.7 |
| EF (mean ± SD) (%) | 53.5 ± 7.5 |
| Previous stroke (%) | 21.7 |
| Previous cerebral Hemorrhage | 5.4 |
| Type of AF (%) | |
| Paroxismal | 13.1 |
| Persistent | 5.4 |
| Longstanding Pers | 5.4 |
| Permanent | 75.9 |
| BARC criteria (%) | |
| I | 0 |
| II | 18.5 |
| III | 71.2 |
| IV | 0 |
| V | 10.1 |
| Lower GI Bleeding (%) | 86 |
| Angiodysplasia (%) | 70.4 |
| Diverticular disease (%) | 7.7 |
| Inflammatory disease (%) | 5.4 |
| Hemorrhoids (%) | 3.1 |
| Upper GI Bleeding (%) | 9.4 |
| Liver failure, portal hypertension, esophageal varices | 6.2 |
| Gastroduedenal erosion | 3.2 |
| Obscure occult (%) | 4.6 |
| Anticoagulant regime before procedure | |
| Anti Vit K (%) | 9.3 |
| DOACs full dose (%) | 77.5 |
| DOACs reduced dose (%) | 13.1 |
| Number of transfusions pre-LAAO/Patient (mean ± SD) | 2.1 ± 1.5 |
| Number of invasive diagnostic procedures/patient (mean ± SD) | 2.5 ± 1.9 |
| TOTAL NUMBER OF PATIENTS | 129 |
| No Stroke or Bleeding During Follow-Up (N = 125) | Stroke or Bleeding During Follow-Up (N = 4) | Total (N = 129) | p Value | |
|---|---|---|---|---|
| Age | 76.6 (7.2) | 79.2 (3.9) | 76.7 (7.2) | 0.464 |
| Gender (M) | 83 (66.4%) | 2 (50.0%) | 85 (65.9%) | 0.605 |
| FE | 53.6 (7.6) | 51.2 (2.5) | 53.5 (7.5) | 0.538 |
| CHADVASC | 3.8 (1.5) | 5.8 (2.1) | 3.9 (1.5) | 0.013 |
| risk_stroke_year | 4.4 (2.3) | 7.4 (3.1) | 4.5 (2.4) | 0.012 |
| HASBLED | 3.3 (1.1) | 4.0 (0.8) | 3.3 (1.1) | 0.185 |
| bleeding_risk_year | 5.4 (3.4) | 8.4 (3.6) | 5.5 (3.5) | 0.084 |
| transfusion_N | 2.0 (1.5) | 1.3 (0.9) | 2.0 (1.5) | 0.351 |
| new_GRC | 0.1 (0.4) | 0.0 (0.0) | 0.1 (0.3) | 0.719 |
| FUP | 26.1 (19.1) | 38.5 (22.8) | 26.5 (19.2) | 0.206 |
| prevEC | 7 (5.6%) | 0 (0.0%) | 7 (5.4%) | 1.000 |
| prev_stroke | 25 (20.0%) | 3 (75.0%) | 28 (21.7%) | 0.032 |
| Diabetes | 27 (21.8%) | 1 (25.0%) | 28 (21.9%) | 1.000 |
| Hypertension | 69 (55.2%) | 3 (75.0%) | 72 (55.8%) | 0.629 |
| Death | 4 (3.2%) | 0 (0.0%) | 4 (3.1%) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branzoli, S.; Marini, M.; Catanzariti, D.; Pravadelli, C.; Pannone, L.; D’Onghia, G.; Fantinel, M.; Guarracini, F.; Franceschini, G.; Zadro, M.; et al. Patients with Gastrointestinal Bleeding and Atrial Fibrillation: Potential Ideal Target for Epicardial Appendage Occlusion. J. Cardiovasc. Dev. Dis. 2025, 12, 173. https://doi.org/10.3390/jcdd12050173
Branzoli S, Marini M, Catanzariti D, Pravadelli C, Pannone L, D’Onghia G, Fantinel M, Guarracini F, Franceschini G, Zadro M, et al. Patients with Gastrointestinal Bleeding and Atrial Fibrillation: Potential Ideal Target for Epicardial Appendage Occlusion. Journal of Cardiovascular Development and Disease. 2025; 12(5):173. https://doi.org/10.3390/jcdd12050173
Chicago/Turabian StyleBranzoli, Stefano, Massimiliano Marini, Domenico Catanzariti, Cecilia Pravadelli, Luigi Pannone, Giovanni D’Onghia, Mauro Fantinel, Fabrizio Guarracini, Gaia Franceschini, Mirco Zadro, and et al. 2025. "Patients with Gastrointestinal Bleeding and Atrial Fibrillation: Potential Ideal Target for Epicardial Appendage Occlusion" Journal of Cardiovascular Development and Disease 12, no. 5: 173. https://doi.org/10.3390/jcdd12050173
APA StyleBranzoli, S., Marini, M., Catanzariti, D., Pravadelli, C., Pannone, L., D’Onghia, G., Fantinel, M., Guarracini, F., Franceschini, G., Zadro, M., Baroni, G., Casagrande, S., Ottaviani, D., Turco, R., Nicolussi Paolaz, S., Annicchiarico, L., Corsini, F., Rordorf, R., Krishnadath, K., ... La Meir, M. (2025). Patients with Gastrointestinal Bleeding and Atrial Fibrillation: Potential Ideal Target for Epicardial Appendage Occlusion. Journal of Cardiovascular Development and Disease, 12(5), 173. https://doi.org/10.3390/jcdd12050173









