Long-Term Outcomes of Mitral Valve Repair Versus Replacement in Patients with Ischemic Mitral Regurgitation: A Retrospective Propensity-Matched Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patients
2.3. Study Variables
2.4. Study Outcomes
2.5. Surgical Techniques
2.6. Statistical Methods
3. Results
3.1. Baseline Demographics and Clinical Characteristics
3.2. Intraoperative Characteristics
3.3. Postoperative Outcomes
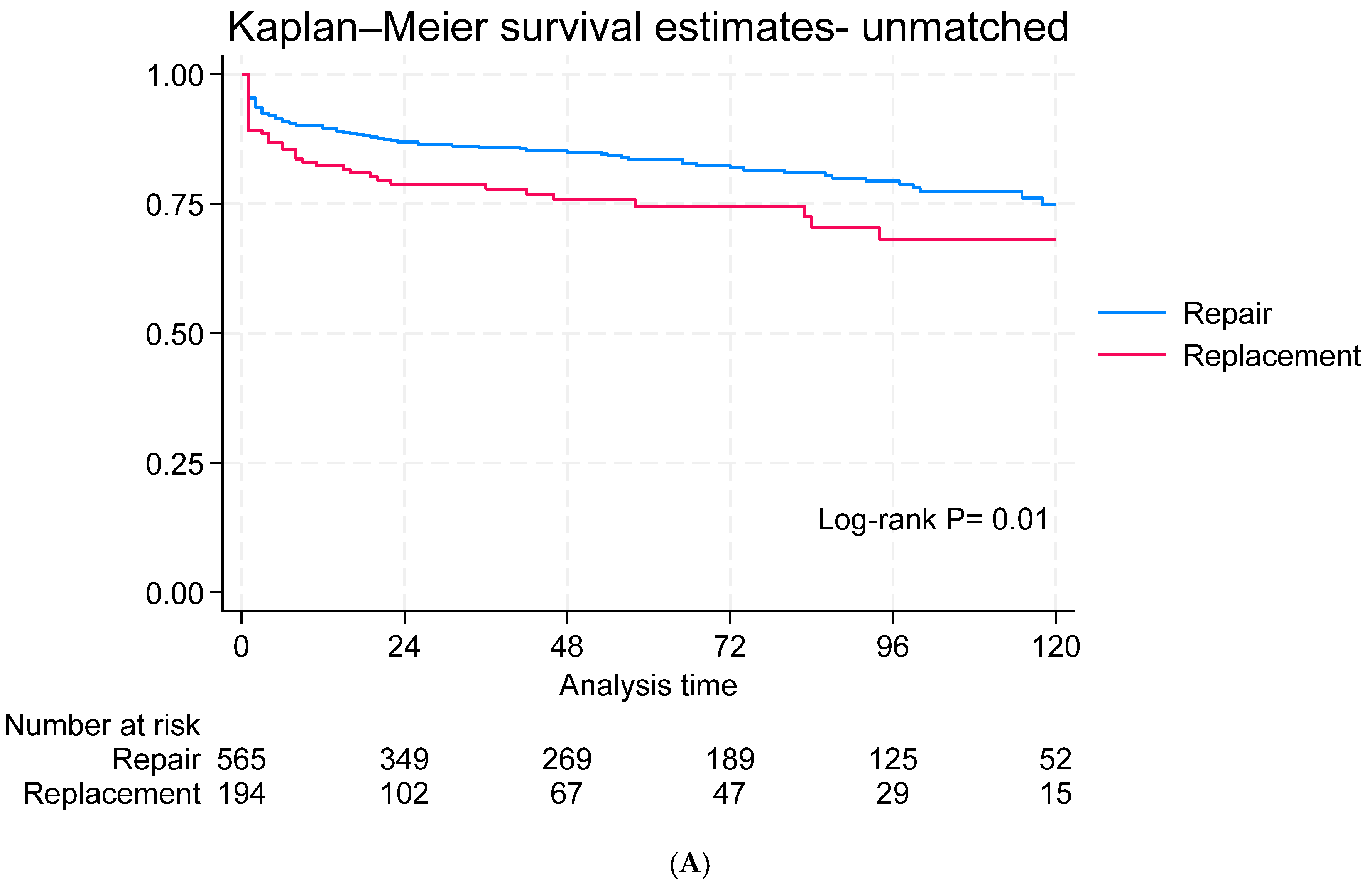
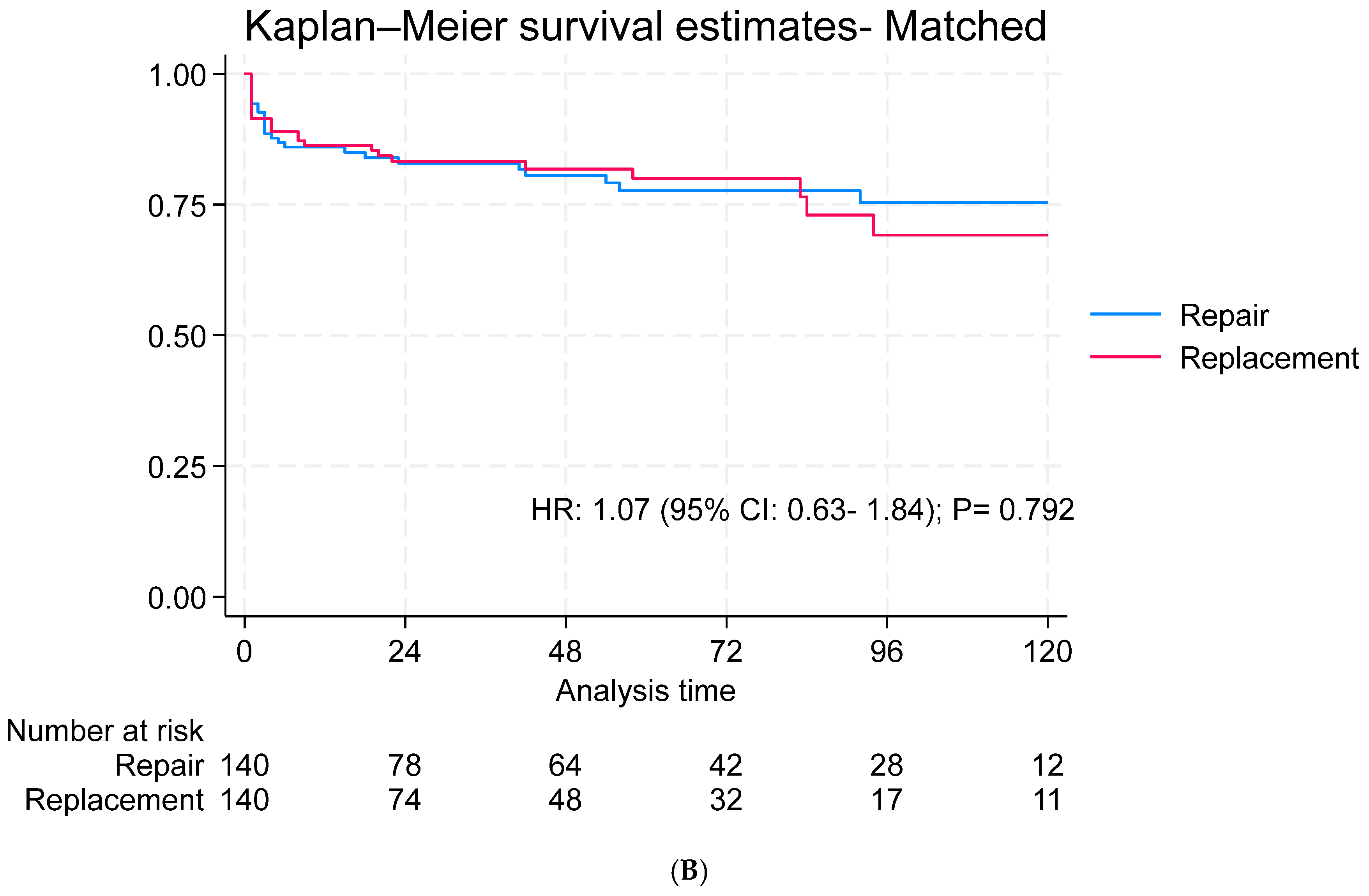
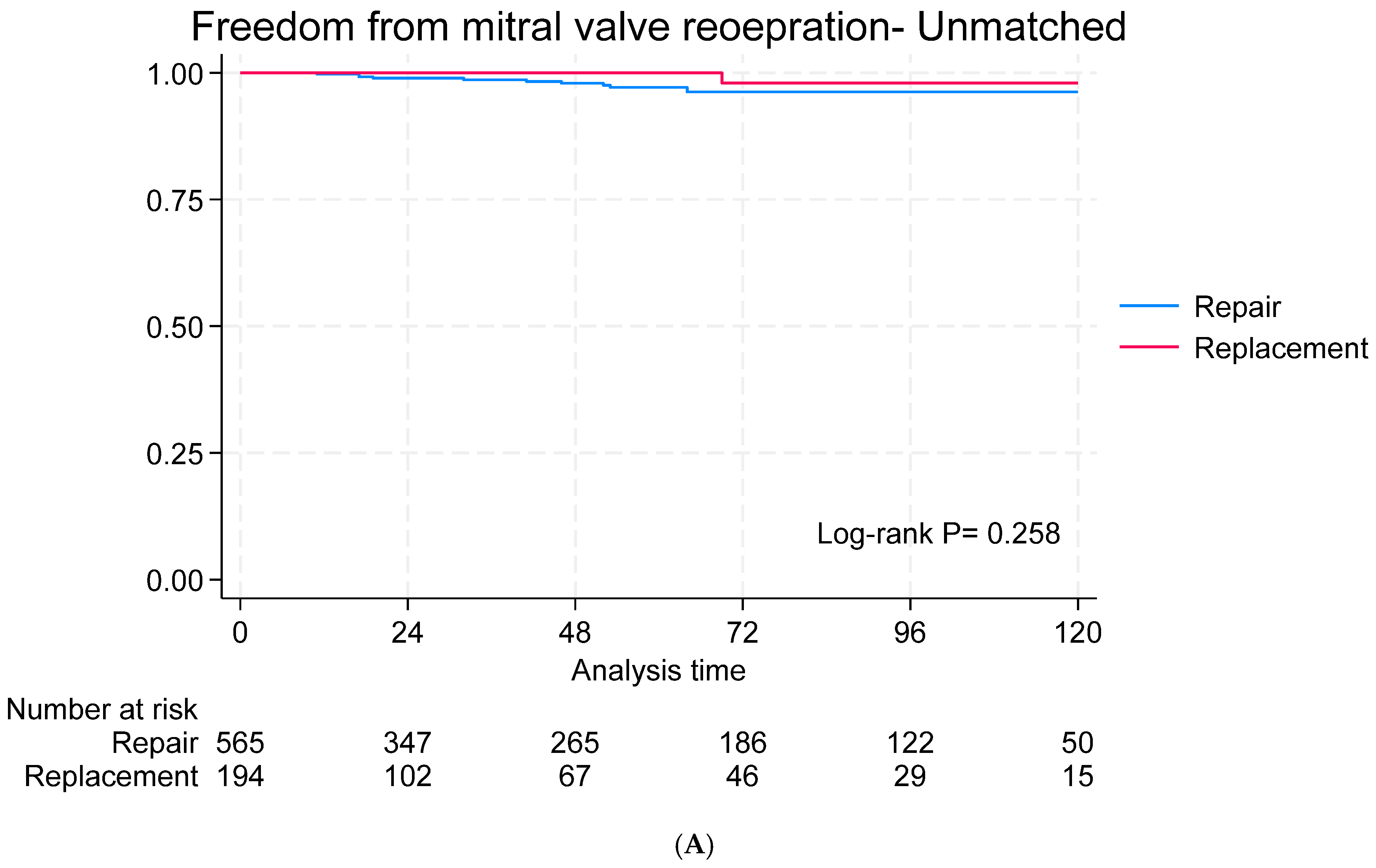
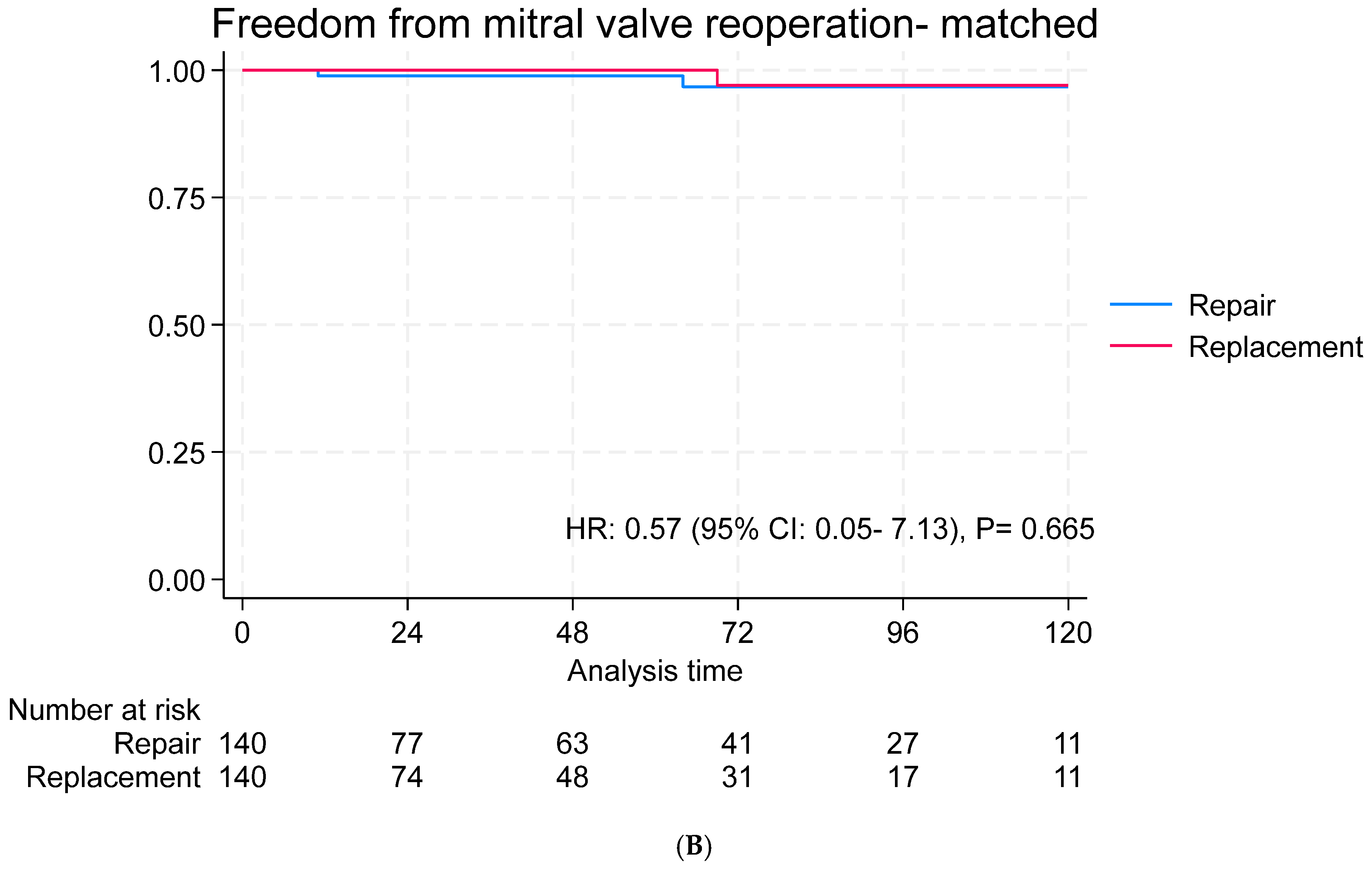
3.4. Long-Term Outcomes
3.5. Subgroup Analysis for Survival
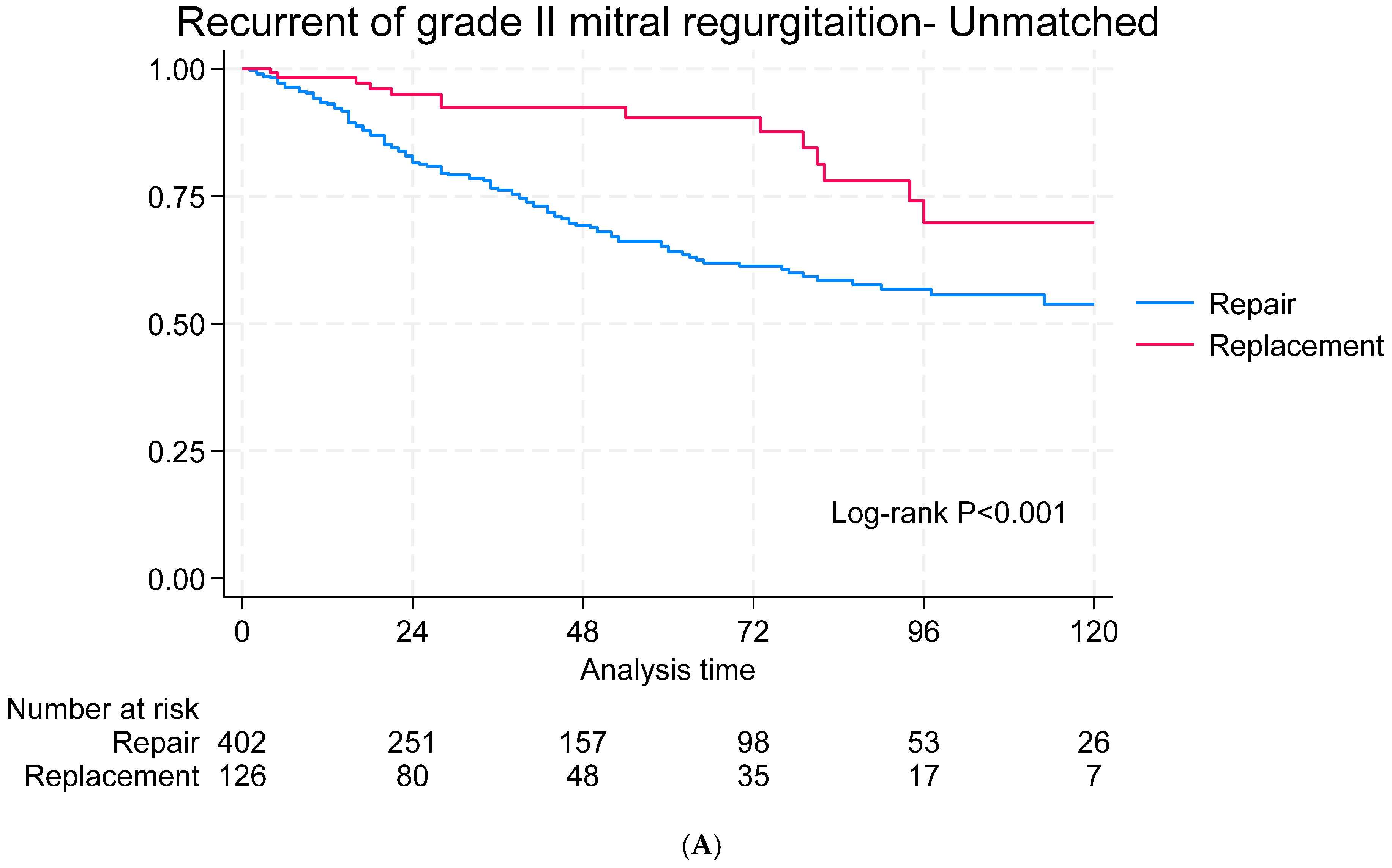
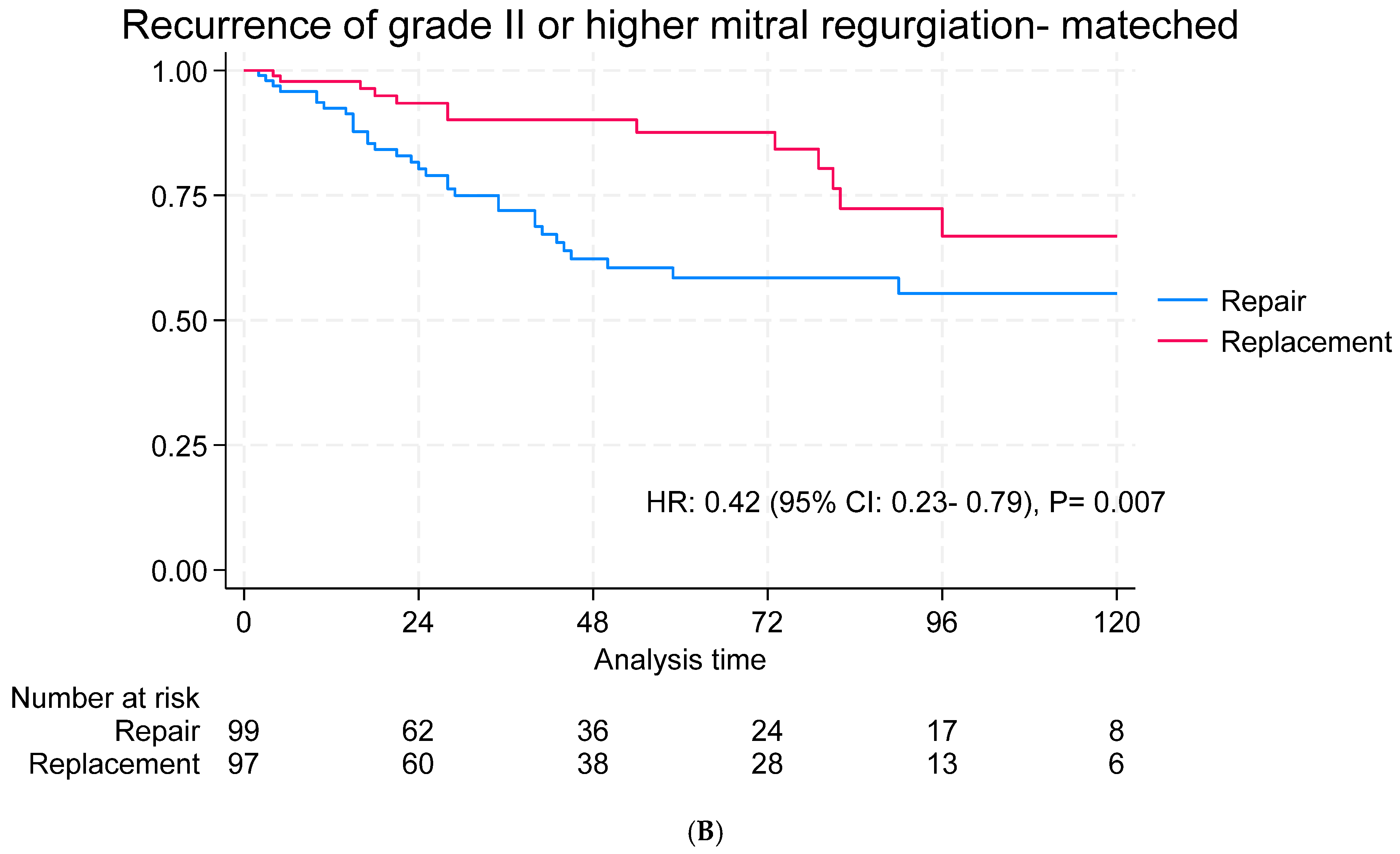
3.6. Echocardiographic Follow-Up
4. Discussion
4.1. Hospital Outcomes
4.2. Long-Term Outcomes
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grigioni, F.; Enriquez-Sarano, M.; Zehr, K.J.; Bailey, K.R.; Tajik, A.J. Ischemic Mitral Regurgitation. Circulation 2001, 103, 1759–1764. [Google Scholar]
- Estévez-Loureiro, R.; Lorusso, R.; Taramasso, M.; Torregrossa, G.; Kini, A.; Moreno, P.R. Management of Severe Mitral Regurgitation in Patients with Acute Myocardial Infarction: JACC Focus Seminar 2/5. J. Am. Coll. Cardiol. 2024, 83, 1799–1817. [Google Scholar] [PubMed]
- Hadjadj, S.; Marsit, O.; Paradis, J.M.; Beaudoin, J. Pathophysiology, Diagnosis, and New Therapeutic Approaches for Ischemic Mitral Regurgitation. Can. J. Cardiol. 2021, 37, 968–979. [Google Scholar] [PubMed]
- Chaput, M.; Handschumacher, M.D.; Tournoux, F.; Hua, L.; Guerrero, J.L.; Vlahakes, G.J.; Levine, R.A. Mitral Leaflet Adaptation to Ventricular Remodeling. Circulation 2008, 118, 845–852. [Google Scholar] [PubMed]
- Huang, A.L.; Dal-Bianco, J.P.; Levine, R.A.; Hung, J.W. Secondary Mitral Regurgitation: Cardiac Remodeling, Diagnosis, and Management. Struct. Heart 2023, 7, 100129. [Google Scholar]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar]
- Nappi, F.; Singh, S.S.A.; Fiore, A.; Ellouze, O. Insight from International Guidelines: Do We Have Satisfactory Recommendations for Secondary Mitral Regurgitation? Rev. Cardiovasc. Med. 2022, 23, 180. [Google Scholar]
- Hu, J.; Lee, A.P.W.; Wei, X.; Cheng, Z.Y.; Ho, A.M.H.; Wan, S. Update on surgical repair in functional mitral regurgitation. J. Card. Surg. 2022, 37, 3328–3335. [Google Scholar]
- Gamal, M.A.; El-Fiky, M.M.; Gamea, M.M.; Ali, I. Mitral valve repair versus replacement in severe ischemic mitral regurgitation systematic review and meta-analysis. J. Card. Surg. 2022, 37, 1591–1598. [Google Scholar]
- Acker, M.A.; Parides, M.K.; Perrault, L.P.; Moskowitz, A.J.; Gelijns, A.C.; Voisine, P.; Smith, P.K.; Hung, J.W.; Blackstone, E.H.; Puskas, J.D.; et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N. Engl. J. Med. 2014, 370, 23–32. [Google Scholar] [PubMed]
- Formica, F.; Gallingani, A.; Tuttolomondo, D.; Hernandez-Vaquero, D.; D’Alessandro, S.; Singh, G.; Benassi, F.; Grassa, G.; Pattuzzi, C.; Maestri, F.; et al. Long-term outcomes comparison of mitral valve repair or replacement for secondary mitral valve regurgitation. An updated systematic review and reconstructed time-to-event study-level meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102636. [Google Scholar]
- Luz, F.M.-H.; Amorim, M.J. Ischemic mitral regurgitation—To repair or replace? looking beyond the valve. Port. J. Card. Thorac. Vasc. Surg. 2022, 29, 25–34. [Google Scholar] [CrossRef]
- Nashef, S.A.M.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardio-Thorac. Surg. 2012, 41, 734–745. [Google Scholar]
- Vassileva, C.M.; Boley, T.; Markwell, S.; Hazelrigg, S. Meta-analysis of short-term and long-term survival following repair versus replacement for ischemic mitral regurgitation. Eur. J. Cardio-Thorac. Surg. 2011, 39, 295–303. [Google Scholar]
- Hannan, E.L.; Samadashvili, Z.; Smith, C.R.; Lahey, S.J.; Gold, J.P.; Jordan, D.; Sundt, T.M.; Girardi, L.; Ashraf, M.H.; Chikwe, J. Mitral valve repair versus replacement for patients with preserved left ventricular function without heart failure symptoms. J. Thorac. Cardiovasc. Surg. 2019, 157, 1432–1439.e2. [Google Scholar]
- Dufendach, K.; Aranda-Michel, E.; Sultan, I.; Gleason, T.G.; Navid, F.; Thoma, F.; Kilic, A. Outcomes of mitral valve surgery for severe ischemic mitral regurgitation. J. Card. Surg. 2020, 35, 390–396. [Google Scholar]
- Takagi, H.; Umemoto, T. Similar Survival After Repair vs Replacement for Ischemic Mitral Regurgitation. Semin. Thorac. Cardiovasc. Surg. 2016, 28, 748–756. [Google Scholar]
- Di Mauro, M.; Cargoni, M.; Liberi, R.; Lorusso, R.; Calafiore, A.M. Mitral valve repair or replacement. How long is this feud to last? J. Card. Surg. 2022, 37, 1599–1601. [Google Scholar]
- Thourani, V.H.; Weintraub, W.S.; Guyton, R.A.; Jones, E.L.; Williams, W.H.; Elkabbani, S.; Craver, J.M. Outcomes and Long-Term Survival for Patients Undergoing Mitral Valve Repair Versus Replacement. Circulation 2003, 108, 298–304. [Google Scholar]
- Deja, M.A.; Malinowski, M.; Widenka, K.; Stożyński, N.; Bartuś, K.; Kapelak, B.; Kuśmierczyk, M.; Hrapkowicz, T.; Suwalski, P.; Jasiński, M.; et al. Repair or Replacement for Secondary Mitral Regurgitation: Results From Polish National Registry. Ann. Thorac. Surg. 2022, 113, 146–156. [Google Scholar] [PubMed]
- Rao, C.; Murphy, M.O.; Saso, S.; Pandis, D.; Grapsa, J.; Nihoyannopoulos, P.; Reeves, B.C.; Athanasiou, T. Mitral Valve Repair or Replacement for Ischaemic Mitral Regurgitation: A Systematic Review. Heart Lung Circ. 2011, 20, 555–565. [Google Scholar]
- Virk, S.A.; Sriravindrarajah, A.; Dunn, D.; Liou, K.P.; Wolfenden, H.D.; Tan, G.M.Y.; Cao, C. A meta-analysis of mitral valve repair versus replacement for ischemic mitral regurgitation. Ann. Cardiothorac. Surg. 2015, 4, 400. [Google Scholar]
- Kron, I.L.; Hung, J.; Overbey, J.R.; Bouchard, D.; Gelijns, A.C.; Moskowitz, A.J.; Voisine, P.; O’Gara, P.T.; Argenziano, M.; Michler, R.E.; et al. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J. Thorac. Cardiovasc. Surg. 2015, 149, 752–761.e1. [Google Scholar] [PubMed]
- Sweeney, J.C.; Alotaibi, A.; Porter, G.D.; Avula, D.; Trivedi, J.R.; Slaughter, M.S.; Ganzel, B.L.; Pahwa, S.V. Ischemic mitral regurgitation: To repair or replace? A single center experience. PLoS ONE 2024, 19, e0307449. [Google Scholar]
- McNeely, C.A.; Vassileva, C.M. Long-term Outcomes of Mitral Valve Repair Versus Replacement for Degenerative Disease: A Systematic Review. Curr. Cardiol. Rev. 2014, 11, 157–162. [Google Scholar]
- Arafat, A.A.; Alghamdi, R.; Alfonso, J.; Shalaby, M.A.A.; Alotaibi, K.; Pragliola, C. Concomitant Mitral Valve Repair vs Replacement During Surgical Ventricular Restoration for Ischemic Cardiomyopathy. Angiology 2023, 75, 331–339. [Google Scholar]
- LaPar, D.J.; Acker, M.; Gelijns, A.C.; Kron, I.L. Repair or replace for severe ischemic mitral regurgitation: Prospective randomized multicenter data. Ann. Cardiothorac. Surg. 2015, 4, 411–416. [Google Scholar]
- Goldstein, D.R.; Moskowitz, A.J.; Gelijns, A.C.; Ailawadi, G.; Parides, M.K.; Perrault, L.P.; Hung, J.W.; Voisine, P.; Dagenais, F.; Gillinov, A.M.; et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N. Engl. J. Med. 2016, 374, 344–353. [Google Scholar]
- Noly, P.E.; Pagani, F.D.; Obadia, J.F.; Bouchard, D.; Bolling, S.F.; Ailawadi, G.; Tang, P.C. The role of surgery for secondary mitral regurgitation and heart failure in the era of transcatheter mitral valve therapies. Rev. Cardiovasc. Med. 2022, 23, 87. [Google Scholar]
| Unmatched | Matched | |||||
|---|---|---|---|---|---|---|
| Mitral Valve Repair (n = 565) | Mitral Valve Replacement (n = 194) | p Value | Mitral Valve Repair (n = 140) | Mitral Valve Replacement (n = 140) | SMD | |
| Age (years) | 62.46 ± 9.28 | 63.13 ± 9.58 | 0.388 | 63.28 ± 9.61 | 63.17 ± 9.24 | 0.01 |
| Male | 426 (75.40%) | 127 (65.46%) | 0.007 | 88 (62.86%) | 86 (61.43%) | −0.03 |
| BMI (kg/m2) | 27 (25–31) (n = 563) | 27 (24–31) | 0.944 | 27 (24–31) | 27 (24–31) | 0.01 |
| Smoker | 59/443 (13.32%) | 19/150 (12.67%) | 0.838 | 12 (8.57%) | 17 (12.14%) | −0.12 |
| EuroSCORE II (%) | 4.56 (2.57–7.58) (n = 535) | 7.13 (3.8–12.18) (n = 191) | <0.001 | 6.1 (3.33–9.57) | 5.89 (3.44–10.09) | 0.02 |
| NYHA III/IV | 446/558 (79.93%) | 161/192 (83.85%) | 0.232 | 118 (84.29%) | 118 (84.29%) | 0 |
| CCS III/IV | 230/560 (41.07%) | 65/192 (33.85%) | 0.077 | 54 (38.57%) | 43 (30.71%) | 0.16 |
| Diabetes mellitus | 421/561 (75.04%) | 132/192 (68.75%) | 0.088 | 101 (72.14%) | 98 (70%) | 0.05 |
| Dialysis | 29/547 (5.30) | 9/185 (4.86) | 0.817 | 7 (5%) | 6 (4.29%) | 0.03 |
| COPD | 19/550 (3.45%) | 5/185 (2.70%) | 0.619 | 4 (2.86%) | 4 (2.86%) | 0 |
| Previous MI | 266/559 (47.58%) | 87/189 (46.03%) | 0.712 | 64 (45.71%) | 54 (38.57%) | 0.14 |
| Atrial fibrillation | 46/534 (8.61) | 22/180 (12.22%) | 0.154 | 17 (12.23%) | 19 (13.87%) | −0.05 |
| Implanted device | 9/544 (1.65%) | 5/179 (2.79%) | 0.337 | 4 (2.96%) | 3 (2.27%) | 0.04 |
| Previous cardiac surgery | 11/557 (1.97) | 8/193 (4.15) | 0.098 | 4 (2.86%) | 4 (2.86%) | 0 |
| Creatinine clearance (mL/min) | 80 (56–102) (n = 543) | 78 (58–95) (n = 189) | 0.387 | 71 (52–95) | 77 (58–95) | −0.05 |
| Preop IABP | 12/552 (2.17%) | 13/187 (6.95%) | 0.002 | 5 (3.60%) | 7 (5%) | −0.07 |
| Inotropes | 74/550 (13.45%) | 19/188 (10.11%) | 0.232 | 17 (12.23%) | 10 (7.14%) | 0.17 |
| Ventilation | 60/550 (10.91%) | 12/187 (6.42%) | 0.074 | 11 (7.91%) | 6 (4.29%) | 0.15 |
| Emergency | 43 (7.61) | 24 (12.37) | 0.044 | 11 (7.86%) | 12 (8.57%) | −0.03 |
| Ejection fraction (%) | 35 (30–45) (n = 556) | 35 (25–45) (n = 194) | 0.103 | 35 (30–45) | 40 (28–48) | −0.12 |
| LVEDD (mm) | 55.12 ± 6.86 (n = 559) | 57.22 ± 8.54 (n = 189) | <0.001 | 56 (52–60) | 56 (52–61) | 0 |
| LVESD (mm) | 41.79 ± 7.96 (n = 556) | 43.65 ± 10.16 (n = 189) | 0.010 | 42 (37–48) | 43 (36–48) | 0.06 |
| RV hypofunction | 38/558 (6.81%) | 38/188 (20.21%) | <0.001 | 24 (17.14%) | 24 (17.14%) | 0 |
| RV dilatation | 28/564 (4.96%) | 35/193 (18.13%) | <0.001 | 14 (10%) | 22 (15.71%) | −0.17 |
| Concomitant CABG | 556 (98.41%) | 190 (97.94%) | 0.664 | 139 (99.29%) | 138 (98.57%) | 0.07 |
| Tricuspid repair | 171 (30.27%) | 120 (61.86%) | <0.001 | 89 (63.57%) | 86 (61.43%) | 0.04 |
| Unmatched | Matched | |||||
|---|---|---|---|---|---|---|
| Mitral Valve Repair (n = 565) | Mitral Valve Replacement (n = 194) | p Value | Mitral Valve Repair (n = 140) | Mitral Valve Replacement (n = 140) | p Value | |
| Cross clamp (min) | 111 (92–130) (n = 507) | 122 (104–142) (n = 182) | <0.001 | 114 (94–130) | 123 (105–143) | <0.001 |
| CPB time (min) | 140 (120–167) (n = 511) | 155 (133–186) (n = 18) | <0.001 | 151 (125–172) | 157 (134–187) | 0.021 |
| New-onset AF | 67/491 (13.65%) | 37/168 (22.02%) | 0.010 | 21/121 (17.36%) | 23/122 (18.85%) | >0.99 |
| IABP | 33/512 (6.45%) | 29/177 (16.38%) | <0.001 | 8/123 (6.50%) | 16/126 (12.70%) | 0.151 |
| ECMO | 11/513 (2.14%) | 12/177 (6.78%) | 0.003 | 1/123 (0.81%) | 5/126 (3.97%) | 0.219 |
| CRRT/Dialysis | 40/509 (7.85%) | 24/167 (14.37%) | 0.013 | 15/121 (12.40%) | 16/119 (13.45%) | 0.832 |
| Stroke | 13/507 (2.56%) | 11/168 (6.55%) | 0.016 | 5/120 (4.17%) | 10/120 (8.33%) | 0.549 |
| Open Sternum | 18/505 (3.56%) | 19/174 (10.92%) | <0.001 | 5/122 (4.10%) | 8/128 (6.25%) | 0.581 |
| Hospital mortality | 38 (6.73%) | 25 (12.89%) | 0.007 | 14 (10%) | 15 (10.71%) | >0.99 |
| HR (95% CI) | p | |
|---|---|---|
| Age | ||
| Age < 60—Replacement vs. Repair | 0.68 (0.20–2.28) | 0.534 |
| Age ≥ 60—Replacement vs. Repair | 1.09 (0.59–2.01) | 0.776 |
| Gender | ||
| Male—Replacement vs. Repair | 0.93 (0.45–1.91) | 0.845 |
| Female—Replacement vs. Repair | 1.29 (0.57–2.90) | 0.536 |
| Ejection fraction | ||
| EF > 40—Replacement vs. Repair | 1.72 (0.75–3.92) | 0.202 |
| EF ≤ 40—Replacement vs. Repair | 0.74 (0.36–1.55) | 0.427 |
| EuroSCORE II | ||
| EuroSCORE < 8—Replacement vs. Repair | 1.25 (0.58–2.67) | 0.564 |
| EuroSCORE ≥ 8—Replacement vs. Repair | 0.86 (0.40–1.84) | 0.693 |
| RV function | ||
| Normal—Replacement vs. Repair | 0.98 (0.54–1.78) | 0.958 |
| Dysfunction—Replacement vs. Repair | 1.44 (0.59–3.56) | 0.425 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elnagar, I.M.; Alghamdi, R.; Alawami, M.H.; Alshammari, A.; Almedimigh, A.A.; Albabtain, M.A.; AlGhamdi, A.; Ismail, H.H.; Shalaby, M.A.; Alotaibi, K.A.; et al. Long-Term Outcomes of Mitral Valve Repair Versus Replacement in Patients with Ischemic Mitral Regurgitation: A Retrospective Propensity-Matched Analysis. J. Cardiovasc. Dev. Dis. 2025, 12, 109. https://doi.org/10.3390/jcdd12040109
Elnagar IM, Alghamdi R, Alawami MH, Alshammari A, Almedimigh AA, Albabtain MA, AlGhamdi A, Ismail HH, Shalaby MA, Alotaibi KA, et al. Long-Term Outcomes of Mitral Valve Repair Versus Replacement in Patients with Ischemic Mitral Regurgitation: A Retrospective Propensity-Matched Analysis. Journal of Cardiovascular Development and Disease. 2025; 12(4):109. https://doi.org/10.3390/jcdd12040109
Chicago/Turabian StyleElnagar, Ismail M., Rawan Alghamdi, Murtadha H. Alawami, Ahmad Alshammari, Abdulmalik A. Almedimigh, Monirah A. Albabtain, Alaa AlGhamdi, Huda H. Ismail, Mostafa A. Shalaby, Khaled A. Alotaibi, and et al. 2025. "Long-Term Outcomes of Mitral Valve Repair Versus Replacement in Patients with Ischemic Mitral Regurgitation: A Retrospective Propensity-Matched Analysis" Journal of Cardiovascular Development and Disease 12, no. 4: 109. https://doi.org/10.3390/jcdd12040109
APA StyleElnagar, I. M., Alghamdi, R., Alawami, M. H., Alshammari, A., Almedimigh, A. A., Albabtain, M. A., AlGhamdi, A., Ismail, H. H., Shalaby, M. A., Alotaibi, K. A., & Arafat, A. A. (2025). Long-Term Outcomes of Mitral Valve Repair Versus Replacement in Patients with Ischemic Mitral Regurgitation: A Retrospective Propensity-Matched Analysis. Journal of Cardiovascular Development and Disease, 12(4), 109. https://doi.org/10.3390/jcdd12040109






