Vo2peak, Ve/VCO2, and Cardiac Remodeling Correlate with Long-Term Cardiovascular Outcome in Heart Failure Patients
Abstract
1. Introduction
2. Methods
2.1. CPET Protocol
2.2. Echocardiography
2.3. Statistical Analysis
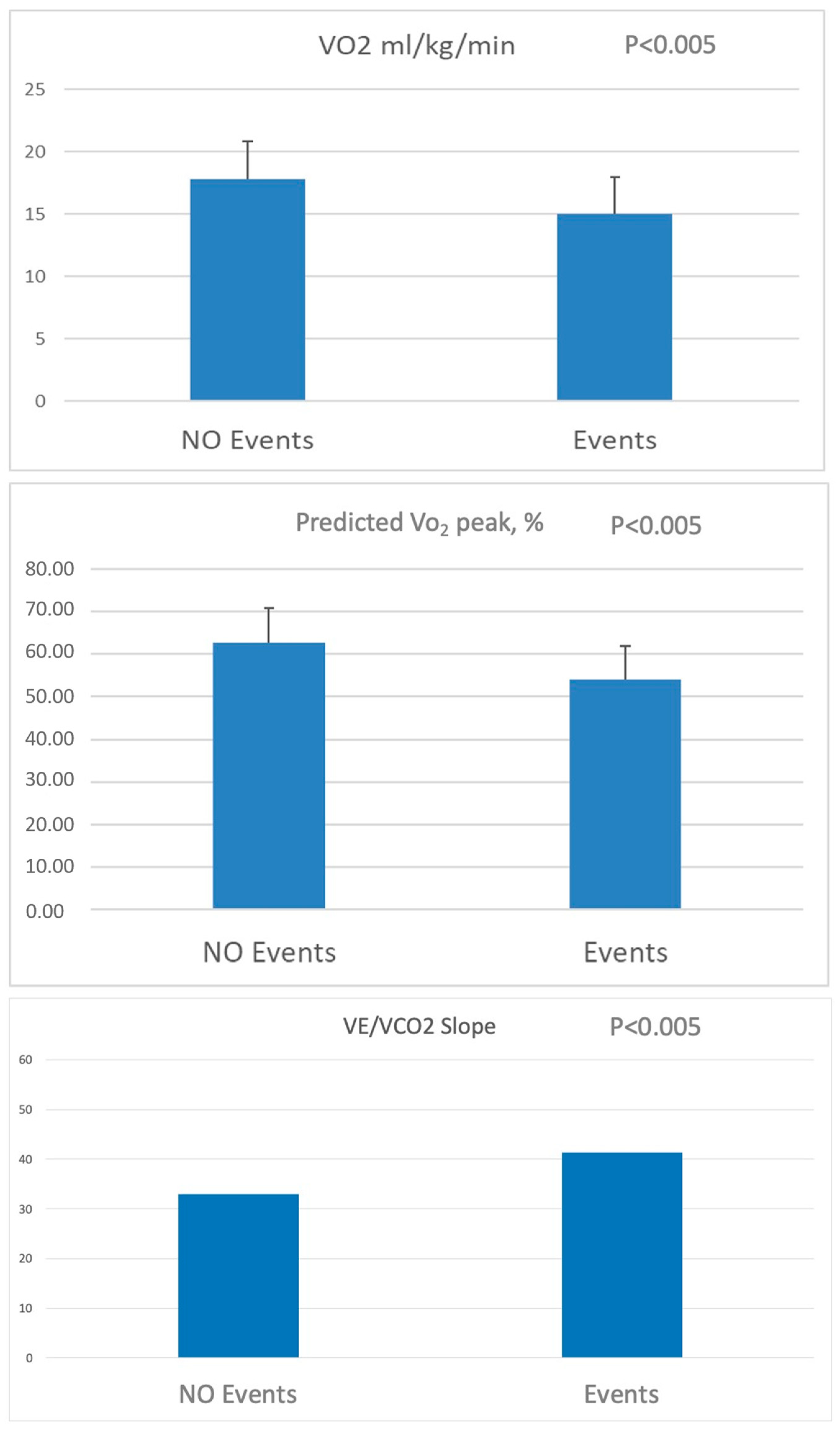
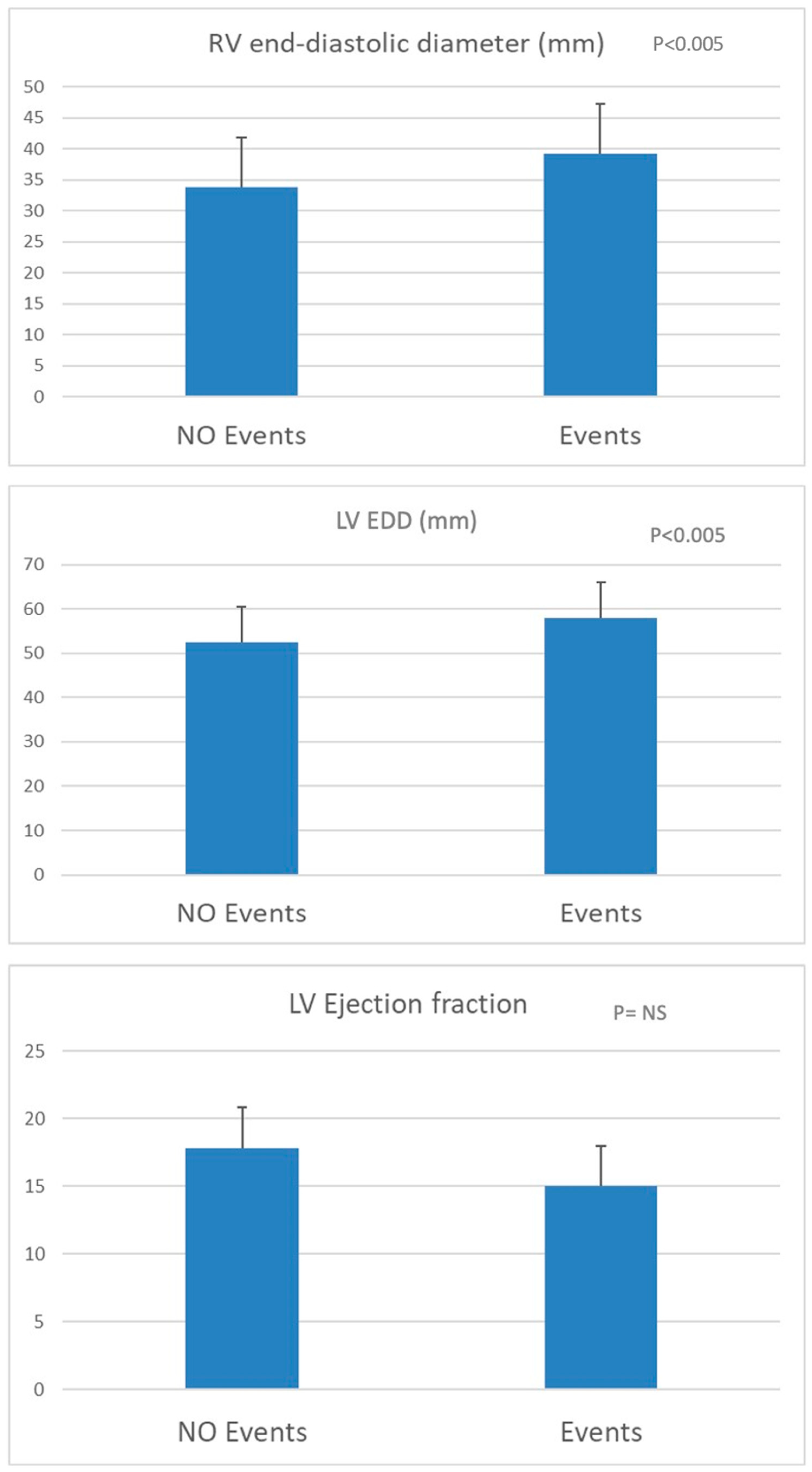
3. Results
3.1. Demographic and Clinical Data
3.2. Follow-Up and Cardiovascular Events
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Authors/Task Force, M.; McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Cikes, M.; Solomon, S.D. Beyond ejection fraction: An integrative approach for assessment of cardiac structure and function in heart failure. Eur. Heart J. 2016, 37, 1642–1650. [Google Scholar] [CrossRef]
- Bhatia, R.S.; Tu, J.V.; Lee, D.S.; Austin, P.C.; Fang, J.; Haouzi, A.; Gong, Y.; Liu, P.P. Outcome of heart failure with preserved ejection fraction in a population-based study. N. Engl. J. Med. 2006, 355, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Corra, U.; Magini, A.; Paolillo, S.; Frigerio, M. Comparison among different multiparametric scores for risk stratification in heart failure patients with reduced ejection fraction. Eur. J. Prev. Cardiol. 2020, 27, 12–18. [Google Scholar] [CrossRef]
- Corra, U.; Piepoli, M.F.; Adamopoulos, S.; Agostoni, P.; Coats, A.J.; Conraads, V.; Lambrinou, E.; Pieske, B.; Piotrowicz, E.; Schmid, J.P.; et al. Cardiopulmonary exercise testing in systolic heart failure in 2014: The evolving prognostic role: A position paper from the committee on exercise physiology and training of the heart failure association of the ESC. Eur. J. Heart Fail. 2014, 16, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef]
- Sartipy, U.; Dahlstrom, U.; Edner, M.; Lund, L.H. Predicting survival in heart failure: Validation of the MAGGIC heart failure risk score in 51,043 patients from the Swedish heart failure registry. Eur. J. Heart Fail. 2014, 16, 173–179. [Google Scholar] [CrossRef]
- Agostoni, P.; Corra, U.; Cattadori, G.; Veglia, F.; La Gioia, R.; Scardovi, A.B.; Emdin, M.; Metra, M.; Sinagra, G.; Limongelli, G.; et al. Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: A multiparametric approach to heart failure prognosis. Int. J. Cardiol. 2013, 167, 2710–2718. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Cavigli, L.; Pagliaro, A.; Focardi, M.; Valente, S.; Cameli, M.; Mandoli, G.E.; Mueller, S.; Dendale, P.; Piepoli, M.; et al. Clinician approach to cardiopulmonary exercise testing for exercise prescription in patients at risk of and with cardiovascular disease. Br. J. Sports Med. 2022, 56, 1180–1187. [Google Scholar] [CrossRef]
- Anselmi, F.; Cavigli, L.; Pagliaro, A.; Valente, S.; Mondillo, S.; Focardi, M.; Cameli, M.; Bonifazi, M.; D’Ascenzi, F. Cardiopulmonary exercise testing: An essential tool for a tailored exercise prescription in patients with cardiac disease. G. Ital. Cardiol. 2021, 22, 716–726. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Abella, J.; Pinkstaff, S.; Brubaker, P.; Moore, B.; Kitzman, D.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; et al. Determining the preferred percent-predicted equation for peak oxygen consumption in patients with heart failure. Circ. Heart Fail. 2009, 2, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Binder, R.K.; Wonisch, M.; Corra, U.; Cohen-Solal, A.; Vanhees, L.; Saner, H.; Schmid, J.P. Methodological approach to the first and second lactate threshold in incremental cardiopulmonary exercise testing. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, F.; Cavigli, L.; Pagliaro, A.; Valente, S.; Valentini, F.; Cameli, M.; Focardi, M.; Mochi, N.; Dendale, P.; Hansen, D.; et al. The importance of ventilatory thresholds to define aerobic exercise intensity in cardiac patients and healthy subjects. Scand. J. Med. Sci. Sports 2021, 31, 1796–1808. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmunds, L.H., Jr.; Wilson, J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef]
- O’Neill, J.O.; Young, J.B.; Pothier, C.E.; Lauer, M.S. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation 2005, 111, 2313–2318. [Google Scholar] [CrossRef]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Fang, J.C.; Ewald, G.A.; Allen, L.A.; Butler, J.; Westlake Canary, C.A.; Colvin-Adams, M.; Dickinson, M.G.; Levy, P.; Stough, W.G.; Sweitzer, N.K.; et al. Advanced (stage D) heart failure: A statement from the Heart Failure Society of America Guidelines Committee. J. Card. Fail. 2015, 21, 519–534. [Google Scholar] [CrossRef]
- Poggio, R.; Arazi, H.C.; Giorgi, M.; Miriuka, S.G. Prediction of severe cardiovascular events by VE/VCO2 slope versus peak VO2 in systolic heart failure: A meta-analysis of the published literature. Am. Heart J. 2010, 160, 1004–1014. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Abella, J.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; Guazzi, M. Development of a ventilatory classification system in patients with heart failure. Circulation 2007, 115, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Gavazzi, A.; Campana, C.; Inserra, C.; Klersy, C.; Sebastiani, R.; Arbustini, E.; Recusani, F.; Tavazzi, L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J. Am. Coll. Cardiol. 2001, 37, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Filippatos, G.S.; Ahmed, M.I.; Iskandrian, A.E.; Bittner, V.; Perry, G.J.; White, M.; Aban, I.B.; Mujib, M.; Dell’Italia, L.J.; et al. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation 2010, 121, 252–258. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Kane, G.C.; Melenovsky, V.; Olson, T.P. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur. Heart J. 2016, 37, 3293–3302. [Google Scholar] [CrossRef]
- Malhotra, R.; Dhakal, B.P.; Eisman, A.S.; Pappagianopoulos, P.P.; Dress, A.; Weiner, R.B.; Baggish, A.L.; Semigran, M.J.; Lewis, G.D. Pulmonary Vascular Distensibility Predicts Pulmonary Hypertension Severity, Exercise Capacity, and Survival in Heart Failure. Circ. Heart Fail. 2016, 9, e003011. [Google Scholar] [CrossRef]
- Johnson, R.L., Jr. Gas exchange efficiency in congestive heart failure II. Circulation 2001, 103, 916–918. [Google Scholar] [CrossRef]
- Methvin, A.B.; Owens, A.T.; Emmi, A.G.; Allen, M.; Wiegers, S.E.; Dries, D.L.; Margulies, K.B.; Forfia, P.R. Ventilatory inefficiency reflects right ventricular dysfunction in systolic heart failure. Chest 2011, 139, 617–625. [Google Scholar] [CrossRef]
- Lewis, G.D.; Shah, R.V.; Pappagianopolas, P.P.; Systrom, D.M.; Semigran, M.J. Determinants of ventilatory efficiency in heart failure: The role of right ventricular performance and pulmonary vascular tone. Circ. Heart Fail. 2008, 1, 227–233. [Google Scholar] [CrossRef]
- Guazzi, M.; Villani, S.; Generati, G.; Ferraro, O.E.; Pellegrino, M.; Alfonzetti, E.; Labate, V.; Gaeta, M.; Sugimoto, T.; Bandera, F. Right Ventricular Contractile Reserve and Pulmonary Circulation Uncoupling During Exercise Challenge in Heart Failure: Pathophysiology and Clinical Phenotypes. JACC Heart Fail. 2016, 4, 625–635. [Google Scholar] [CrossRef]
- Klaassen, S.H.C.; Liu, L.C.Y.; Hummel, Y.M.; Damman, K.; van der Meer, P.; Voors, A.A.; Hoendermis, E.S.; van Veldhuisen, D.J. Clinical and Hemodynamic Correlates and Prognostic Value of VE/VCO(2) Slope in Patients With Heart Failure With Preserved Ejection Fraction and Pulmonary Hypertension. J. Card. Fail. 2017, 23, 777–782. [Google Scholar] [CrossRef]
- Nadruz, W., Jr.; West, E.; Sengelov, M.; Santos, M.; Groarke, J.D.; Forman, D.E.; Claggett, B.; Skali, H.; Shah, A.M. Prognostic Value of Cardiopulmonary Exercise Testing in Heart Failure With Reduced, Midrange, and Preserved Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e006000. [Google Scholar] [CrossRef] [PubMed]
- Bourantas, C.V.; Loh, H.P.; Bragadeesh, T.; Rigby, A.S.; Lukaschuk, E.I.; Garg, S.; Tweddel, A.C.; Alamgir, F.M.; Nikitin, N.P.; Clark, A.L.; et al. Relationship between right ventricular volumes measured by cardiac magnetic resonance imaging and prognosis in patients with chronic heart failure. Eur. J. Heart Fail. 2011, 13, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Benes, J.; Kotrc, M.; Wohlfahrt, P.; Kroupova, K.; Tupy, M.; Kautzner, J.; Melenovsky, V. Right ventricular global dysfunction score: A new concept of right ventricular function assessment in patients with heart failure with reduced ejection fraction (HFrEF). Front. Cardiovasc. Med. 2023, 10, 1194174. [Google Scholar] [CrossRef]
- Ito, K.; Li, S.; Homma, S.; Thompson, J.L.P.; Buchsbaum, R.; Matsumoto, K.; Anker, S.D.; Qian, M.; Di Tullio, M.R.; Investigators, W. Left ventricular dimensions and cardiovascular outcomes in systolic heart failure: The WARCEF trial. ESC Heart Fail. 2021, 8, 4997–5009. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Anavekar, N.; Skali, H.; McMurray, J.J.; Swedberg, K.; Yusuf, S.; Granger, C.B.; Michelson, E.L.; Wang, D.; Pocock, S.; et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005, 112, 3738–3744. [Google Scholar] [CrossRef]
- Cameli, M.; Lisi, M.; Righini, F.M.; Tsioulpas, C.; Bernazzali, S.; Maccherini, M.; Sani, G.; Ballo, P.; Galderisi, M.; Mondillo, S. Right ventricular longitudinal strain correlates well with right ventricular stroke work index in patients with advanced heart failure referred for heart transplantation. J. Card. Fail. 2012, 18, 208–215. [Google Scholar] [CrossRef]
| Total Patients, n | 170 |
| Age (years) | 55 ± 10 |
| Sex, Male, n (%) | 149 (88) |
| Weight (kg) | 81.4 ± 13.4 |
| Height (cm) | 173 ± 8 |
| Ischemic DCM n (%) | 63 (37) |
| NIDCM, n (%) | 107 (63) |
| ICD, n (%) | 65 (38) |
| CRT-D, n (%) | 68 (40) |
| CRT-P, n (%) | 1 (1) |
| Βetablockers, n (%) | 154 (91) |
| ACE-inhibitors/ARBs, n (%) | 148 (87) |
| ARNI, n (%) | 3 (2) |
| MRI, n (%) | 139 (82) |
| Diuretics, n (%) | 143 (84) |
| Diuretic dose (mg) | 75.9 ± 60.0 |
| Ivabradine, n (%) | 26 (15) |
| VO2 mL/min | 1367.7 ± 422.0 |
| VO2 mL/kg/min | 17.1 ± 4.6 |
| %ppVO2 | 61.1 ± 14.0 |
| VE/VCO2 slope | 34.8 ± 8.7 |
| RER | 1.08 ± 0.09 |
| VO2/Work mL/min/watt | 9.5 ± 1.4 |
| OUES L/min | 1.7 ± 0.6 |
| pSBP mmHg | 132 ± 24 |
| HRR, bpm | 13 ± 7 |
| LV EDD, mm | 66 ± 9 |
| LV ESD, mm | 53 ± 11 |
| LVEF, % | 30 ± 7 |
| RV EDD, mm | 35 ± 7 |
| TAPSE, mm | 18 ± 4 |
| PAPs, mmHg | 34 ± 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagliaro, A.; Cavigli, L.; Molle, R.; Iardino, E.; Anselmi, F.; Righini, F.; Martini, L.; Zacà, V.; Mandoli, G.E.; Pastore, M.C.; et al. Vo2peak, Ve/VCO2, and Cardiac Remodeling Correlate with Long-Term Cardiovascular Outcome in Heart Failure Patients. J. Cardiovasc. Dev. Dis. 2025, 12, 174. https://doi.org/10.3390/jcdd12050174
Pagliaro A, Cavigli L, Molle R, Iardino E, Anselmi F, Righini F, Martini L, Zacà V, Mandoli GE, Pastore MC, et al. Vo2peak, Ve/VCO2, and Cardiac Remodeling Correlate with Long-Term Cardiovascular Outcome in Heart Failure Patients. Journal of Cardiovascular Development and Disease. 2025; 12(5):174. https://doi.org/10.3390/jcdd12050174
Chicago/Turabian StylePagliaro, Antonio, Luna Cavigli, Roberta Molle, Elisabetta Iardino, Francesca Anselmi, Francesca Righini, Luca Martini, Valerio Zacà, Giulia Elena Mandoli, Maria Concetta Pastore, and et al. 2025. "Vo2peak, Ve/VCO2, and Cardiac Remodeling Correlate with Long-Term Cardiovascular Outcome in Heart Failure Patients" Journal of Cardiovascular Development and Disease 12, no. 5: 174. https://doi.org/10.3390/jcdd12050174
APA StylePagliaro, A., Cavigli, L., Molle, R., Iardino, E., Anselmi, F., Righini, F., Martini, L., Zacà, V., Mandoli, G. E., Pastore, M. C., Focardi, M., Cameli, M., Bernazzali, S., Maccherini, M., Chiostri, M., D’Ascenzi, F., & Valente, S. (2025). Vo2peak, Ve/VCO2, and Cardiac Remodeling Correlate with Long-Term Cardiovascular Outcome in Heart Failure Patients. Journal of Cardiovascular Development and Disease, 12(5), 174. https://doi.org/10.3390/jcdd12050174










