The Possibilities and Importance of Assessing the Left Atrioventricular Coupling Index Using Various Diagnostic Imaging Methods in an Adult Population: A Comprehensive Review
Abstract
1. Introduction
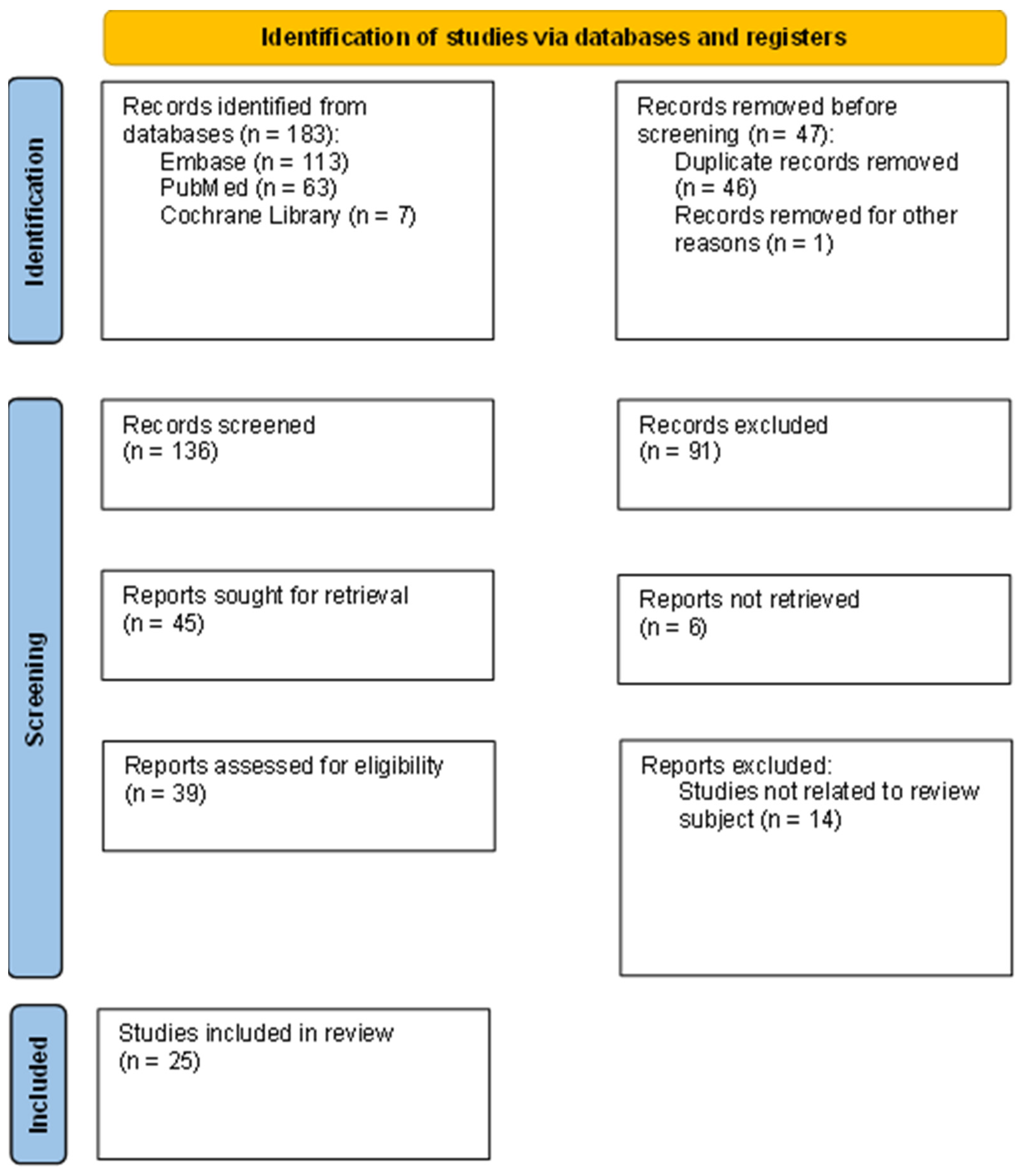
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Literature Selection
3. Results and Discussion
3.1. Heart Imaging Techniques
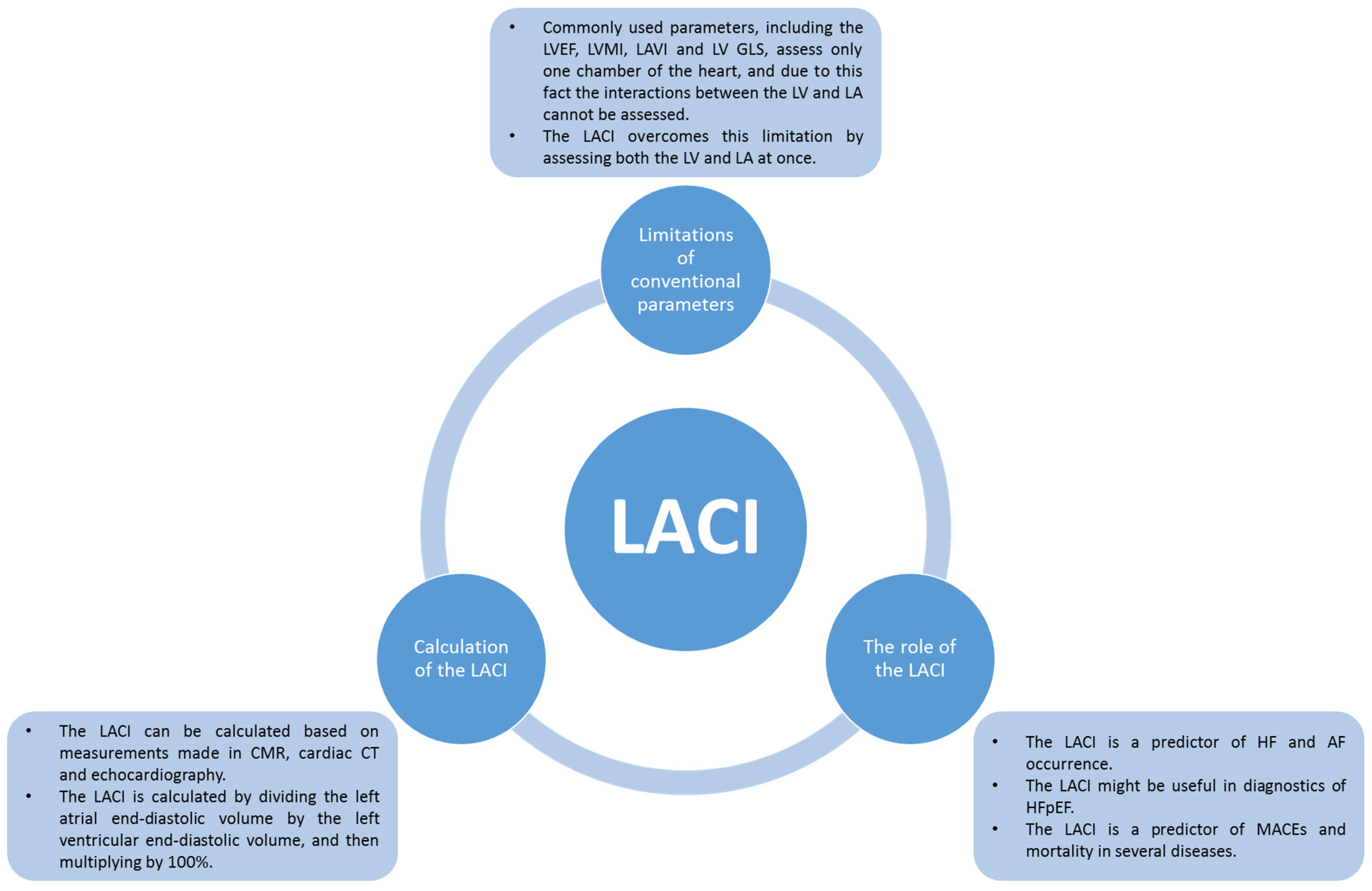
3.2. Left Atrioventricular Coupling Index
3.3. The Role of the Left Atrioventricular Coupling Index in Heart Failure
3.4. The Role of Left Atrioventricular Coupling Index in Atrial Fibrillation
3.5. The Role of Left Atrioventricular Coupling Index in Diabetes Mellitus and Hypertension
3.6. The Role of the Left Atrioventricular Coupling Index in Other Conditions
3.6.1. Cardiomyopathies and Myocarditis
3.6.2. Myocardial Infarction
3.6.3. Beta-Thalassemia Major
3.6.4. Metabolic Syndrome
3.6.5. Light-Chain Amyloidosis
3.6.6. Sodium Intake
3.6.7. Menopause
3.7. The Cut-Off Value of the Left Atrioventricular Coupling Index
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Health Estimates: Leading Causes of Death. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 11 January 2025).
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Ristow, B.; Ali, S.; Whooley, M.A.; Schiller, N.B. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul Study). Am. J. Cardiol. 2008, 102, 70–76. [Google Scholar] [CrossRef]
- Gillam, L.D.; Marcoff, L. Echocardiography: Past, Present, and Future. Circ. Cardiovasc. Imaging 2024, 17, e016517. [Google Scholar] [CrossRef] [PubMed]
- Litmanovich, D.E.; Kirsch, J. Computed Tomography of Cardiac Valves: Review. Radiol. Clin. N. Am. 2019, 57, 141–164. [Google Scholar] [CrossRef]
- Cao, C.-F.; Ma, K.-L.; Shan, H.; Liu, T.-F.; Zhao, S.-Q.; Wan, Y.; Zhang, J.; Wang, H.-Q. CT Scans and Cancer Risks: A Systematic Review and Dose-response Meta-analysis. BMC Cancer 2022, 22, 1238. [Google Scholar] [CrossRef]
- Rehman, R.; Yelamanchili, V.S.; Makaryus, A.N. Cardiac Imaging. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sala, M.L.; Bizino, M.B.; Amersfoort, J.; de Roos, A.; Lamb, H.J. Computed tomography evaluation of cardiac structure and function. J. Thorac. Imaging 2014, 29, 173–184. [Google Scholar] [CrossRef]
- Teis, A.; Delgado, V. Artificial Intelligence, Left Atrial Ventricular Coupling Index, and Stress Cardiac Magnetic Resonance: An Interesting Combination. JACC Cardiovasc. Imaging 2023, 16, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Pezel, T.; Venkatesh, B.A.; De Vasconcellos, H.D.; Kato, Y.; Shabani, M.; Xie, E.; Heckbert, S.R.; Post, W.S.; Shea, S.J.; Allen, N.B.; et al. Left Atrioventricular Coupling Index as a Prognostic Marker of Cardiovascular Events: The MESA Study. Hypertension 2021, 78, 661–671. [Google Scholar] [CrossRef]
- Pezel, T.; Dillinger, J.-G.; Toupin, S.; Mirailles, R.; Logeart, D.; Cohen-Solal, A.; Unger, A.; Canuti, E.S.; Beauvais, F.; Lafont, A.; et al. Left atrioventricular coupling index assessed using cardiac CT as a prognostic marker of cardiovascular death. Diagn. Interv. Imaging 2023, 104, 594–604. [Google Scholar] [CrossRef]
- Nguyen Ngoc Dang, H.; Viet Luong, T.; Khanh Tran, H.; Ha Tuyet Le, N.; Hoang Nhat Nguyen, M.; Chi Doan, T.; Minh Nguyen, H. Left Atrioventricular Coupling Index in Heart Failure Patients Using Echocardiography: A Simple Yet Effective Metric. medRxiv 2024. [Google Scholar] [CrossRef]
- Lange, T.; Backhaus, S.J.; Schulz, A.; Evertz, R.; Kowallick, J.T.; Bigalke, B.; Hasenfuß, G.; Thiele, H.; Stiermaier, T.; Eitel, I.; et al. Cardiovascular magnetic resonance-derived left atrioventricular coupling index and major adverse cardiac events in patients following acute myocardial infarction. J. Cardiovasc. Magn. Reson. 2023, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Pezel, T.; Garot, P.; Toupin, S.; Sanguineti, F.; Hovasse, T.; Unterseeh, T.; Champagne, S.; Morisset, S.; Chitiboi, T.; Jacob, A.J.; et al. AI-Based Fully Automated Left Atrioventricular Coupling Index as a Prognostic Marker in Patients Undergoing Stress CMR. JACC Cardiovasc. Imaging 2023, 16, 1288–1302. [Google Scholar] [CrossRef]
- Vîjîiac, A.; Scărlătescu, A.I.; Petre, I.G.; Vîjîiac, C.; Vătășescu, R.G. Three-Dimensional Combined Atrioventricular Coupling Index-A Novel Prognostic Marker in Dilated Cardiomyopathy. Biomedicines 2024, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Fortuni, F.; Bernetti, C.; Carluccio, E. The emerging role of left atrioventricular coupling index in heart failure: A new frontier for cardiac magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2025, jeaf047. [Google Scholar] [CrossRef]
- Leiner, T. Left Atrioventricular Coupling for Early Prediction of Incident Atrial Fibrillation. Radiology 2022, 303, 327–328. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726, Erratum in Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. About Heart Failure. Available online: https://www.cdc.gov/heart-disease/about/heart-failure.html (accessed on 11 January 2025).
- Backhaus, S.J.; Lange, T.; Schulz, A.; Evertz, R.; Frey, S.M.; Hasenfuß, G.; Schuster, A. Cardiovascular magnetic resonance rest and exercise-stress left atrioventricular coupling index to detect diastolic dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H686–H695. [Google Scholar] [CrossRef]
- Pezel, T.; Venkatesh, B.A.; Kato, Y.; De Vasconcellos, H.D.; Heckbert, S.R.; Wu, C.O.; Post, W.S.; Bluemke, D.A.; Cohen-Solal, A.; Henry, P.; et al. Left Atrioventricular Coupling Index to Predict Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 704611. [Google Scholar] [CrossRef]
- Lange, T.; Backhaus, S.J.; Schulz, A.; Hashemi, D.; Evertz, R.; Kowallick, J.T.; Hasenfuß, G.; Kelle, S.; Schuster, A. CMR-based cardiac phenotyping in different forms of heart failure. Int. J. Cardiovasc. Imaging 2024, 40, 1585–1596. [Google Scholar] [CrossRef]
- American Heart Association. What Is Atrial Fibrillation? Available online: https://www.heart.org/en/health-topics/atrial-fibrillation/what-is-atrial-fibrillation-afib-or-af (accessed on 11 January 2025).
- Centers for Disease Control and Prevention. About Atrial Fibrillation. Available online: https://www.cdc.gov/heart-disease/about/atrial-fibrillation.html (accessed on 11 January 2025).
- United Kingdom National Health Service. Atrial Fibrillation. Available online: https://www.nhs.uk/conditions/atrial-fibrillation/ (accessed on 11 January 2025).
- Pezel, T.; Ambale-Venkatesh, B.; Quinaglia, T.; Heckbert, S.R.; Kato, Y.; de Vasconcellos, H.D.; Wu, C.O.; Post, W.S.; Henry, P.; Bluemke, D.A.; et al. Change in Left Atrioventricular Coupling Index to Predict Incident Atrial Fibrillation: The Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2022, 303, 317–326. [Google Scholar] [CrossRef]
- Li, A.; Zhang, M.; Ning, B. Predictive value of the left atrioventricular coupling index for recurrence after radiofrequency ablation of paroxysmal atrial fibrillation. J. Cardiothorac. Surg. 2024, 19, 552. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.W.; Esmat, H.A.; Bahee, M.D. Prevalence of hypertension in Type-2 diabetes mellitus. Ann. Med. Surg. 2022, 78, 103758. [Google Scholar] [CrossRef]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Al Ghorani, H.; Götzinger, F.; Böhm, M.; Mahfoud, F. Arterial hypertension—Clinical trials update 2021. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 21–31. [Google Scholar] [CrossRef]
- Laakso, M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms: The Kelly West Award Lecture 2008. Diabetes Care 2010, 33, 442–449. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Z.; Gao, Y.; Li, G.; Zhan, Y.; Liu, S.; Zhao, Z.; Pohost, G.M.; Sun, K.; Li, K. Assessment of left atrial function and left atrioventricular coupling via cardiac magnetic resonance in individuals with prediabetes and diabetes. Acta Diabetol. 2024. [Google Scholar] [CrossRef]
- Dang, H.N.N.; Luong, T.V.; Ho, B.A. Evaluation of the relationship between left atrial stiffness, left ventricular stiffness, and left atrioventricular coupling index in type 2 diabetes patients: A speckle tracking echocardiography study. Front. Cardiovasc. Med. 2024, 11, 1372181. [Google Scholar] [CrossRef]
- Shi, R.; Jiang, Y.-N.; Qian, W.-L.; Guo, Y.-K.; Gao, Y.; Shen, L.-T.; Jiang, L.; Li, X.-M.; Yang, Z.-G.; Li, Y. Assessment of left atrioventricular coupling and left atrial function impairment in diabetes with and without hypertension using CMR feature tracking. Cardiovasc. Diabetol. 2023, 22, 295. [Google Scholar] [CrossRef]
- Girard, A.A.; Denney, T.S.; Gupta, H.; Dell’italia, L.J.; Calhoun, D.A.; Oparil, S.; Sharifov, O.F.; Lloyd, S.G. Spironolactone improves left atrial function and atrioventricular coupling in patients with resistant hypertension. Int. J. Cardiovasc. Imaging 2024, 40, 487–497. [Google Scholar] [CrossRef]
- Meucci, M.C.; Fortuni, F.; Galloo, X.; Bootsma, M.; Crea, F.; Bax, J.J.; Marsan, N.A.; Delgado, V. Left atrioventricular coupling index in hypertrophic cardiomyopathy and risk of new-onset atrial fibrillation. Int. J. Cardiol. 2022, 363, 87–93. [Google Scholar] [CrossRef]
- Matsuura, H.; Yamada, A.; Sugimoto, K.; Sugimoto, K.; Iwase, M.; Ishikawa, T.; Ishii, J.; Ozaki, Y. Clinical implication of LAVI over A’ ratio in patients with acute coronary syndrome. Heart Asia 2018, 10, e011038. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, V.; Raafs, A.; Manca, P.; Henkens, M.T.; Gregorio, C.; Boscutti, A.; Verdonschot, J.; Hazebroek, M.; Knackstedt, C.; Merlo, M.; et al. Left Atrial Reverse Remodeling in Dilated Cardiomyopathy. J. Am. Soc. Echocardiogr. 2023, 36, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, N.; Zhao, W.; Sun, Z.; Liu, J.; Liu, D.; Wen, Z.; Xu, L. Incremental Prognostic Value of Left Atrial Strain in Patients with Suspected Myocarditis and Preserved Left Ventricular Ejection Fraction. J. Magn. Reson. Imaging 2025, 61, 899–908. [Google Scholar] [CrossRef]
- Meloni, A.; Saba, L.; Positano, V.; Pistoia, L.; Spasiano, A.; Putti, M.C.; Casini, T.; Cossu, A.; Corigliano, E.; Massa, A.; et al. Left and right atrioventricular coupling index in patients with beta-thalassemia major. Int. J. Cardiovasc. Imaging 2024, 40, 1631–1640. [Google Scholar] [CrossRef]
- Huang, S.; Shi, K.; Li, Y.; Wang, J.; Jiang, L.; Gao, Y.; Yan, W.; Shen, L.; Yang, Z. Effect of Metabolic Dysfunction-Associated Fatty Liver Disease on Left Ventricular Deformation and Atrioventricular Coupling in Patients with Metabolic Syndrome Assessed by MRI. J. Magn. Reson. Imaging 2023, 58, 1098–1107. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Peng, L.; Gao, Y.; Shi, K.; Qian, W.; Yan, W.; Yang, Z. Effect of Metabolic Syndrome on Left Atrial and Left Ventricular Deformation and Atrioventricular Interactions in Patients With Myocardial Infarction. J. Magn. Reson. Imaging 2025, 61, 235–247. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, K.; Wan, K.; Liu, J.; He, W.; Li, X.; Huang, L.; Peng, L.; Chen, Y. Cardiovascular magnetic resonance-derived left atrioventricular coupling index as a novel prognostic marker for light-chain amyloidosis. Int. J. Cardiol. 2025, 418, 132630. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Mei, J.; Dong, J.; Qu, X.; Jiang, Y. Association of sodium intake with adverse left atrial function and left atrioventricular coupling in Chinese. J. Hypertens. 2023, 41, 159–170. [Google Scholar] [CrossRef]
- Pezel, T.; Michos, E.D.; Varadarajan, V.; Shabani, M.; Venkatesh, B.A.; Vaidya, D.; Kato, Y.; De Vasconcellos, H.D.; Heckbert, S.R.; Wu, C.O.; et al. Prognostic value of a left atrioventricular coupling index in pre- and post-menopausal women from the Multi-Ethnic Study of Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 1066849. [Google Scholar] [CrossRef]

| Feature | Echocardiography | Cardiac CT | Cardiac MR |
|---|---|---|---|
| Accessibility | Very high | High | Low |
| Bedside examination | Yes | No | No |
| Real-time examination | Yes | No | No |
| Time of examination | Short | Short | Long |
| Contrast | No | Yes, iodinated | Yes, gadolinium |
| Ionizing radiation | No | Yes | No |
| Safe in pregnancy | Yes | No | Yes |
| Cost | Cheap | Moderate | Expensive |
| Authors and Year of Publication | Imaging Technique | Studied Group | Main Findings | References |
|---|---|---|---|---|
| Pezel et al., 2021 | CMR | People without recognized cardiovascular diseases (MESA population) | The LACI is a predictor of HF, AF, coronary heart disease death and hard cardiovascular disease in people without recognized cardiovascular diseases. The LACI has greater prognostic value than LA and LV parameters individually. | [10] |
| Pezel et al., 2023 | Cardiac CT | People without recognized cardiovascular diseases (MESA population) | The LACI is independently associated with cardiovascular and all-cause death in people without cardiovascular diseases. | [11] |
| Backhaus et al., 2023 | CMR | Patients with HFpEF or noncardiac dyspnoea | The LACI can distinguish patients with HFpEF from patients with noncardiac dyspnoea. A correlation was found between the LACI and PCPW during rest and exercise. High values of the LACI are associated with an increased risk of hospitalization within 24 months. | [20] |
| Pezel et al., 2021 | CMR | People without recognized cardiovascular diseases (MESA population) | Elevated LACI and ΔLACI values are associated with HF incidents. | [21] |
| Lange et al., 2024 | CMR | Patients with heart failure | The LACI was increased in patients with HFpEF when compared to healthy controls. No difference in the LACI was found between HFmrEF and HFrEF patients and healthy controls. | [22] |
| Dang et al., 2024 | Echocardiography | Patients with heart failure | Patients with HF have increased LACI variability when compared to healthy people. LACI variability is highest in patients with HFpEF. The LACI may have use in HFpEF diagnostics. | [12] |
| Pezel et al., 2023 | CMR | Patients with normal or abnormal stress CMR | The LACI is independently associated with cardiovascular death and hospitalization due to acute HF in patients with normal or abnormal stress CMR. | [14] |
| Pezel et al., 2022 | CMR | People without recognized cardiovascular diseases (MESA population) | The LACI and ΔLACI were predictors for AF occurrence in a population without diagnosed CVD. The LACI and ΔLACI have greater predictive value than traditional parameters in predicting AF. | [26] |
| Li et al., 2024 | Echocardiography | Patients with paroxysmal atrial fibrillation after catheter ablation | A high value of the LACI is significantly associated with a higher risk of AF recurrence after catheter ablation. The LACI is a better predictor of AF recurrence after catheter ablation than traditional parameters. | [27] |
| Zhou et al., 2024 | CMR | Patients with prediabetes and diabetes | The LACI does not significantly correlate with HbA1C level. The LACI does not significantly correlate with LA deformation parameters. | [32] |
| Dang et al., 2024 | Echocardiography | Patients with diabetes | LACI values were significantly higher in patients with DM type 2 when compared to healthy people. The LACI correlates with left atrial and left ventricular stiffness. The LACI has prognostic value for detecting cardiac changes in patients with DM type 2. | [33] |
| Shi et al., 2023 | CMR | Patients with diabetes with and without coexisting hypertension | The LACI was significantly higher in patients with coexisting hypertension in a population of diabetic patients. | [34] |
| Girard et al., 2023 | CMR | Patients with resistant hypertension using spironolactone | The LACI was significantly lower after using spironolactone in patients with resistant hypertension. There is a positive significant correlation between the LACI and LV mass. | [35] |
| Meucci et al., 2022 | Echocardiography | Patients with hypertrophic cardiomyopathy | The LACI is associated with an increased risk of AF in patients with HCM. | [36] |
| Vîjîiac et al., 2024 | Echocardiography | Patients with dilated cardiomyopathy | The LACI was elevated in patients with DCM who experienced MACEs, and can be an independent predictor of MACEs. | [15] |
| Chen et al., 2024 | CMR | Patients with suspected myocarditis and preserved LVEF | The LACI is not associated with MACEs in patients with suspected myocarditis. | [39] |
| Lange et al., 2023 | CMR | Patients after acute myocardial infarction treated with primary percutaneous coronary intervention | The LACI was significantly higher in patients who experienced MACEs when compared to those who did not. | [13] |
| Meloni et al., 2024 | CMR | Patients with beta-thalassemia major | The LACI is significantly higher in beta-thalassemia major patients. The LACI is significantly higher in patients with beta-thalassemia after splenectomy compared to those with a spleen. An LACI >23.6% can be a good predictor of cardiac complications. | [40] |
| Huang et al., 2023 | CMR | Patients with metabolic syndrome with and without metabolic dysfunction-associated fatty liver disease | The LACI is significantly higher in patients with metabolic syndrome. Metabolic dysfunction-associated fatty liver disease is an independent factor influencing the LACI. | [41] |
| Liu et al., 2024 | CMR | Patients after myocardial infarction with and without metabolic syndrome | The LACI is significantly higher in patients after myocardial infarction. There is no significant correlation between the presence of metabolic syndrome and the LACI. | [42] |
| Wang et al., 2025 | CMR | Patients with light-chain amyloidosis | The LACI is an independent all-cause mortality predictor in light-chain amyloidosis in patients with Mayo stages IIIa and IIIb. The LACI may be useful in risk assessment in advanced light-chain amyloidosis. | [43] |
| Yin et al., 2023 | CMR | Volunteers with higher economic status | The LACI is significantly higher in patients with high urinary sodium. The LACI significantly correlates with BMI. | [44] |
| Pezel et al., 2022 | CMR | Perimenopausal women without recognized cardiovascular diseases (MESA population) | The LACI is significantly higher in postmenopausal women than in premenopausal women. The LACI is significantly lower in perimenopausal women taking hormone therapy. The LACI is an independent predictor of atrial fibrillation, heart failure, coronary heart disease death and other hard cardiovascular disease. | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poręba, M.; Kraik, K.; Zasoński, I.; Ratajczyk, O.; Paździerz, Ł.; Chachaj, A.; Poręba, R.; Gać, P. The Possibilities and Importance of Assessing the Left Atrioventricular Coupling Index Using Various Diagnostic Imaging Methods in an Adult Population: A Comprehensive Review. J. Cardiovasc. Dev. Dis. 2025, 12, 110. https://doi.org/10.3390/jcdd12040110
Poręba M, Kraik K, Zasoński I, Ratajczyk O, Paździerz Ł, Chachaj A, Poręba R, Gać P. The Possibilities and Importance of Assessing the Left Atrioventricular Coupling Index Using Various Diagnostic Imaging Methods in an Adult Population: A Comprehensive Review. Journal of Cardiovascular Development and Disease. 2025; 12(4):110. https://doi.org/10.3390/jcdd12040110
Chicago/Turabian StylePoręba, Małgorzata, Krzysztof Kraik, Igor Zasoński, Oskar Ratajczyk, Łukasz Paździerz, Angelika Chachaj, Rafał Poręba, and Paweł Gać. 2025. "The Possibilities and Importance of Assessing the Left Atrioventricular Coupling Index Using Various Diagnostic Imaging Methods in an Adult Population: A Comprehensive Review" Journal of Cardiovascular Development and Disease 12, no. 4: 110. https://doi.org/10.3390/jcdd12040110
APA StylePoręba, M., Kraik, K., Zasoński, I., Ratajczyk, O., Paździerz, Ł., Chachaj, A., Poręba, R., & Gać, P. (2025). The Possibilities and Importance of Assessing the Left Atrioventricular Coupling Index Using Various Diagnostic Imaging Methods in an Adult Population: A Comprehensive Review. Journal of Cardiovascular Development and Disease, 12(4), 110. https://doi.org/10.3390/jcdd12040110






