Myocardial Viability: Evolving Insights and Challenges in Revascularization and Functional Recovery
Abstract
1. Introduction
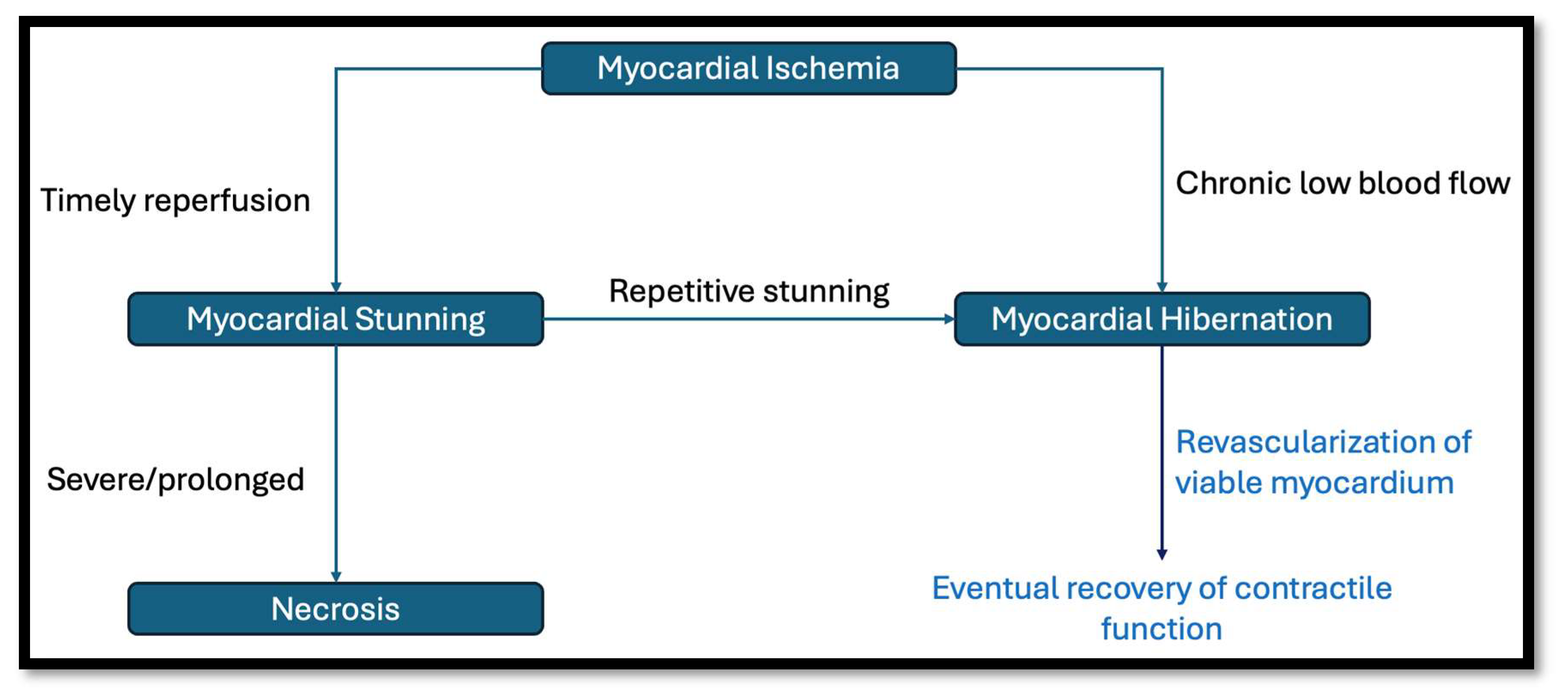
1.1. What Is Myocardial Viability?
1.2. Assessing Myocardial Viability
1.2.1. SPECT/PET
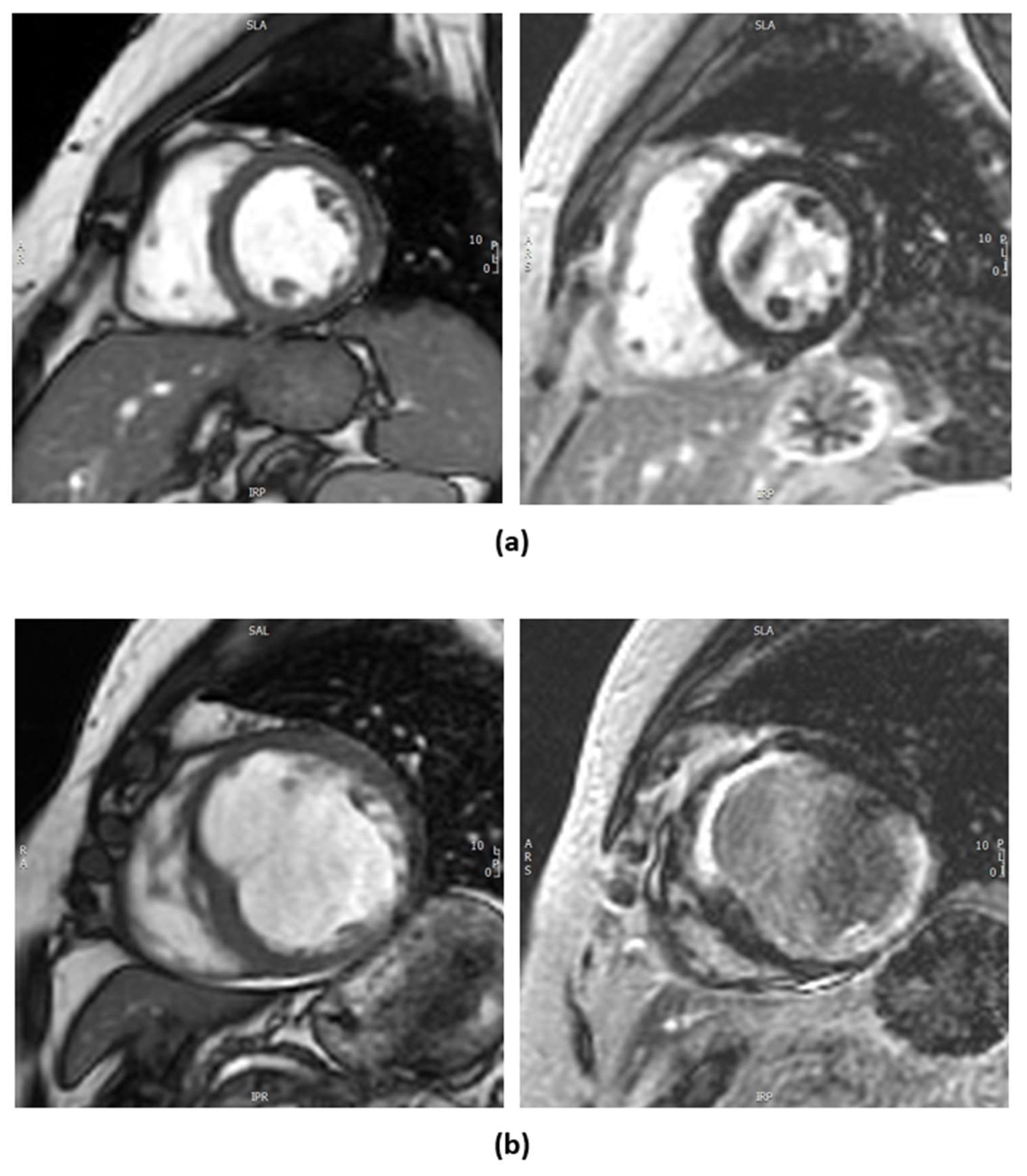
1.2.2. Cardiac Magnetic Resonance Imaging
1.2.3. Dobutamine Stress Echocardiography
1.3. Does the Presence of Myocardial Viability Translate to Better Outcomes Following Revascularization?
2. Limitations
Future Directions
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LV | Left ventricle |
| CMRI | Cardiac Magnetic Resonance Imaging |
| SPECT | Single-Photon Emission Computed Tomography |
| PET | Positron Emission Tomography |
| 18F-FDG | 18F-Fluorodeoxyglucose |
| LAD | Left anterior descending artery |
| LCx | Left circumflex artery |
| 99mTc-MIBI | 99m-technicium-sestamibi |
| LGE | Late gadolinium enhancement |
| DSE | Dobutamine stress echocardiography |
| STE | Speckle-tracking echocardiography |
| LDD | Low-dose dobutamine |
| EF | Ejection fraction |
| MI | Myocardial infarction |
| GDMT | Guideline-directed medical therapy |
| CAD | Coronary artery disease |
| PCI | Percutaneous coronary intervention |
| CABG | Coronary Artery Bypass Grafting |
| OMT | Optimal medical therapy |
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E.; Kloner, R.A. The stunned myocardium: Prolonged, postischemic ventricular dysfunction. Circulation 1982, 66, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Jamiel, A.; Ebid, M.; Ahmed, A.M.; Ahmed, D.; Al-Mallah, M.H. The Role of Myocardial Viability in Contemporary Cardiac Practice; Springer: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Gunning, M.G.; Kaprielian, R.R.; Pepper, J.; Pennell, D.J.; Sheppard, M.N.; Severs, N.J.; Underwood, S.R. The histology of viable and hibernating myocardium in relation to imaging characteristics. J. Am. Coll. Cardiol. 2002, 39, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Morgan, H.; Chiribiri, A.; Nagel, E.; Cleland, J.; Perera, D. Myocardial Viability Testing: All Stiched Up, or About to Be Revived? Oxford University Press: Oxford, UK, 2022. [Google Scholar] [CrossRef]
- Garcia, M.J.; Kwong, R.Y.; Scherrer-Crosbie, M.; Taub, C.C.; Blankstein, R.; Lima, J.; Bonow, R.O.; Eshtehardi, P.; Bois, J.P. State of the Art: Imaging for Myocardial Viability: A Scientific Statement from the American Heart Association. Circ. Cardiovasc. Imaging 2020, 13, e000053. [Google Scholar] [CrossRef]
- Kloner, R.A. Stunned and Hibernating Myocardium: Where Are We Nearly 4 Decades Later? J. Am. Heart Assoc. 2020, 9, e015502. [Google Scholar] [CrossRef]
- Weil, B.R.; Young, R.F.; Shen, X.; Suzuki, G.; Qu, J.; Malhotra, S.; Canty, J.M., Jr. Brief Myocardial Ischemia Produces Cardiac Troponin I Release and Focal Myocyte Apoptosis in the Absence of Pathological Infarction in Swine. JACC. Basic Transl. Sci. 2017, 2, 105–114. [Google Scholar] [CrossRef]
- Rahimtoola, S.H. The hibernating myocardium. Am. Heart J. 1989, 117, 211–221. [Google Scholar] [CrossRef]
- Conti, C.R. The stunned and hibernating myocardium: A brief review. Clin. Cardiol 1991, 14, 708–712. [Google Scholar] [CrossRef]
- Fedele, F.A.; Gewirtz, H.; Capone, R.J.; Sharaf, B.; Most, A.S. Metabolic response to prolonged reduction of myocardial blood flow distal to a severe coronary artery stenosis. Circulation 1988, 78, 729–735. [Google Scholar] [CrossRef]
- Ryan, M.J.; Perera, D. Identifying and Managing Hibernating Myocardium: What’s New and What Remains Unknown? Curr. Heart Fail. Rep. 2018, 15, 214–223. [Google Scholar] [CrossRef]
- Kim, S.J.; Peppas, A.; Hong, S.K.; Yang, G.; Huang, Y.; Diaz, G.; Vatner, S. Persistent stunning induces myocardial hibernation and protection: Flow/function and metabolic mechanisms. Circ. Res. 2003, 92, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Fallavollita, J.A.; Canty, J.M. Differential 18 F-2-Deoxyglucose Uptake in Viable Dysfunctional Myocardium with Normal Resting Perfusion Evidence for Chronic Stunning in Pigs. Circulation 1999, 99, 2798–2805. [Google Scholar] [CrossRef]
- Canty, J.M.; Fallavollita, J.A. Chronic hibernation and chronic stunning: A continuum. J. Nucl. Cardiol. 2000, 7, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Canty, J.M.; Fallavollita, J.A. Lessons from experimental models of hibernating myocardium. Coron. Artery Dis. 2001, 12, 371–380. [Google Scholar] [CrossRef]
- Nihoyannopoulos, P.; Vanoverschelde, J.L. Myocardial ischaemia and viability: The pivotal role of echocardiography. Eur. Heart J. 2011, 32, 810–819. [Google Scholar] [CrossRef]
- Trimarchi, G.; Trimarchi, G.; Teresi, L.; Licordari, R.; Pingitore, A.; Pizzino, F.; Grimaldi, P.; Di Bella, G. Transient Left Ventricular Dysfunction from Cardiomyopathies to Myocardial Viability: When and Why Cardiac Function Recovers. Biomedicines 2024, 12, 1051. [Google Scholar] [CrossRef]
- Madsen, S.; Dias, A.H.; Lauritsen, K.M.; Bouchelouche, K.; Tolbod, L.P.; Gormsen, L.C. Myocardial Viability Testing by Positron Emission Tomography: Basic Concepts, Mini-Review of the Literature and Experience From a Tertiary PET Center. Semin. Nucl. Med. 2020, 50, 248–259. [Google Scholar] [CrossRef]
- Schinkel, A.F.L.; Bax, J.J.; Poldermans, D.; Elhendy, A.; Ferrari, R.; Rahimtoola, S.H. Hibernating Myocardium: Diagnosis and Patient Outcomes. Curr. Probl. Cardiol. 2007, 32, 375–420. [Google Scholar] [CrossRef]
- Al-Sabeq, B.; Nabi, F.; Shah, D.J. Assessment of myocardial viability by cardiac MRI. Curr. Opin. Cardiol. 2019, 34, 502–509. [Google Scholar] [CrossRef]
- Ker, W.D.S.; Nunes, T.H.P.; Nacif, M.S.; Mesquita, C.T. Practical implications of myocardial viability studies. Arq. Bras. Cardiol. 2018, 110, 278–288. [Google Scholar] [CrossRef]
- Wende, A.R.; Brahma, M.K.; McGinnis, G.R.; Young, M.E. Metabolic Origins of Heart Failure. JACC Basic Transl. Sci. 2017, 2, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, M.; Schildt, J.; Ahonen, A.; Nikkinen, P.; Lauerma, K.; Sinisalo, J.; Pöyhiä, R. Combining FDG-PET and 99mTc-SPECT topredict functional outcome after coronary artery bypass surgery. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.J.; Fieno, D.S.; Parrish, T.B.; Harris, K.; Chen, E.L.; Simonetti, O.; Bundy, J.; Finn, J.P.; Klocke, F.J.; Judd, R.M. Relationship of MRI Delayed Contrast Enhancement to Irreversible Injury, Infarct Age, and Contractile Function. Circulation 1999, 100, 1992–2002. [Google Scholar] [CrossRef]
- Shah, D.J.; Kim, H.W.; James, O.; Parker, M.; Wu, E.; Bonow, R.O.; Kim, R.J. Prevalence of regional myocardial thinning and relationship with myocardial scarring in patients with coronary artery disease. JAMA 2013, 309, 909–918. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Arruda-Olson, A.; Chaudhry, F.A.; Chen, M.H.; Marshall, J.E.; Porter, T.R.; Sawada, S.G. Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 1–41.e8. [Google Scholar] [CrossRef]
- Bonow, R.O. Identification of viable myocardium. Circulation 1996, 94, 2674–2680. [Google Scholar] [CrossRef]
- La Canna, G.; Alfieri, O.; Giubbini, R.; Gargano, M.; Ferrari, R.; Visioli, O. Echocardiography during infusion of dobutamine for identification of reversible dyfunction in patients with chronic coronary artery disease. J. Am. Coll. Cardiol. 1994, 23, 617–626. [Google Scholar] [CrossRef]
- Perera, D.; Ryan, M.; Morgan, H.P.; Greenwood, J.P.; Petrie, M.C.; Dodd, M.; Weerackody, R.; O’Kane, P.D.; Masci, P.G.; Nazir, M.S.; et al. Viability and Outcomes with Revascularization or Medical Therapy in Ischemic Ventricular Dysfunction: A Prespecified Secondary Analysis of the REVIVED-BCIS2 Trial. JAMA Cardiol. 2023, 8, 1154–1161. [Google Scholar] [CrossRef]
- Panza, J.A.; Ellis, A.M.; Al-Khalidi, H.R.; Holly, T.A.; Berman, D.S.; Oh, J.K.; Pohost, G.M.; Sopko, G.; Chrzanowski, L.; Mark, D.B.; et al. Myocardial Viability and Long-Term Outcomes in Ischemic Cardiomyopathy. N. Engl. J. Med. 2019, 381, 739–748. [Google Scholar] [CrossRef]
- Ardle, B.M.; Shukla, T.; Nichol, G.; deKemp, R.A.; Bernick, J.; Guo, A.; Beanlands, R.S. Long-Term Follow-Up of Outcomes with F-18-Fluorodeoxyglucose Positron Emission Tomography Imaging-Assisted Management of Patients with Severe Left Ventricular Dysfunction Secondary to Coronary Disease. Circ. Cardiovasc. Imaging 2016, 9, e004331. [Google Scholar] [CrossRef]
- Khemka, A.; Sawada, S.G. Dobutamine echocardiography for assessment of viability in the current era. Curr. Opin. Cardiol. 2019, 34, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Collier, P.; Phelan, D.; Klein, A. The Present and Future Review Topic of The Week a Test in Context: Myocardial Strain Measured by Speckle-Tracking Echocardiography. J. Am. Coll. Cardiol 2017, 69, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Ballo, H.; Doghman, F.; Hartikainen, J.; Saraste, A.; Knuuti, J. Speckle-tracking echocardiography for predicting improvement of myocardial contractile function after revascularization: A meta-analysis of prospective trials. Int. J. Cardiovasc. Imaging 2023, 39, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Allman, K.C.; Shaw, L.J.; Hachamovitch, R.; Udelson, J.E. Coronary Revascularization Myocardial Viability Testing and Impact of Revascularization on Prognosis in Patients with Coronary Artery Disease and Left Ventricular Dysfunction: A Meta-Analysis. J. Am. Coll. Cardiol. 2002, 39, 1151–1158. [Google Scholar] [CrossRef]
- Beanlands, R.S.; Nichol, G.; Huszti, E.; Humen, D.; Racine, N.; Freeman, M.; PARR-2 Investigators. F-18-Fluorodeoxyglucose Positron Emission Tomography Imaging-Assisted Management of Patients with Severe Left Ventricular Dysfunction and Suspected Coronary Disease: A Randomized, Controlled Trial (PARR-2). J. Am. Coll. Cardiol. 2007, 50, 2002–2012. [Google Scholar] [CrossRef]
- Liga, R.; Colli, A.; Taggart, D.P.; Boden, W.E.; De Caterina, R. Myocardial Revascularization in Patients with Ischemic Cardiomyopathy: For Whom and How. J. Am. Heart Assoc. 2023, 12, e026943. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Lee, K.L.; Deja, M.A.; Jain, A.; Sopko, G.; Marchenko, A.; Rouleau, J.L. Coronary-Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. N. Engl. J. Med. 2011, 364, 1607–1616. [Google Scholar] [CrossRef]
- STICH Myocardial Viability at 10 Years: Still No Link with CABG Effects|tctmd.com. Available online: https://www.tctmd.com/news/stich-myocardial-viability-10-years-still-no-link-cabg-effects (accessed on 17 November 2024).
- Allman, K.C. Noninvasive assessment myocardial viability: Current status and future directions. J. Nucl. Cardiol. 2013, 20, 618–637. [Google Scholar] [CrossRef]
- Whittington, B.; Dweck, M.R.; van Beek, E.J.R.; Newby, D.; Williams, M.C. PET-MRI of Coronary Artery Disease. J. Am. Coll. Cardiol 2023, 57, 1301–1311. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Zembala, M.O. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Zwischenberger, B.A. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiolog. 2022, 145, e18–e114. [Google Scholar] [CrossRef]
- Trimarchi, G.; Carerj, S.; Di Bella, G.; Manganaro, R.; Pizzino, F.; Restelli, D.; Zito, C. Clinical Applications of Myocardial Work in Echocardiography: A Comprehensive Review. J. Cardiovasc. Echogr. 2024, 34, 99–113. [Google Scholar] [CrossRef]
| Modality | Key Features | Advantages | Limitations |
|---|---|---|---|
| SPECT/PET | SPECT evaluates perfusion; PET measures glucose metabolism. Mismatch between perfusion and metabolism suggests viability. | High sensitivity for viability. Assesses for presence of intact cellular metabolism. | Ionizing radiation. PET less available due to infrastructure needs (e.g., cyclotron for some tracers). SPECT has lower spatial resolution. |
| Cardiac MRI (CMRI) | Identifies scar burden by measuring contrast uptake in extracellular space. | High spatial resolution. No ionizing radiation. | Expensive. Requires expertise. Not suitable for all patients (e.g., claustrophobia, metal implants). |
| Dobutamine Stress Echo | Evaluates contractile reserve by assessing wall motion response to stress. | Widely available. No radiation. Lower cost. | Operator-dependent. Limited spatial resolution. Challenging in poor acoustic windows. |
| Trial | PARR-2 (Long-Term Follow-Up) | STITCH (Long-Term Follow-Up) | REVIVED-BCIS2 |
|---|---|---|---|
| Patient numbers | 392 patients for long term follow-up (197 PET-assisted vs. 195 standard care) | 1212 with 601 undergoing viability testing | 700 patients. 610 patients with LVEF ≤ 35%, extensive coronary artery disease and evidence of viability in at least 4 myocardial segments |
| Viability testing modality | FDG-PET | SPECT or DSE | CMRI or DSE |
| Mean LVEF at enrollment | 27% | 28% | 27% |
| Primary outcome | Cardiovascular death, MI, hospital admission due to cardiac cause | All-cause mortality | All-cause mortality, heart failure hospitalization |
| Secondary outcomes | Time to primary outcome, time to cardiovascular death | Cardiovascular death, death by any cause, hospitalization for cardiac cause | All-cause death, cardiovascular death, heart failure hospitalization, and improved LV function at 6 months |
| Revascularization technique | CABG and PCI | CABG | PCI |
| Median follow-up | 5 years | 10.4 years | 3.4 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ralota, K.K.; Layland, J.; Han Win, K.T.; Htun, N.M. Myocardial Viability: Evolving Insights and Challenges in Revascularization and Functional Recovery. J. Cardiovasc. Dev. Dis. 2025, 12, 106. https://doi.org/10.3390/jcdd12030106
Ralota KK, Layland J, Han Win KT, Htun NM. Myocardial Viability: Evolving Insights and Challenges in Revascularization and Functional Recovery. Journal of Cardiovascular Development and Disease. 2025; 12(3):106. https://doi.org/10.3390/jcdd12030106
Chicago/Turabian StyleRalota, Kristoffer Ken, Jamie Layland, Kyi Thar Han Win, and Nay M. Htun. 2025. "Myocardial Viability: Evolving Insights and Challenges in Revascularization and Functional Recovery" Journal of Cardiovascular Development and Disease 12, no. 3: 106. https://doi.org/10.3390/jcdd12030106
APA StyleRalota, K. K., Layland, J., Han Win, K. T., & Htun, N. M. (2025). Myocardial Viability: Evolving Insights and Challenges in Revascularization and Functional Recovery. Journal of Cardiovascular Development and Disease, 12(3), 106. https://doi.org/10.3390/jcdd12030106







