Nonlinear Association Between Atherogenic Index of Plasma and Unstable Carotid Plaque: A Single-Center Retrospective Study
Abstract
1. Introduction
2. Methods
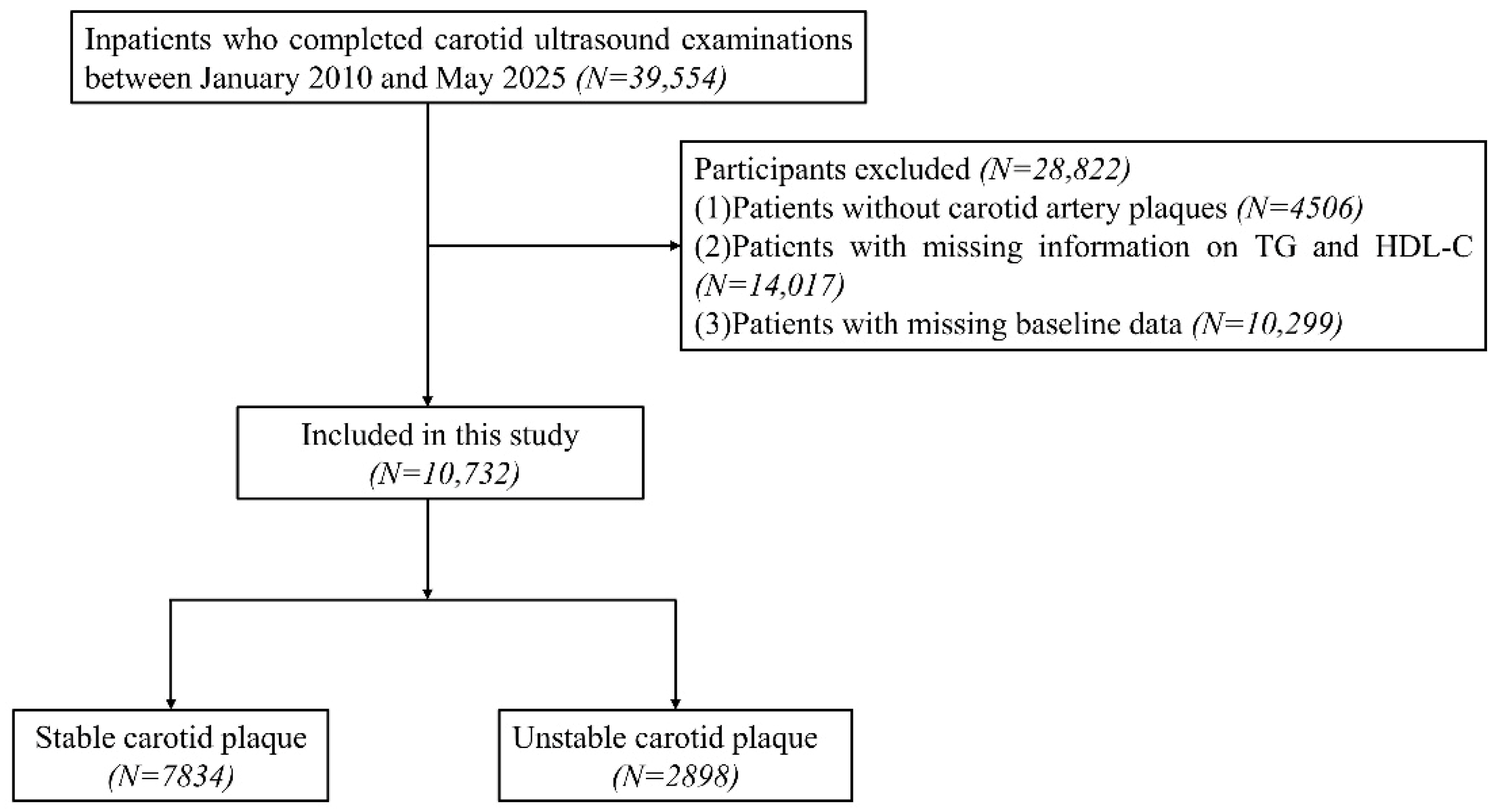
2.1. Study Population
2.2. Data Collection and Definition
2.3. Assessment of Stability of Carotid Plaque
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Association Between AIP and Unstable Carotid Plaque
3.3. RCS Analysis
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saba, L.; Nardi, V.; Cau, R.; Gupta, A.; Kamel, H.; Suri, J.S.; Balestrieri, A.; Congiu, T.; Butler, A.P.H.; Gieseg, S.; et al. Carotid Artery Plaque Calcifications: Lessons from Histopathology to Diagnostic Imaging. Stroke 2022, 53, 290–297. [Google Scholar] [CrossRef]
- Heck, D.; Jost, A. Carotid stenosis, stroke, and carotid artery revascularization. Prog. Cardiovasc. Dis. 2021, 65, 49–54. [Google Scholar] [CrossRef]
- Kopczak, A.; Schindler, A.; Sepp, D.; Bayer-Karpinska, A.; Malik, R.; Koch, M.L.; Zeller, J.; Strecker, C.; Janowitz, D.; Wollenweber, F.A.; et al. Complicated Carotid Artery Plaques and Risk of Recurrent Ischemic Stroke or TIA. J. Am. Coll. Cardiol. 2022, 79, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kesavabhotla, K.; Baradaran, H.; Kamel, H.; Pandya, A.; Giambrone, A.E.; Wright, D.; Pain, K.J.; Mtui, E.E.; Suri, J.S.; et al. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: A systematic review and meta-analysis. Stroke 2015, 46, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xing, Y.; Wang, L.; Liu, K.; Chen, H.; Li, C.; Chen, Y. Carotid Intraplaque Neovascularization and Future Vascular Events in Patients with Asymptomatic Carotid Stenosis. Front. Pharmacol. 2022, 13, 804810. [Google Scholar] [CrossRef] [PubMed]
- Nardi, V.; Benson, C.; Bois, M.C.; Saba, L.; Larson, A.S.; Özcan, I.; Ahmad, A.; Morse, D.W.; Meyer, F.B.; Brinjikji, W.; et al. Carotid Plaques from Symptomatic Patients with Mild Stenosis is Associated with Intraplaque Hemorrhage. Hypertens 2022, 79, 271–282. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, B.; Wu, S.; Liu, Z.; Wang, P.; Lv, Y.; Wu, R.; Ji, B.; Peng, Z.; Lu, C.; et al. Comparison of stable carotid plaques in patients with mild-to-moderate carotid stenosis with vulnerable plaques in patients with significant carotid stenosis. Medicine 2024, 103, e40613. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Luo, Y.; Jiao, L. Identification Markers of Carotid Vulnerable Plaques: An Update. Biomolecules 2022, 12, 1192. [Google Scholar] [CrossRef]
- van Dam-Nolen, D.H.K.; Truijman, M.T.B.; van der Kolk, A.G.; Liem, M.I.; Schreuder, F.; Boersma, E.; Daemen, M.; Mess, W.H.; van Oostenbrugge, R.J.; van der Steen, A.F.W.; et al. Carotid Plaque Characteristics Predict Recurrent Ischemic Stroke and TIA: The PARISK (Plaque at RISK) Study. JACC Cardiovasc. Imaging 2022, 15, 1715–1726. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, Y.; Xie, J.; Wang, J.; He, L.; Huang, M.; Xu, F. Carotid plaque components and other carotid artery features associated with risk of stroke: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2022, 31, 106857. [Google Scholar] [CrossRef]
- Willey, J.Z.; Pasterkamp, G. The Role of the Vulnerable Carotid Plaque in Embolic Stroke of Unknown Source. J. Am. Coll. Cardiol. 2022, 79, 2200–2202. [Google Scholar] [CrossRef] [PubMed]
- Sandesara, P.B.; Virani, S.S.; Fazio, S.; Shapiro, M.D. The Forgotten Lipids: Triglycerides, Remnant Cholesterol, and Atherosclerotic Cardiovascular Disease Risk. Endocr. Rev. 2019, 40, 537–557. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Tian, F.; Xu, J.; Du, X.; Zhang, S.; Liu, L. New insight into dyslipidemia-induced cellular senescence in atherosclerosis. Biol. Rev. Camb. Philos. Soc. 2022, 97, 1844–1867. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Davidson, M.H.; Hirsh, B.J.; Kathiresan, S.; Gaudet, D. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J. Am. Coll. Cardiol. 2014, 64, 2525–2540. [Google Scholar] [CrossRef]
- Dobiásová, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Macías, J.C.; Ochoa-Martínez, A.C.; Varela-Silva, J.A.; Pérez-Maldonado, I.N. Atherogenic Index of Plasma: Novel Predictive Biomarker for Cardiovascular Illnesses. Arch. Med. Res. 2019, 50, 285–294. [Google Scholar] [CrossRef]
- Nam, J.S.; Kim, M.K.; Nam, J.Y.; Park, K.; Kang, S.; Ahn, C.W.; Park, J.S. Association between atherogenic index of plasma and coronary artery calcification progression in Korean adults. Lipids Health Dis. 2020, 19, 157. [Google Scholar] [CrossRef]
- Chen, M.; Fang, C.Y.; Guo, J.C.; Pang, L.M.; Zhou, Y.; Hong, Y.; Yang, L.F.; Zhang, J.; Zhang, T.; Zhou, B.F.; et al. Predictive value of atherogenic index of plasma and atherogenic index of plasma combined with low-density lipoprotein cholesterol for the risk of acute myocardial infarction. Front. Cardiovasc. Med. 2023, 10, 1117362. [Google Scholar] [CrossRef]
- Zhu, X.W.; Deng, F.Y.; Lei, S.F. Meta-analysis of Atherogenic Index of Plasma and other lipid parameters in relation to risk of type 2 diabetes mellitus. Prim. Care Diabetes 2015, 9, 60–67. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Z.; Wei, M.; Huang, Q.; Feng, J.; Liu, Z.; Xia, J. The atherogenic index of plasma and carotid atherosclerosis in a community population: A population-based cohort study in China. Cardiovasc. Diabetol. 2023, 22, 125. [Google Scholar] [CrossRef]
- Wang, A.; Wu, L.; Liu, X.; Su, Z.; Luo, Y.; Chen, S.; Li, H.; Liu, X.; Tao, L.; Guo, J.; et al. The prevalence of carotid plaque with different stability and its association with metabolic syndrome in China: The Asymptomatic Polyvascular Abnormalities Community study. Medicine 2016, 95, e4619. [Google Scholar] [CrossRef]
- Sztajzel, R. Ultrasonographic assessment of the morphological characteristics of the carotid plaque. Swiss Med. Wkly. 2005, 135, 635–643. [Google Scholar] [CrossRef]
- Raitakari, O.; Pahkala, K.; Magnussen, C.G. Prevention of atherosclerosis from childhood. Nat. Rev. Cardiol. 2022, 19, 543–554. [Google Scholar] [CrossRef]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Bułdak, Ł. Cardiovascular Diseases—A Focus on Atherosclerosis, Its Prophylaxis, Complications and Recent Advancements in Therapies. Int. J. Mol. Sci. 2022, 23, 4695. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.; Martorell, J.; Riambau, V. Review of serum biomarkers in carotid atherosclerosis. J. Vasc. Surg. 2020, 71, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef]
- Marnane, M.; Prendeville, S.; McDonnell, C.; Noone, I.; Barry, M.; Crowe, M.; Mulligan, N.; Kelly, P.J. Plaque inflammation and unstable morphology are associated with early stroke recurrence in symptomatic carotid stenosis. Stroke 2014, 45, 801–806. [Google Scholar] [CrossRef]
- Skagen, K.; Skjelland, M.; Zamani, M.; Russell, D. Unstable carotid artery plaque: New insights and controversies in diagnostics and treatment. Croat. Med. J. 2016, 57, 311–320. [Google Scholar] [CrossRef]
- Jurin, I.; Paić, F.; Bulimbašić, S.; Rudež, I.; Đerek, L.; Jurin, H.; Knežević, A.; Starcevic, B.; Ajduk, M. Association between Circulatory and Plaque Resistin Levels with Carotid Plaque Instability and Ischemic Stroke Events. Heart Surg. Forum. 2018, 21, E448–E463. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, D.; Yao, Z.; Zeng, Y.; Zheng, J.; Tang, Y.; Huang, J.; Liu, Z.; Huo, G. Insulin resistance, vulnerable plaque and stroke risk in patients with carotid artery stenosis. Sci. Rep. 2024, 14, 30453. [Google Scholar] [CrossRef] [PubMed]
- Coffman, E.; Richmond-Bryant, J. Multiple biomarker models for improved risk estimation of specific cardiovascular diseases related to metabolic syndrome: A cross-sectional study. Popul. Health Metr. 2015, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Taqui, S.; Daniels, L.B. Putting it into perspective: Multimarker panels for cardiovascular disease risk assessment. Biomark Med. 2013, 7, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; Grupper, M.F.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef]
- Kuklina, E.V.; Tong, X.; George, M.G.; Bansil, P. Epidemiology and prevention of stroke: A worldwide perspective. Expert Rev. Neurother. 2012, 12, 199–208. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, K.; Wu, W.; Chen, G.; Chen, Z.; Cai, Z.; Cai, Z.; Lan, Y.; Wu, S.; Chen, Y. Relationship between the cumulative exposure to atherogenic index of plasma and ischemic stroke: A retrospective cohort study. Cardiovasc. Diabetol. 2023, 22, 313. [Google Scholar] [CrossRef]
- Won, K.B.; Heo, R.; Park, H.B.; Lee, B.K.; Lin, F.Y.; Hadamitzky, M.; Kim, Y.J.; Sung, J.M.; Conte, E.; Andreini, D.; et al. Atherogenic index of plasma and the risk of rapid progression of coronary atherosclerosis beyond traditional risk factors. Atherosclerosis 2021, 324, 46–51. [Google Scholar] [CrossRef]
- Alifu, J.; Xiang, L.; Zhang, W.; Qi, P.; Chen, H.; Liu, L.; Yin, G.; Mohammed, A.Q.; Lv, X.; Shi, T.; et al. Association between the atherogenic index of plasma and adverse long-term prognosis in patients diagnosed with chronic coronary syndrome. Cardiovasc. Diabetol. 2023, 22, 255. [Google Scholar] [CrossRef]
- Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Qu, L.; Fang, S.; Lan, Z.; Xu, S.; Jiang, J.; Pan, Y.; Xu, Y.; Zhu, X.; Jin, J. Association between atherogenic index of plasma and new-onset stroke in individuals with different glucose metabolism status: Insights from CHARLS. Cardiovasc. Diabetol. 2024, 23, 215. [Google Scholar] [CrossRef]
- Liu, H.; Liu, K.; Pei, L.; Li, S.; Zhao, J.; Zhang, K.; Zong, C.; Zhao, L.; Fang, H.; Wu, J.; et al. Atherogenic Index of Plasma Predicts Outcomes in Acute Ischemic Stroke. Front. Neurol. 2021, 12, 741754. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.S.; Kim, M.K.; Park, K.; Choi, A.; Kang, S.; Ahn, C.W.; Park, J.S. The Plasma Atherogenic Index is an Independent Predictor of Arterial Stiffness in Healthy Koreans. Angiology 2022, 73, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Wu, Z.; Yao, J.; Wang, S.; Duan, L.; Liu, S.; Zhang, M.; Luo, Y.; Ye, D.; Huang, Y.; et al. Association between atherogenic index of plasma control level and incident cardiovascular disease in middle-aged and elderly Chinese individuals with abnormal glucose metabolism. Cardiovasc. Diabetol. 2024, 23, 54. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, M.; Shi, W.; Li, Y.; Zhang, T.; Shi, W. Atherogenic index of plasma, high sensitivity C-reactive protein and incident diabetes among middle-aged and elderly adults in China: A national cohort study. Cardiovasc. Diabetol. 2025, 24, 103. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, H.; Hou, Q.; Zhou, Y.; Zhang, Y. Non-traditional lipid parameters as potential predictors of carotid plaque vulnerability and stenosis in patients with acute ischemic stroke. Neurol. Sci. 2023, 44, 835–843. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A.; Cicero, A.F.G.; Janez, A.; Stoian, A.P.; Sonmez, A.; Rizzo, M. Atherosclerosis Development and Progression: The Role of Atherogenic Small, Dense LDL. Medicina 2022, 58, 299. [Google Scholar] [CrossRef]
- Quispe, R.; Manalac, R.J.; Faridi, K.F.; Blaha, M.J.; Toth, P.P.; Kulkarni, K.R.; Nasir, K.; Virani, S.S.; Banach, M.; Blumenthal, R.S.; et al. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: The Very Large Database of Lipids-4 (VLDL-4) study. Atherosclerosis 2015, 242, 243–250. [Google Scholar] [CrossRef]
- Salazar, M.R.; Carbajal, H.A.; Espeche, W.G.; Leiva Sisnieguez, C.E.; March, C.E.; Balbín, E.; Dulbecco, C.A.; Aizpurúa, M.; Marillet, A.G.; Reaven, G.M. Comparison of the abilities of the plasma triglyceride/high-density lipoprotein cholesterol ratio and the metabolic syndrome to identify insulin resistance. Diab. Vasc. Dis. Res. 2013, 10, 346–352. [Google Scholar] [CrossRef]
- Ding, P.F.; Zhang, H.S.; Wang, J.; Gao, Y.Y.; Mao, J.N.; Hang, C.H.; Li, W. Insulin resistance in ischemic stroke: Mechanisms and therapeutic approaches. Front. Endocrinol. 2022, 13, 1092431. [Google Scholar] [CrossRef]
- Deng, X.L.; Liu, Z.; Wang, C.; Li, Y.; Cai, Z. Insulin resistance in ischemic stroke. Metab. Brain Dis. 2017, 32, 1323–1334. [Google Scholar] [CrossRef]
- Li, J.; Hou, D.; Li, J.; Li, R.; Sun, M. Association between the atherogenic index of plasma and the systemic immuno-inflammatory index using NHANES data from 2005 to 2018. Sci. Rep. 2025, 15, 11245. [Google Scholar] [CrossRef]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, R.; Berg, A.R.; Hong, C.G.; Parel, P.M.; Mehta, N.N.; Teague, H.L. Systemic consequences of abnormal cholesterol handling: Interdependent pathways of inflammation and dyslipidemia. Front. Immunol. 2022, 13, 972140. [Google Scholar] [CrossRef]
- Averill, M.M.; Bornfeldt, K.E. Lipids versus glucose in inflammation and the pathogenesis of macrovascular disease in diabetes. Curr. Diab. Rep. 2009, 9, 18–25. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Role of lipids and intraplaque hypoxia in the formation of neovascularization in atherosclerosis. Ann. Med. 2017, 49, 661–677. [Google Scholar] [CrossRef]
- Crowther, M.A. Pathogenesis of atherosclerosis. Hematol. Am. Soc. Hematol. Educ. Program 2005, 2005, 436–441. [Google Scholar] [CrossRef]
- Kammar-García, A.; López-Moreno, P.; Hernández-Hernández, M.E.; Ortíz-Bueno, A.M.; Martínez-Montaño, M.L.C. Atherogenic index of plasma as a marker of cardiovascular risk factors in Mexicans aged 18 to 22 years. Bayl. Univ. Med. Cent. Proc. 2020, 34, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lioy, B.; Webb, R.J.; Amirabdollahian, F. The Association between the Atherogenic Index of Plasma and Cardiometabolic Risk Factors: A Review. Healthcare 2023, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.B.; Blaha, M.J.; Boord, J.B.; Cariou, B.; Chait, A.; Fein, H.G.; Ginsberg, H.N.; Goldberg, I.J.; Murad, M.H.; Subramanian, S.; et al. Lipid Management in Patients with Endocrine Disorders: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2020, 105, 3613–3682. [Google Scholar] [CrossRef]
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Bornfeldt, K.E.; Tall, A.R. Atherosclerosis: Successes, Surprises, and Future Challenges. Circ. Res. 2016, 118, 531–534. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Overall | Stable Carotid Plaque | Unstable Carotid Plaque | p-Value |
|---|---|---|---|---|
| N = 10,732 | N = 7834 | N = 2898 | ||
| Sex | <0.001 | |||
| Men | 5900 (55.0%) | 4101 (52.3%) | 1799 (62.1%) | |
| Women | 4832 (45.0%) | 3733 (47.7%) | 1099 (37.9%) | |
| Age, year | 71.13 ± 10.70 | 70.85 ± 10.90 | 71.87 ± 10.11 | <0.001 |
| Hypertension | 8809 (82.1%) | 6403 (81.7%) | 2406 (83.0%) | 0.122 |
| Diabetes | 7886 (73.5%) | 5806 (74.1%) | 2080 (71.8%) | 0.015 |
| Dyslipidemia | 9871 (92.0%) | 7096 (90.6%) | 2775 (95.8%) | <0.001 |
| Hypertension medications | 7174 (66.8%) | 5165 (65.9%) | 2009 (69.3%) | <0.001 |
| Diabetes medications | 5806 (54.1%) | 4331 (55.3%) | 1475 (50.9%) | <0.001 |
| Dyslipidemia medications | 8615 (80.3%) | 5986 (76.4%) | 2629 (90.7%) | <0.001 |
| SBP, mmHg | 143.33 ± 22.80 | 143.46 ± 22.88 | 142.95 ± 22.55 | 0.298 |
| DBP, mmHg | 76.96 ± 17.04 | 77.14 ± 18.17 | 76.47 ± 13.50 | 0.040 |
| Height, m | 1.62 ± 0.19 | 1.62 ± 0.21 | 1.63 ± 0.14 | 0.023 |
| Weight, kg | 65.00 (56.50, 72.00) | 65.00 (56.00, 72.00) | 65.00 (57.00, 72.00) | 0.444 |
| BMI, kg/m2 | 24.03 (21.96, 26.30) | 24.12 (22.03, 26.37) | 23.88 (21.78, 26.13) | 0.004 |
| FPG, mmol/L | 5.68 (4.94, 7.15) | 5.73 (4.97, 7.21) | 5.54 (4.87, 6.97) | <0.001 |
| TC, mmol/L | 4.31 ± 1.16 | 4.36 ± 1.16 | 4.18 ± 1.13 | <0.001 |
| TG, mmol/L | 1.28 (0.90, 1.85) | 1.31 (0.91, 1.88) | 1.21 (0.87, 1.73) | <0.001 |
| HDL-C, mmol/L | 1.17 ± 0.31 | 1.17 ± 0.31 | 1.16 ± 0.31 | 0.094 |
| LDL-C, mmol/L | 2.73 ± 1.04 | 2.79 ± 1.04 | 2.59 ± 1.02 | <0.001 |
| HbA1c, % | 7.19 ± 1.95 | 7.20 ± 1.97 | 7.15 ± 1.92 | 0.235 |
| AIP | 0.15 ± 0.69 | 0.17 ± 0.70 | 0.11 ± 0.67 | <0.001 |
| Characteristic | Event, n | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | ||
| AIP (per 1 unit) | 2898 | 0.89 | 0.83, 0.94 | <0.001 | 0.85 | 0.80, 0.91 | <0.001 | 0.95 | 0.89, 1.02 | 0.162 |
| AIP quartile | ||||||||||
| Q1 | 768 | Ref | Ref | Ref | ||||||
| Q2 | 765 | 0.99 | 0.88, 1.12 | 0.914 | 0.96 | 0.85, 1.08 | 0.473 | 1.10 | 0.97, 1.24 | 0.140 |
| Q3 | 699 | 0.88 | 0.78, 0.99 | 0.033 | 0.82 | 0.73, 0.93 | 0.002 | 1.01 | 0.89, 1.15 | 0.868 |
| Q4 | 666 | 0.82 | 0.73, 0.93 | 0.002 | 0.76 | 0.67, 0.87 | <0.001 | 0.97 | 0.85, 1.11 | 0.679 |
| p for trend | <0.001 | <0.001 | 0.425 | |||||||
| Characteristic | Case/Total | OR | 95%CI | p |
|---|---|---|---|---|
| Unstable carotid plaque | ||||
| Total | 2898/10,732 | 0.95 | 0.89, 1.02 | 0.162 |
| The inflection points of the AIP | 0.10 | |||
| <0.10 | 1476/5164 | 1.24 | 1.07, 1.44 | 0.004 |
| ≥0.10 | 1422/5568 | 0.78 | 0.70, 0.88 | <0.001 |
| Log likelihood ratio | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, G.; Tang, Y.; Zhou, D. Nonlinear Association Between Atherogenic Index of Plasma and Unstable Carotid Plaque: A Single-Center Retrospective Study. J. Cardiovasc. Dev. Dis. 2025, 12, 443. https://doi.org/10.3390/jcdd12110443
Huo G, Tang Y, Zhou D. Nonlinear Association Between Atherogenic Index of Plasma and Unstable Carotid Plaque: A Single-Center Retrospective Study. Journal of Cardiovascular Development and Disease. 2025; 12(11):443. https://doi.org/10.3390/jcdd12110443
Chicago/Turabian StyleHuo, Guijun, Yao Tang, and Dayong Zhou. 2025. "Nonlinear Association Between Atherogenic Index of Plasma and Unstable Carotid Plaque: A Single-Center Retrospective Study" Journal of Cardiovascular Development and Disease 12, no. 11: 443. https://doi.org/10.3390/jcdd12110443
APA StyleHuo, G., Tang, Y., & Zhou, D. (2025). Nonlinear Association Between Atherogenic Index of Plasma and Unstable Carotid Plaque: A Single-Center Retrospective Study. Journal of Cardiovascular Development and Disease, 12(11), 443. https://doi.org/10.3390/jcdd12110443







