Oral Anticoagulation Choice and Dosage in Very Elderly Patients with Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Study Endpoints
2.3. Definition of Covariates
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Prediction of OAC Discharge Prescription
3.3. Association of OAC Type (DOACs vs. VKA) with Clinical Outcomes
3.4. Prediction of the Prescription of Full vs. Reduced DOAC Dose
3.5. Association of DOAC Dose (Full vs. Reduced) with Clinical Outcomes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
References
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Wolf, P.A.; Benjamin, E.J.; Levy, D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am. J. Cardiol. 1998, 82, 2N–9N. [Google Scholar] [CrossRef]
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.-H.; McAnulty, J.H., Jr.; Zheng, Z.-J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, A.S.; Kartas, A.; Moysidis, D.V.; Tsagkaris, C.; Papadakos, S.P.; Bekiaridou, A.; Samaras, A.; Karagiannidis, E.; Papadakis, M.; Giannakoulas, G. Glycemic control and atrial fibrillation: An intricate relationship, yet under investigation. Cardiovasc. Diabetol. 2022, 21, 39. [Google Scholar] [CrossRef]
- Hanon, O.; Jeandel, C.; Jouanny, P.; Paccalin, M.; Puisieux, F.; Krolak-Salmon, P.; Berrut, G. Anticoagulant treatment in elderly patients with atrial fibrillation: A position paper. Geriatr. Psychol. Neuropsychiatr. Vieil. 2019, 17, 341–354. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016, 18, 1609–1678. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, A.S.; Moysidis, D.V.; Kartas, A.; Bekiaridou, A.; Samaras, A.; Giannakoulas, G. Oral anticoagulation challenges and therapeutic dilemmas in the very elderly: To treat and how to treat octogenarians and nonagenarians? Pol. Arch. Intern. Med. 2023, 133, 16508. [Google Scholar] [CrossRef]
- Besford, M.; Graham, S.; Sammon, C.; Mehmud, F.; Allan, V.; Alikhan, R.; Ramagopalan, S. Factors associated with non-prescription of oral anticoagulant treatment in non-valvular atrial fibrillation patients with dementia: A CPRD-HES study. Age Ageing 2020, 49, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Foley, G.; Zhang, N.; Zhou, Y.; Crawford, S.; McManus, D.; Gurwitz, J. Geriatric Conditions Predict Discontinuation of Anticoagulation in Long-Term Care Residents With Atrial Fibrillation. J. Am. Geriatr. Soc. 2020, 68, 717–724. [Google Scholar] [CrossRef]
- Okumura, K.; Yamashita, T.; Akao, M.; Atarashi, H.; Ikeda, T.; Koretsune, Y.; Shimizu, W.; Suzuki, S.; Tsutsui, H.; Toyoda, K.; et al. Oral Anticoagulants in Very Elderly Nonvalvular Atrial Fibrillation Patients With High Bleeding Risks: ANAFIE Registry. JACC Asia 2022, 2, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S.; Kim, H.-J.; Kim, T.-H.; Uhm, J.-S.; Joung, B.; Lee, M.-H.; Pak, H.-N. Non-vitamin K antagonist oral anticoagulants have better efficacy and equivalent safety compared to warfarin in elderly patients with atrial fibrillation: A systematic review and meta-analysis. J. Cardiol. 2018, 72, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Choi, E.K.; Park, C.S.; Cha, M.-J.; Lee, S.-Y.; Kwon, J.-M.; Oh, S. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in octogenarian patients with non-valvular atrial fibrillation. PLoS ONE 2019, 14, e0211766. [Google Scholar] [CrossRef]
- Lai, C.L.; Chen, H.M.; Liao, M.T.; Lin, T.T. Dabigatran, Rivaroxaban, and Warfarin in the Oldest Adults with Atrial Fibrillation in Taiwan. J. Am. Geriatr. Soc. 2018, 66, 1567–1574. [Google Scholar] [CrossRef]
- Lee, S.; Monz, B.U.; Clemens, A.; Brueckmann, M.; Lip, G.Y.H. Representativeness of the dabigatran, apixaban and rivaroxaban clinical trial populations to real-world atrial fibrillation patients in the United Kingdom: A cross-sectional analysis using the General Practice Research Database. BMJ Open 2012, 2, e001768. [Google Scholar] [CrossRef] [PubMed]
- Oqab, Z.; Pournazari, P.; Sheldon, R.S. What is the Impact of Frailty on Prescription of Anticoagulation in Elderly Patients with Atrial Fibrillation? A Systematic Review and Meta-Analysis. J. Atr. Fibrillation 2018, 10, 1870. [Google Scholar] [CrossRef]
- Samaras, A.; Kartas, A.; Vasdeki, D.; Dividis, G.; Forozidou, E.; Fotos, G.; Kotsi, E.; Paschou, E.; Tsoukra, P.; Goulas, I.; et al. Rationale and design of a randomized study comparing Motivational Interviewing to Support Oral Anticoagulation adherence versus usual care in patients with nonvalvular atrial fibrillation: The MISOAC-AF trial. Hell. J. Cardiol. 2020, 61, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Tzikas, A.; Samaras, A.; Kartas, A.; Vasdeki, D.; Fotos, G.; Dividis, G.; Paschou, E.; Forozidou, E.; Tsoukra, P.; Kotsi, E.; et al. Motivational Interviewing to Support Oral AntiCoagulation adherence in patients with non-valvular Atrial Fibrillation (MISOAC-AF): A randomized clinical trial. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, f63–f71. [Google Scholar] [CrossRef] [PubMed]
- Patsiou, V.; Samaras, A.; Kartas, A.; Moysidis, D.V.; Papazoglou, A.S.; Bekiaridou, A.; Baroutidou, A.; Ziakas, A.; Tzikas, A.; Giannakoulas, G. Prognostic implications of adherence to oral anticoagulants among patients with atrial fibrillation: Insights from MISOAC-AF trial. J. Cardiol. 2023, 81, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, D.; Nunes-Ferreira, A.; Rodrigues, R.; Vicente, E.; Pinto, F.J.; Ferreira, J.J. Non-vitamin K antagonist oral anticoagulants in elderly patients with atrial fibrillation: A systematic review with meta-analysis and trial sequential analysis. Arch. Gerontol. Geriatr. 2019, 81, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.; Lucerna, M.; Pecen, L.; Siller-Matula, J.M.; Cavallari, I.; Kirchhof, P.; De Caterina, R. Thromboembolic Risk, Bleeding Outcomes and Effect of Different Antithrombotic Strategies in Very Elderly Patients With Atrial Fibrillation: A Sub-Analysis From the PREFER in AF (PREvention oF Thromboembolic Events–European Registry in Atrial Fibrillation). J. Am. Heart Assoc. 2017, 6, e005657. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Wallentin, L.; Connolly, S.J.; Ezekowitz, M.; Healey, J.S.; Oldgren, J.; Yang, S.; Alings, M.; Kaatz, S.; Hohnloser, S.H.; et al. Risk of Bleeding With 2 Doses of Dabigatran Compared With Warfarin in Older and Younger Patients With Atrial Fibrillation. Circulation 2011, 123, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Halperin, J.L.; Hankey, G.J.; Wojdyla, D.M.; Piccini, J.P.; Lokhnygina, Y.; Patel, M.R.; Breithardt, G.; Singer, D.E.; Becker, R.C.; Hacke, W.; et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the rivaroxaban once daily, oral, direct factor xa inhibition compared with vitamin k antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation 2014, 130, 138–146. [Google Scholar] [CrossRef]
- Halvorsen, S.; Atar, D.; Yang, H.; De Caterina, R.; Erol, C.; Garcia, D.; Granger, C.B.; Hanna, M.; Held, C.; Husted, S.; et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: Observations from the ARISTOTLE trial. Eur. Heart J. 2014, 35, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, E1–E156. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Antonucci, E.; Ageno, W.; Bertù, L.; Migliaccio, L.; Martinese, L.; Pilato, G.; Testa, S.; Palareti, G. Oral anticoagulation in very elderly patients with atrial fibrillation: Results from the prospective multicenter START2-REGISTER study. PLoS ONE 2019, 14, e0216831. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, O.C.W.; Jonasson, C.; Ghanima, W.; Söderdahl, F.; Halvorsen, S. Effectiveness and safety of oral anticoagulants in elderly patients with atrial fibrillation. Heart 2022, 108, 345–352. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Joosten, L.P.T.; Van Doorn, S.; Van De Ven, P.M.; Köhlen, B.T.; Nierman, M.C.; Koek, H.L.; Hemels, M.E.; Huisman, M.V.; Kruip, M.; Faber, L.M.; et al. Safety of Switching From a Vitamin K Antagonist to a Non-Vitamin K Antagonist Oral Anticoagulant in Frail Older Patients With Atrial Fibrillation: Results of the FRAIL-AF Randomized Controlled Trial. Circulation 2024, 149, 279–289. [Google Scholar] [CrossRef]
- Ding, J.; Sun, Y.; Zhang, K.; Huang, W.; Tang, M.; Zhang, D.; Xing, Y. Effects of Anticoagulant Therapy and Frailty in the Elderly Patients with Atrial Fibrillation. Clin. Interv. Aging 2024, 19, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Cools, F.; Virdone, S.; Bassand, J.-P.; Fitzmaurice, D.A.; Fox, K.A.A.; Goldhaber, S.Z.; Goto, S.; Haas, S.; Mantovani, L.G.; et al. Mortality in Patients With Atrial Fibrillation Receiving Nonrecommended Doses of Direct Oral Anticoagulants. J. Am. Coll. Cardiol. 2020, 76, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Kartas, A.; Samaras, A.; Vasdeki, D.; Dividis, G.; Fotos, G.; Paschou, E.; Forozidou, E.; Tsoukra, P.; Kotsi, E.; Goulas, I.; et al. Flaws in Anticoagulation Strategies in Patients With Atrial Fibrillation at Hospital Discharge. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Tzeis, S.; Savvari, P.; Skiadas, I.; Patsilinakos, S.; Stamatelopoulos, K.; Kourouklis, S.; Kyrikos, S.; Tsatiris, K.; Menegas, D.; Hahalis, G.; et al. Right drug, wrong dosage: Insights from the PAVE-AF antithrombotic study in older patients with atrial fibrillation. J. Thromb. Thrombolysis 2021, 51, 81. [Google Scholar] [CrossRef]
- Pilotto, A.; Veronese, N.; Polidori, M.C.; Strandberg, T.; Topinkova, E.; Cruz-Jentoft, A.J.; Custodero, C.; Maggi, S.; on behalf of the EUROSAF Study Investigators. The role of prognostic stratification on prescription of anticoagulants in older patients with atrial fibrillation: A multicenter, observational, prospective European study (EUROSAF). Ann. Med. 2022, 54, 2411. [Google Scholar] [CrossRef] [PubMed]

| All Patients Under OACs (n = 450, 100%) | Patients Under VKA (n = 164, 36.4%) | Patients Under DOAC (n = 286, 63.6%) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Male gender, n (%) | 213 (47.3) | 77 (47) | 136 (47.6) | 0.90 |
| Age, mean (SD) | 81.9 (4.3) | 81 (4.2) | 81.2 (4.3) | 0.85 |
| Type of AF | ||||
| Temporal type of AF, n (%) | 445 | 163 | 282 | |
| First diagnosed AF, n (%) | 40 (9) | 10 (2.2) | 30 (6.7) | 0.29 |
| Paroxysmal AF or atrial flutter, n (%) | 137 (30.8) | 42 (9.4) | 95 (21.3) | 0.29 |
| Persistent or permanent AF, n (%) | 268 (60.2) | 111 (24.9) | 157 (35.3) | 0.29 |
| Cardiovascular risk factors and comorbidities | ||||
| BMI (kg/m2), mean (SD) | 28 (4.8) | 27.6 (4.9) | 28 (4.8) | 0.84 |
| History of smoking (prior or current), n (%) | 234 (53.3) | 84 (52.2) | 150 (54) | 0.72 |
| Serum creatinine (mg/dL), mean (SD) | 1.33 (0.88) | 1.37 (0.86) | 1.16 (0.41) | <0.01 |
| CHA2DS2-VASc score, mean (SD) | 5.4 (1.4) | 5.8 (1.6) | 5.3 (1.4) | <0.01 |
| HAS-BLED score, mean (SD) | 12 (0.9) | 2.43 (1.0) | 1.7 (0.8) | <0.01 |
| HAS-BLED ≥ 3, n (%) | 118 (26.2) | 77 (47) | 41 (14.3) | <0.01 |
| Estimated GFR with Cockcroft–Gault formula, mean (SD) | 27.5 (29.69) | 24 (27) | 28.6 (30.8) | 0.02 |
| Estimated GFR with CKD-EPI formula, mean (SD) | 53.4 (19.9) | 51.9 (19.5) | 56.3 (18.3) | 0.43 |
| Hemoglobin (g/dL), mean (SD) | 12.30 (2.32) | 12.3 (2.3) | 12.4 (2.5) | 0.29 |
| Coronary artery disease, n (%) | 270 (60.9) | 117 (71.8) | 153 (54.6) | <0.01 |
| Heart failure, n (%) | 270 (60.9) | 117 (71.8) | 153 (54.6) | <0.01 |
| Prior myocardial infarction, n (%) | 270 (60.9) | 117 (71.8) | 153 (54.6) | <0.01 |
| Pacemaker/ ICD, n (%) | 48 (2.5) | 25 (5.7) | 23 (5.2) | 0.07 |
| Hypertension, n (%) | 389 (87.4) | 131 (80.9) | 258 (91.2) | <0.01 |
| Dyslipidemia, n (%) | 218 (49.2) | 86 (52.8) | 132 (47.1) | 0.25 |
| Diabetes mellitus, n (%) | 165 (37.1) | 68 (41.7) | 97 (34.4) | 0.12 |
| Peripheral artery disease, n (%) | 217 (49.3) | 95 (59) | 122 (43.7) | <0.01 |
| Prior ischemic stroke (%) | 57 (12.8) | 25 (15.5) | 32 (11.2) | 0.19 |
| Prior systemic thromboembolic disease, n (%) | 38 (8.5) | 19 (11.8) | 19 (6.6) | 0.06 |
| Prior intracranial bleeding, n (%) | 3 (0.7) | 2 (1.2) | 1 (0.4) | 0.27 |
| Prior upper gastrointestinal bleeding, n (%) | 35 (7.9) | 14 (8.7) | 21 (7.4) | 0.62 |
| Prior lower gastrointestinal bleeding, n (%) | 36 (8.1) | 13 (2.9) | 23 (8.1) | 0.99 |
| Prior bleeding episodes, n (%) | 147 (33) | 57 (35.4) | 90 (31.7) | 0.42 |
| Chronic kidney disease, n (%) | 269 (60.6) | 107 (66) | 162 (57.4) | 0.07 |
| Hepatic disease, n (%) | 9 (2.1) | 3 (1.9) | 6 (2.2) | 0.83 |
| Thyroid disease, n (%) | 96 (21.8) | 32 (19.9) | 64 (22.9) | 0.45 |
| Medication | ||||
| Use of rate control medication at discharge (b-blocker, digoxin, or both), n (%) | 362 (80.4) | 128 (78) | 234 (81.8) | 0.33 |
| Use of antiplatelets at discharge (aspirin, clopidogrel, or both), n (%) | 75 (17.1) | 49 (29.9) | 26 (9.5) | <0.01 |
| Use of rhythm control medication at discharge (propafenone, amiodarone, sotalol), n (%) | 79 (17.6) | 27 (16.5) | 52 (18.2) | 0.65 |
| All Patients Under DOACs (N = 286, 100%) | Reduced Dose of DOACs (N = 190, 66.4%) | Full Dose of DOACs (N = 96, 33.6%) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Male gender, n (%) | 134 (46.9) | 97 (51.1) | 37 (38.5) | 0.04 |
| Age (years), mean (SD) | 81.2 (4.3) | 81.9 (4.4) | 79.9 (3.7) | <0.01 |
| Type of AF | ||||
| First diagnosed AF, n (%) | 30 (10.8) | 15 (16) | 15 (8.1) | 0.12 |
| Paroxysmal AF or atrial flutter, n (%) | 94 (33.7) | 66 (35.7) | 28 (29.8) | 0.12 |
| Persistent or permanent AF, n (%) | 155 (55.6) | 104 (56.2) | 51 (54.3) | 0.12 |
| Cardiovascular risk factors and comorbidities | ||||
| BMI (kg/m2), mean (SD) | 28.0 (4.8) | 27.6 (4.7) | 28.9 (4.8) | 0.03 |
| History of smoking (prior or current), n (%) | 148 (53.8) | 106 (58.2) | 42 (45.2) | 0.04 |
| Serum creatinine (mg/dL), mean (SD) | 1.16 (0.4) | 1.2 (0.4) | 1 (0.3) | <0.01 |
| CHA2DS2-VASc score, mean (SD) | 5.3 (1.4) | 5.3 (1.3) | 5.2 (1.4) | 0.47 |
| HAS-BLED score, mean (SD) | 1.7 (0.8) | 1.7 (0.8) | 1.7 (0.8) | 0.94 |
| HAS-BLED ≥ 3, n (%) | 41 (14.5) | 29 (15.4) | 12 (12.6) | 0.53 |
| Estimated GFR with Cockcroft–Gault formula, mean (SD) | 28.6 (30.8) | 29.2 (29.2) | 27.1 (34.0) | 0.59 |
| Estimated GFR with CKD-EPI formula, mean (SD) | 56.3 (18.3) | 52 (17.7) | 64.5 (16.5) | <0.01 |
| Hemoglobin (g/dL), mean (SD) | 12.4 (2.5) | 12.4 (2.7) | 12.4 (2.0) | 0.91 |
| Coronary artery disease, n (%) | 91 (32.4) | 72 (38.7) | 19 (20.0) | <0.01 |
| Heart failure, n (%) | 164 (58) | 116 (61.7) | 48 (50.5) | 0.07 |
| Prior myocardial infarction, n (%) | 48 (17.3) | 41 (22.3) | 7 (7.5) | <0.01 |
| Pacemaker/ ICD, n (%) | 23 (8.3) | 17 (9.2) | 6 (6.5) | 0.28 |
| Hypertension, n (%) | 256 (91.4) | 169 (90.9) | 87 (92.6) | 0.63 |
| Dyslipidemia, n (%) | 130 (46.9) | 84 (45.7) | 46 (49.5) | 0.55 |
| Diabetes mellitus, n (%) | 97 (34.8) | 62 (33.3) | 35 (37.6) | 0.48 |
| Peripheral artery disease, n (%) | 120 (43.5) | 87 (47.5) | 33 (35.5) | 0.06 |
| Prior ischemic or hemorrhagic stroke or transient ischemic attack, n (%) | 47 (16.7) | 34 (18.1) | 13 (13.8) | 0.63 |
| Systemic thromboembolic disease, n (%) | 19 (6.7) | 14 (7.4) | 5 (5.3) | 0.49 |
| Prior intracranial bleeding, n (%) | 1 (0.4) | 1 (0.5) | 0 (0.0) | 0.47 |
| Prior upper gastrointestinal bleeding, n (%) | 21 (7.5) | 13 (7.0) | 8 (8.4) | 0.67 |
| Prior lower gastrointestinal bleeding, n (%) | 23 (8.2) | 15 (8.1) | 8 (8.4) | 0.92 |
| Prior bleeding episodes, n (%) | 90 (32.0) | 56 (30.1) | 34 (35.8) | 0.33 |
| Chronic kidney disease, n (%) | 161 (57.7) | 126 (68.1) | 35 (37.2) | <0.01 |
| Hepatic disease | 6 (2.2) | 4 (2.2) | 2 (2.2) | 0.98 |
| Medication | ||||
| Use of rate control medication at discharge (b-blocker, digoxin, or both), n (%) | 231 (81.6) | 152 (80.9) | 79 (83.2) | 0.90 |
| Use of antiplatelets at discharge (aspirin, clopidogrel, or both), n (%) | 25 (9.1) | 21 (11.4) | 4 (4.4) | 0.10 |
| Use of rhythm control medication at discharge (propafenone, amiodarone, sotalol), n (%) | 52 (18.3) | 33 (20) | 19 (6.7) | 0.35 |
| Univariate p-Value | Multivariate p-Value | Adjusted OR (95% CIs) | |
|---|---|---|---|
| Female gender | 0.90 | 0.94 | 1.02 (0.55–1.90) |
| Age | 0.63 | 0.71 | 0.99 (0.94–1.05) |
| Smoking (ever) | 0.72 | 0.81 | 0.94 (0.57–1.55) |
| Body mass index | 0.69 | 0.62 | 0.99 (0.94–1.04) |
| CHA2DS2-VASc score | <0.01 | 0.02 | 0.69 (0.50–0.95) |
| HAS-BLED score | <0.01 | <0.01 | 0.33 (0.23–0.48) |
| Coronary artery disease | <0.01 | 0.80 | 0.93 (0.52–1.66) |
| Hypertension | <0.01 | <0.01 | 4.80 (2.30–10.00) |
| Diabetes mellitus | 0.12 | 0.24 | 1.43 (0.79–2.58) |
| Chronic kidney disease | 0.08 | 0.09 | 0.64 (0.39–1.07) |
| Prior stroke or TIA | 0.05 | <0.01 | 4.90 (1.8–13.44) |
| Prior major bleeding | 0.94 | 0.01 | 2.72 (1.26–5.88) |
| Antiplatelet use | <0.01 | 0.05 | 0.74 (0.54–1.00) |
| Outcome | Rates of Occurrence in VKA | Rates of Occurrence in DOACs | Log-Rank Test p-Value | Univariate HR Cis (95%) | Multivariate HR * Cis (95%) |
|---|---|---|---|---|---|
| All-cause mortality | 90 (54.9) | 113 (39.5) | 0.004 | 0.69 (0.50–0.88) | 0.79 (0.58–1.06) |
| CV mortality | 69 (42.1) | 80 (28.0) | 0.003 | 0.62 (0.45–0.85) | 0.76 (0.53–1.07) |
| Bleeding | 5 (3.4) | 16 (6.1) | 0.302 | 1.69 (0.62–4.60) | 1.50 (0.52–4.27 |
| Stroke | 11 (7.5) | 11 (4.2) | 0.110 | 0.51 (0.22–1.17) | 0.43 (0.17–1.08) |
| AF- or HF-related hospitalization or CV death | 116 (70.7) | 163 (57.0) | 0.297 | 0.95 (0.64–1.43) | 0.84 (0.63–1.11) |
| Covariate | Univariate p-Value | Multivariate p-Value | Adjusted OR (95% CIs) |
|---|---|---|---|
| Diabetes mellitus | 0.477 | 0.449 | 0.74 (0.35–1.60) |
| Age | <0.001 | 0.033 | 1.09 (1.01–1.17) |
| Female gender | 0.044 | 0.450 | 0.73 (0.32–1.65) |
| Body mass index | 0.035 | 0.969 | 1.00 (0.94–1.07) |
| Smoking (ever) | 0.040 | 0.098 | 1.68 (0.91–3.12) |
| Heart failure | 0.073 | 0.639 | 1.20 (0.56–2.56) |
| Coronary artery disease | 0.002 | 0.730 | 1.18 (0.46–3.05) |
| Prior acute myocardial infarction | 0.003 | 0.069 | 3.01 (0.92–9.88) |
| Chronic kidney disease | <0.001 | <0.001 | 3.53 (1.92–6.49) |
| History of any stroke or TIA | 0.366 | 0.924 | 1.07 (0.26–4.45) |
| History of bleeding | 0.335 | 0.180 | 0.64 (0.33–1.23) |
| HAS_BLED > 3 | 0.528 | 0.854 | 1.09 (0.42–2.82) |
| CHA2DS2_VASC | 0.334 | 0.887 | 1.04 (0.64–1.67) |
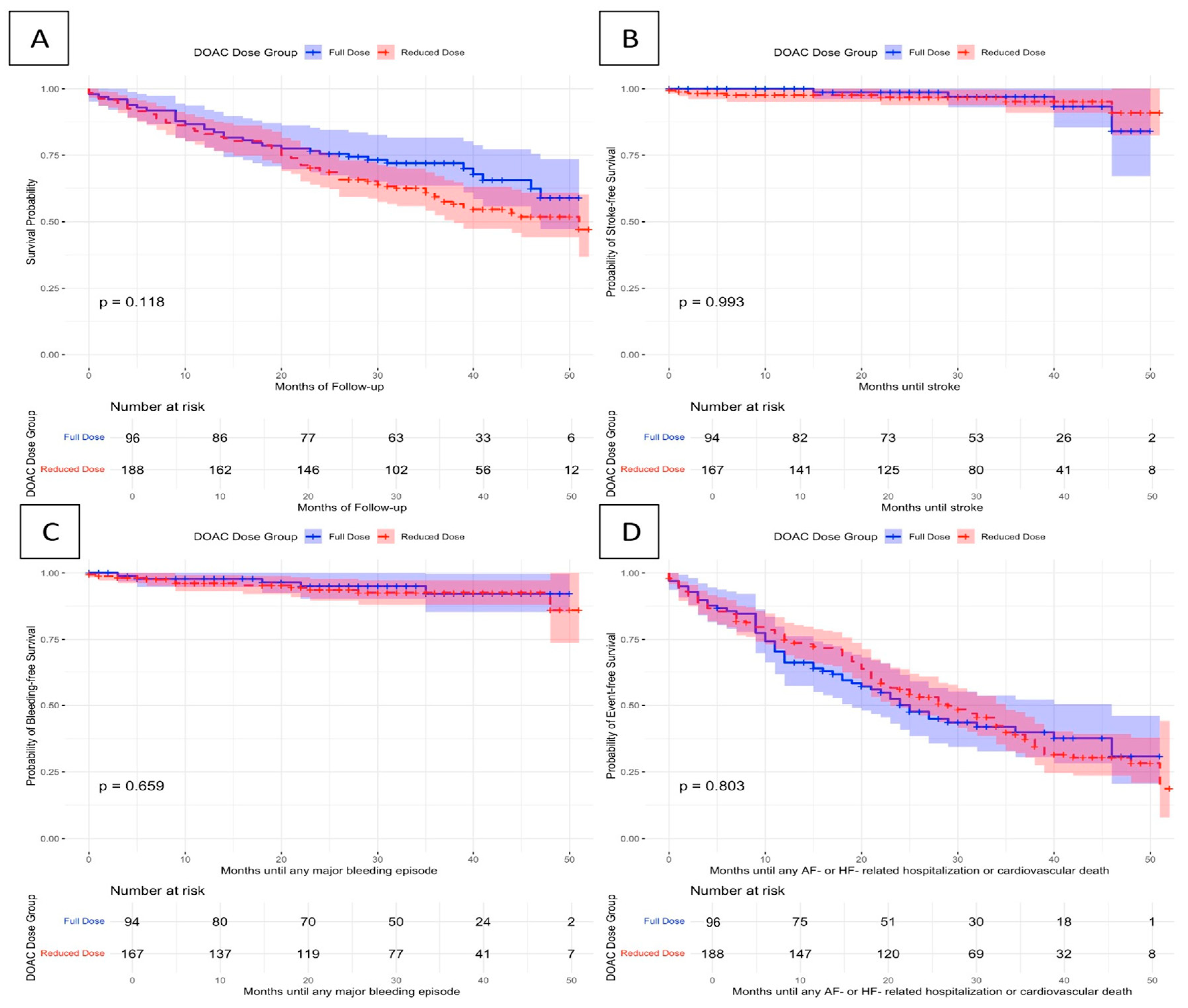
| Outcome | Rates of Occurrence in DOAC Reduced Dose | Rates of Occurrence in DOAC Full Dose | Log-Rank Test p-Value | Univariate HR (95% CIs) | Multivariate aHR * (95% CIs) |
|---|---|---|---|---|---|
| All-cause mortality | 81 (43.1) | 32 (33.7) | 0.118 | 1.32 (0.88–1.99) | 0.96 (0.60–1.53) |
| CV mortality | 59 (31.4) | 21 (22.1) | 0.137 | 1.46 (0.89–2.40) | 0.84 (0.47–1.50) |
| Bleeding | 11 (6.6) | 5 (5.5) | 0.659 | 1.24 (0.43–3.56) | 1.32 (0.42–4.16) |
| Stroke | 7 (4.2) | 4 (4.4) | 0.993 | 0.97 (0.28–3.31) | 1.24 (0.27–5.70) |
| AF- or HF-related hospitalization or CV death | 93 (49.5) | 39 (41.1) | 0.803 | 1.09 (0.75–1.59) | 0.79 (0.50–1.22) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zergioti, M.; Kyriakou, M.; Papazoglou, A.S.; Kartas, A.; Moysidis, D.V.; Samaras, A.; Karagiannidis, E.; Kamperidis, V.; Ziakas, A.; Giannakoulas, G. Oral Anticoagulation Choice and Dosage in Very Elderly Patients with Atrial Fibrillation. J. Cardiovasc. Dev. Dis. 2025, 12, 86. https://doi.org/10.3390/jcdd12030086
Zergioti M, Kyriakou M, Papazoglou AS, Kartas A, Moysidis DV, Samaras A, Karagiannidis E, Kamperidis V, Ziakas A, Giannakoulas G. Oral Anticoagulation Choice and Dosage in Very Elderly Patients with Atrial Fibrillation. Journal of Cardiovascular Development and Disease. 2025; 12(3):86. https://doi.org/10.3390/jcdd12030086
Chicago/Turabian StyleZergioti, Martha, Melina Kyriakou, Andreas S. Papazoglou, Anastasios Kartas, Dimitrios V. Moysidis, Athanasios Samaras, Efstratios Karagiannidis, Vasileios Kamperidis, Antonios Ziakas, and George Giannakoulas. 2025. "Oral Anticoagulation Choice and Dosage in Very Elderly Patients with Atrial Fibrillation" Journal of Cardiovascular Development and Disease 12, no. 3: 86. https://doi.org/10.3390/jcdd12030086
APA StyleZergioti, M., Kyriakou, M., Papazoglou, A. S., Kartas, A., Moysidis, D. V., Samaras, A., Karagiannidis, E., Kamperidis, V., Ziakas, A., & Giannakoulas, G. (2025). Oral Anticoagulation Choice and Dosage in Very Elderly Patients with Atrial Fibrillation. Journal of Cardiovascular Development and Disease, 12(3), 86. https://doi.org/10.3390/jcdd12030086









