Pharmacological and Interventional Prevention and Treatment of Microvascular Obstruction Following Primary PCI in STEMI
Abstract
1. Introduction
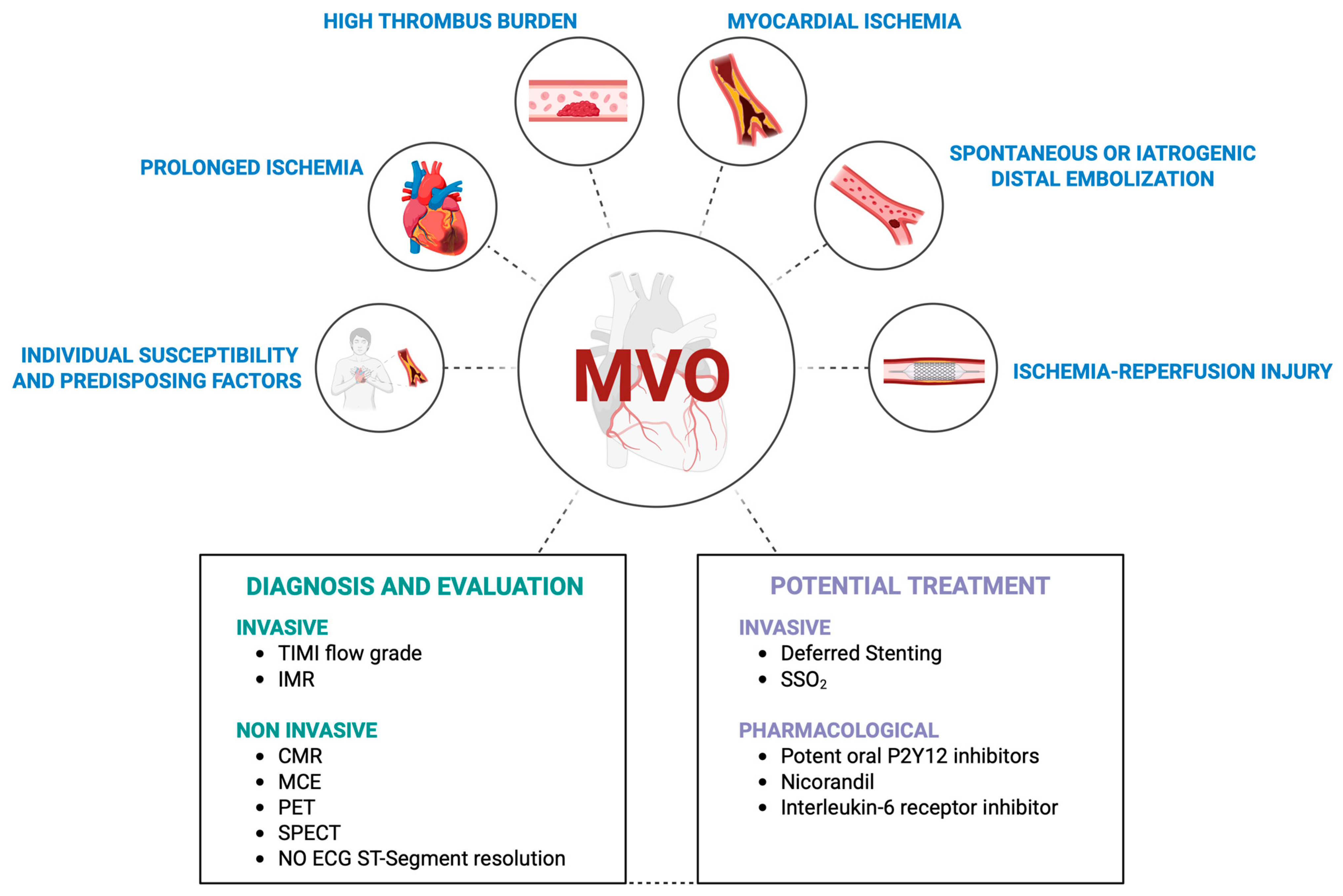
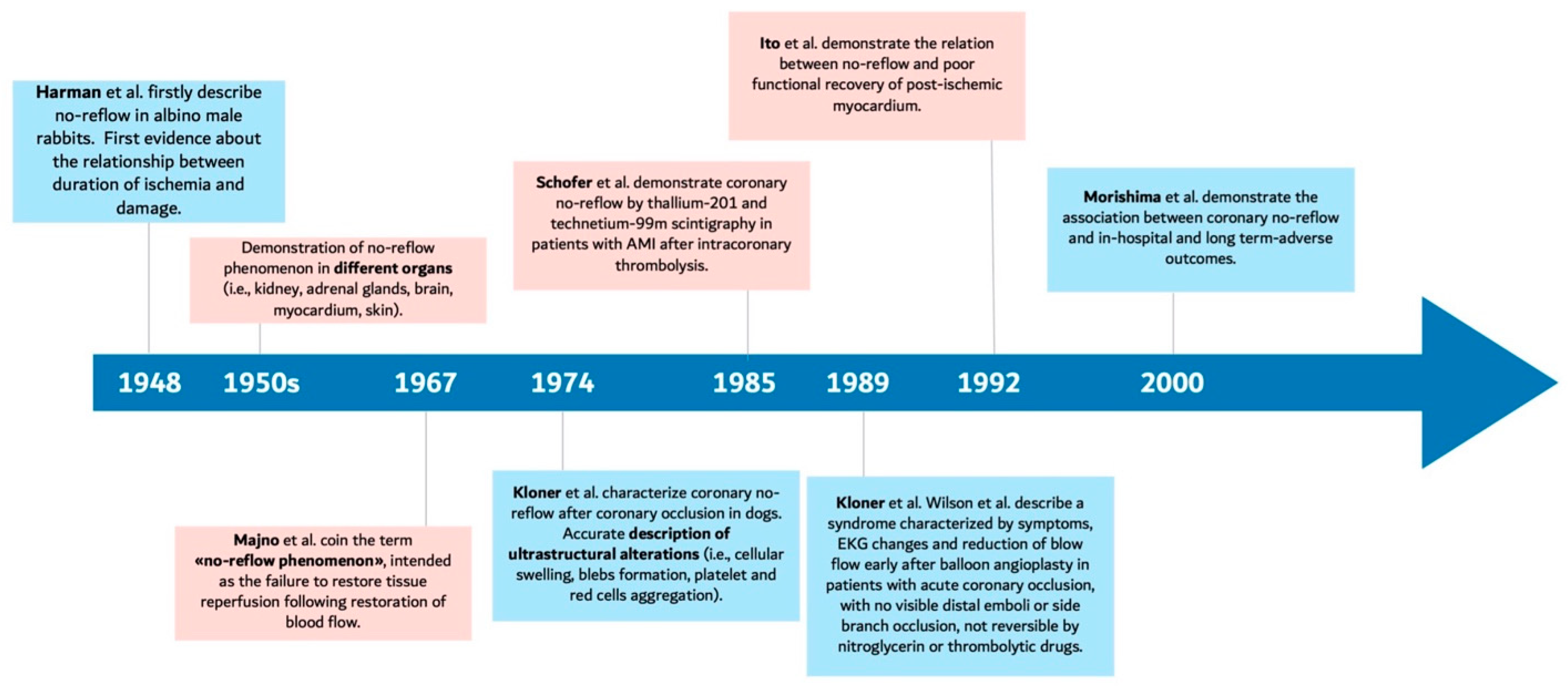
2. Mechanisms of Microvascular Obstruction
3. Diagnosis of Microvascular Obstruction
4. Pharmacological Prevention and Treatment of Microvascular Obstruction
4.1. Adenosine
4.2. Calcium Channel Blockers
4.3. Epinephrine
4.4. Intracoronary Thrombolytic Agents
4.5. Nicorandil
4.6. Oral Antiplatelet Agents
4.7. Parenteral Antiplatelet Agents
4.8. Sodium Nitroprusside
4.9. Other Pharmacological Strategies from Recent Randomized Trials
5. Interventional Prevention and Treatment of Microvascular Obstruction
5.1. Direct Stenting
5.2. Specific Stent Platforms
5.3. Deferred Stenting
5.4. Aspiration Thrombectomy
5.5. Stent-Based Mechanical Thrombectomy Devices
5.6. Distal Protection Devices
5.7. Excimer Laser Coronary Atherectomy
5.8. Pressure-Controlled Intermittent Coronary Sinus Occlusion (Picso)
5.9. Supersaturated Oxygen
5.10. Other Treatment Strategies
6. Ongoing Trials and Future Directions
6.1. New Trials Exploring Novel Cardioprotective Approaches in STEMI
6.2. Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Eeckhout, E.; Kern, M.J. The coronary no-reflow phenomenon: A review of mechanisms and therapies. Eur. Heart J. 2001, 22, 729–739. [Google Scholar] [CrossRef]
- Harman, J.W. The significance of local vascular phenomena in the production of ischemic necrosis in skeletal muscle. Am. J. Pathol. 1948, 24, 625–641. [Google Scholar]
- Morishima, I.; Sone, T.; Okumura, K.; Tsuboi, H.; Kondo, J.; Mukawa, H.; Matsui, H.; Toki, Y.; Ito, T.; Hayakawa, T.; et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J. Am. Coll. Cardiol. 2000, 36, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Marc, M.C.; Iancu, A.C.; Balanescu, S.; Dregoesc, M.I. Microvascular obstruction in acute myocardial infarction: An old and unsolved mystery. Med. Pharm. Rep. 2019, 92, 216–219. [Google Scholar] [CrossRef]
- Durante, A.; Camici, P.G. Novel insights into an “old” phenomenon: The no reflow. Int. J. Cardiol. 2015, 187, 273–280. [Google Scholar] [CrossRef] [PubMed]
- de Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W.; et al. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef]
- Spagnolo, M.; Occhipinti, G.; Laudani, C.; Greco, A.; Capodanno, D. Periprocedural myocardial infarction and injury. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 433–445. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Kastrati, A. Coronary No-Reflow after Primary Percutaneous Coronary Intervention-Current Knowledge on Pathophysiology, Diagnosis, Clinical Impact and Therapy. J. Clin. Med. 2023, 12, 5592. [Google Scholar] [CrossRef]
- Galli, M.; Niccoli, G.; De Maria, G.; Brugaletta, S.; Montone, R.A.; Vergallo, R.; Benenati, S.; Magnani, G.; D’Amario, D.; Porto, I.; et al. Coronary microvascular obstruction and dysfunction in patients with acute myocardial infarction. Nat. Rev. Cardiol. 2024, 21, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Caiazzo, G.; Musci, R.L.; Frediani, L.; Umińska, J.; Wanha, W.; Filipiak, K.J.; Kubica, J.; Navarese, E.P. State of the Art: No-Reflow Phenomenon. Cardiol. Clin. 2020, 38, 563–573. [Google Scholar]
- Vaidya, K.; Tucker, B.; Patel, S.; Ng, M.K.C. Acute Coronary Syndromes (ACS)-Unravelling Biology to Identify New Therapies-The Microcirculation as a Frontier for New Therapies in ACS. Cells 2021, 10, 2188. [Google Scholar] [CrossRef] [PubMed]
- Soeda, T.; Higuma, T.; Abe, N.; Yamada, M.; Yokoyama, H.; Shibutani, S.; Ong, D.-S.; Vergallo, R.; Minami, Y.; Lee, H.; et al. Morphological predictors for no reflow phenomenon after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction caused by plaque rupture. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Schofer, J.; Montz, R.; Mathey, D.G. Scintigraphic evidence of the “no reflow” phenomenon in human beings after coronary thrombolysis. J. Am. Coll. Cardiol. 1985, 5, 593–598. [Google Scholar] [CrossRef]
- Bulluck, H.; Foin, N.; Tan, J.W.; Low, A.F.; Sezer, M.; Hausenloy, D.J. Invasive Assessment of the Coronary Microcirculation in Reperfused ST-Segment-Elevation Myocardial Infarction Patients: Where Do We Stand? Circ. Cardiovasc. Interv. 2017, 10, e004373. [Google Scholar] [CrossRef]
- Vergallo, R.; Lombardi, M.; Besis, G.; Benedetto, L.; Di Vito, L.; Porto, I.; Burzotta, F.; Aurigemma, C.; Galiuto, L.; Crea, F.; et al. Pre-stenting residual thrombotic volume assessed by dual quantitative coronary angiography predicts microvascular obstruction in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Minerva Cardiol. Angiol. 2023, 71, 421–430. [Google Scholar] [CrossRef]
- Vicente, J.; Mewton, N.; Croisille, P.; Staat, P.; Bonnefoy-Cudraz, E.; Ovize, M.; Revel, D. Comparison of the angiographic myocardial blush grade with delayed-enhanced cardiac magnetic resonance for the assessment of microvascular obstruction in acute myocardial infarctions. Catheter. Cardiovasc. Interv. 2009, 74, 1000–1007. [Google Scholar] [CrossRef]
- Nijveldt, R.; van der Vleuten, P.A.; Hirsch, A.; Beek, A.M.; Tio, R.A.; Tijssen, J.G.; Piek, J.J.; van Rossum, A.C.; Zijlstra, F. Early electrocardiographic findings and MR imaging-verified microvascular injury and myocardial infarct size. JACC Cardiovasc. Imaging 2009, 2, 1187–1194. [Google Scholar] [CrossRef]
- Henriques, J.P.; Zijlstra, F.; van ’t Hof, A.W.; de Boer, M.J.; Dambrink, J.H.; Gosselink, M.; Hoorntje, J.C.; Suryapranata, H. Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation 2003, 107, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Dharmakumar, R.; Arai, A.E.; Berry, C.; Hausenloy, D.J. Cardiovascular Magnetic Resonance in Acute ST-Segment-Elevation Myocardial Infarction: Recent Advances, Controversies, and Future Directions. Circulation 2018, 137, 1949–1964. [Google Scholar] [CrossRef]
- Ibanez, B.; Aletras, A.H.; Arai, A.E.; Arheden, H.; Bax, J.; Berry, C.; Bucciarelli-Ducci, C.; Croisille, P.; Dall’Armellina, E.; Dharmakumar, R.; et al. Cardiac MRI Endpoints in Myocardial Infarction Experimental and Clinical Trials: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 74, 238–256. [Google Scholar] [CrossRef]
- Modonesi, E.; Balbi, M.; Bezante, G.P. Limitations and potential clinical application on contrast echocardiography. Curr. Cardiol. Rev. 2010, 6, 24–30. [Google Scholar] [CrossRef] [PubMed]
- McGeoch, R.; Watkins, S.; Berry, C.; Steedman, T.; Davie, A.; Byrne, J.; Hillis, S.; Lindsay, M.; Robb, S.; Dargie, H.; et al. The index of microcirculatory resistance measured acutely predicts the extent and severity of myocardial infarction in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 2010, 3, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Maznyczka, A.M.; Oldroyd, K.G.; McCartney, P.; McEntegart, M.; Berry, C. The Potential Use of the Index of Microcirculatory Resistance to Guide Stratification of Patients for Adjunctive Therapy in Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2019, 12, 951–966. [Google Scholar] [CrossRef]
- Schröder, R.; Dissmann, R.; Brüggemann, T.; Wegscheider, K.; Linderer, T.; Tebbe, U.; Neuhaus, K.L. Extent of early ST segment elevation resolution: A simple but strong predictor of outcome in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 1994, 24, 384–391. [Google Scholar] [CrossRef]
- Layland, J.; Carrick, D.; Lee, M.; Oldroyd, K.; Berry, C. Adenosine: Physiology, pharmacology, and clinical applications. JACC Cardiovasc. Interv. 2014, 7, 581–591. [Google Scholar] [CrossRef]
- Vinten-Johansen, J.; Thourani, V.H.; Ronson, R.S.; Jordan, J.E.; Zhao, Z.Q.; Nakamura, M.; Velez, D.; Guyton, R.A. Broad-spectrum cardioprotection with adenosine. Ann. Thorac. Surg. 1999, 68, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Methner, C.; Cao, Z.; Mishra, A.; Kaul, S. Mechanism and potential treatment of the “no reflow” phenomenon after acute myocardial infarction: Role of pericytes and GPR39. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H1030–H1041. [Google Scholar] [CrossRef]
- O’Farrell, F.M.; Mastitskaya, S.; Hammond-Haley, M.; Freitas, F.; Wah, W.R.; Attwell, D. Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. eLife 2017, 6, e29280. [Google Scholar] [CrossRef]
- Niccoli, G.; Scalone, G.; Lerman, A.; Crea, F. Coronary microvascular obstruction in acute myocardial infarction. Eur. Heart J. 2016, 37, 1024–1033. [Google Scholar] [CrossRef]
- Mahaffey, K.W.; Puma, J.A.; Barbagelata, N.A.; DiCarli, M.F.; Leesar, M.A.; Browne, K.F.; Eisenberg, P.R.; Bolli, R.; Casas, A.C.; Molina-Viamonte, V.; et al. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: Results of a multicenter, randomized, placebo-controlled trial: The Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J. Am. Coll. Cardiol. 1999, 34, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.M.; Gibbons, R.J.; Stone, G.W.; Kloner, R.A.; Alexander, R.W.; Investigators, A.-I. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J. Am. Coll. Cardiol. 2005, 45, 1775–1780. [Google Scholar] [CrossRef]
- Quintana, M.; Hjemdahl, P.; Sollevi, A.; Kahan, T.; Edner, M.; Rehnqvist, N.; Swahn, E.; Kjerr, A.-C.; Näsman, P. Left ventricular function and cardiovascular events following adjuvant therapy with adenosine in acute myocardial infarction treated with thrombolysis, results of the ATTenuation by Adenosine of Cardiac Complications (ATTACC) study. Eur. J. Clin. Pharmacol. 2003, 59, 1–9. [Google Scholar] [CrossRef]
- De Marco, C.; Charron, T.; Rousseau, G. Adenosine in Acute Myocardial Infarction-Associated Reperfusion Injury: Does it Still. Have a Role? Front. Pharmacol. 2022, 13, 856747. [Google Scholar] [CrossRef]
- Lasley, R.D. Adenosine Receptor-Mediated Cardioprotection-Current Limitations and Future Directions. Front. Pharmacol. 2018, 9, 310. [Google Scholar] [CrossRef]
- Stoel, M.G.; Marques, K.M.; de Cock, C.C.; Bronzwaer, J.G.; von Birgelen, C.; Zijlstra, F. High dose adenosine for suboptimal myocardial reperfusion after primary PCI: A randomized placebo-controlled pilot study. Catheter. Cardiovasc. Interv. 2008, 71, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Fokkema, M.L.; Vlaar, P.J.; Vogelzang, M.; Gu, Y.L.; Kampinga, M.A.; de Smet, B.J.; Jessurun, G.A.; Anthonio, R.L.; van den Heuvel, A.F.; Tan, E.S.; et al. Effect of high-dose intracoronary adenosine administration during primary percutaneous coronary intervention in acute myocardial infarction: A randomized controlled trial. Circ. Cardiovasc. Interv. 2009, 2, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Rigattieri, S.; De Vita, M.R.; Valgimigli, M.; Corvo, P.; Fabbiocchi, F.; Romagnoli, E.; De Caterina, A.R.; La Torre, G.; Lo Schiavo, P.; et al. Open-label, randomized, placebo-controlled evaluation of intracoronary adenosine or nitroprusside after thrombus aspiration during primary percutaneous coronary intervention for the prevention of microvascular obstruction in acute myocardial infarction: The REOPEN-AMI study (Intracoronary Nitroprusside Versus Adenosine in Acute Myocardial Infarction). JACC Cardiovasc. Interv. 2013, 6, 580–589. [Google Scholar]
- Nazir, S.A.; Khan, J.N.; Mahmoud, I.Z.; Greenwood, J.P.; Blackman, D.J.; Kunadian, V.; Been, M.; Abrams, K.R.; Wilcox, R.; Adgey, J.A.J.; et al. The REFLO-STEMI (REperfusion Facilitated by LOcal adjunctive therapy in ST-Elevation Myocardial Infarction) trial: A randomised controlled trial comparing intracoronary administration of adenosine or sodium nitroprusside with control for attenuation of microvascular obstruction during primary percutaneous coronary intervention. Southampton 2016, 3, 1–48. [Google Scholar]
- Laborante, R.; Bianchini, E.; Restivo, A.; Ciliberti, G.; Galli, M.; Vergallo, R.; Rodolico, D.; Zito, A.; Princi, G.; Leone, A.M.; et al. Adenosine as adjunctive therapy in acute coronary syndrome: A meta-analysis of randomized controlled trials. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 173–182. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, B.; Guo, Y.; Zheng, F. Efficacy of Adenosine in Patients with Acute Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: A PRISMA-Compliant Meta-Analysis. Medicine 2015, 94, e1279. [Google Scholar] [CrossRef]
- Frishman, W.H. Calcium channel blockers: Differences between subclasses. Am. J. Cardiovasc. Drugs 2007, 7 (Suppl. 1), 17–23. [Google Scholar] [CrossRef]
- Taniyama, Y.; Ito, H.; Iwakura, K.; Masuyama, T.; Hori, M.; Takiuchi, S.; Nishikawa, N.; Higashino, Y.; Fujii, K.; Minamino, T.; et al. Beneficial effect of intracoronary verapamil on microvascular and myocardial salvage in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 1997, 30, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Weyrens, F.J.; Mooney, J.; Lesser, J.; Mooney, M.R. Intracoronary diltiazem for microvascular spasm after interventional therapy. Am. J. Cardiol. 1995, 75, 849–850. [Google Scholar] [CrossRef]
- Su, Q.; Nyi, T.S.; Li, L. Adenosine and verapamil for no-reflow during primary percutaneous coronary intervention in people with acute myocardial infarction. Cochrane Database Syst. Rev. 2015, 2015, CD009503. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, Z.; Gu, Y.; Peng, D. Short-Term Effects of Verapamil and Diltiazem in the Treatment of No Reflow Phenomenon: A Meta-Analysis of Randomized Controlled Trials. Biomed. Res. Int. 2015, 2015, 382086. [Google Scholar] [CrossRef]
- Skelding, K.A.; Goldstein, J.A.; Mehta, L.; Pica, M.C.; O’Neill, W.W. Resolution of refractory no-reflow with intracoronary epinephrine. Catheter. Cardiovasc. Interv. 2002, 57, 305–309. [Google Scholar] [CrossRef]
- Navarese, E.P.; Frediani, L.; Kandzari, D.E.; Caiazzo, G.; Cenname, A.M.; Cortese, B.; Piva, T.; Muçaj, A.; Tumscitz, C.; Paparoni, F.; et al. Efficacy and safety of intracoronary epinephrine versus conventional treatments alone in STEMI patients with refractory coronary no-reflow during primary PCI: The RESTORE observational study. Catheter. Cardiovasc. Interv. 2021, 97, 602–611. [Google Scholar] [CrossRef]
- Khan, K.A.; Qamar, N.; Saghir, T.; Sial, J.A.; Kumar, D.; Kumar, R.; Qayyum, D.; Yasin, U.; Jalbani, J.; Karim, M.; et al. Comparison of Intracoronary Epinephrine and Adenosine for No-Reflow in Normotensive Patients with Acute Coronary Syndrome (COAR Trial). Circ. Cardiovasc. Interv. 2022, 15, e011408. [Google Scholar] [CrossRef] [PubMed]
- Jafari Afshar, E.; Samimisedeh, P.; Tayebi, A.; Shafiabadi Hassani, N.; Rastad, H.; Yazdani, S. Efficacy and safety of intracoronary epinephrine for the management of the no-reflow phenomenon following percutaneous coronary interventions: A systematic-review study. Ther. Adv. Cardiovasc. Dis. 2023, 17, 17539447231154654. [Google Scholar] [CrossRef]
- Alyamani, M.; Campbell, S.; Navarese, E.; Welsh, R.C.; Bainey, K.R. Safety and Efficacy of Intracoronary Thrombolysis as Adjunctive Therapy to Primary PCI in STEMI: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2021, 37, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Kulick, N.; Friede, K.A.; Stouffer, G.A. Safety and efficacy of intracoronary thrombolytic agents during primary percutaneous coronary intervention for STEMI. Expert. Rev. Cardiovasc. Ther. 2023, 21, 165–175. [Google Scholar] [CrossRef]
- Sato, T.; Sasaki, N.; O’Rourke, B.; Marban, E. Nicorandil, a potent cardioprotective agent, acts by opening mitochondrial ATP-dependent potassium channels. J. Am. Coll. Cardiol. 2000, 35, 514–518. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, Y.; Dong, W.; Jiang, Z.C.; Li, T.; Cheng, L.Q.; Zou, Y.T.; Jiang, X.S.; Zhou, H.; A, X.; et al. Effects of Nicorandil Administration on Infarct Size in Patients with ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: The CHANGE Trial. J. Am. Heart Assoc. 2022, 11, e026232. [Google Scholar] [CrossRef]
- Xu, L.; Wang, L.; Li, K.; Zhang, Z.; Sun, H.; Yang, X. Nicorandil prior to primary percutaneous coronary intervention improves clinical outcomes in patients with acute myocardial infarction: A meta-analysis of randomized controlled trials. Drug Des. Dev. Ther. 2019, 13, 1389–1400. [Google Scholar] [CrossRef]
- Yang, X.M.; Liu, Y.; Cui, L.; Yang, X.; Liu, Y.; Tandon, N.; Kambayashi, J.; Downey, J.M.; Cohen, M.V. Platelet P2Y(1)(2) blockers confer direct postconditioning-like protection in reperfused rabbit hearts. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 251–262. [Google Scholar] [CrossRef]
- Song, Y.B.; Hahn, J.Y.; Gwon, H.C.; Chang, S.A.; Lee, S.C.; Choe, Y.H.; Choi, S.H.; Choi, J.H.; Lee, S.H.; Oh, J.K. A high loading dose of clopidogrel reduces myocardial infarct size in patients undergoing primary percutaneous coronary intervention: A magnetic resonance imaging study. Am. Heart J. 2012, 163, 500–507. [Google Scholar] [CrossRef]
- Vlachojannis, G.J.; Wilschut, J.M.; Vogel, R.F.; Lemmert, M.E.; Delewi, R.; Diletti, R.; van der Waarden, N.W.P.L.; Nuis, R.J.; Paradies, V.; Alexopoulos, D.; et al. Effect of Prehospital Crushed Prasugrel Tablets in Patients with ST-Segment-Elevation Myocardial Infarction Planned for Primary Percutaneous Coronary Intervention: The Randomized COMPARE CRUSH Trial. Circulation 2020, 142, 2316–2328. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, M.A.H.; van der Hoeven, N.W.; Janssens, G.N.; Everaars, H.; Nap, A.; Lemkes, J.S.; de Waard, G.A.; van de Ven, P.M.; van Rossum, A.C.; Ten Cate, T.J.F.; et al. Evaluation of Microvascular Injury in Revascularized Patients with ST-Segment-Elevation Myocardial Infarction Treated with Ticagrelor Versus Prasugrel. Circulation 2019, 139, 636–646. [Google Scholar] [CrossRef]
- Keating, G.M. Cangrelor: A Review in Percutaneous Coronary Intervention. Drugs 2015, 75, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.M.; Sivaraman, V.; Kunuthur, S.P.; Cohen, M.V.; Downey, J.M.; Yellon, D.M. Cardioprotective Properties of the Platelet P2Y12 Receptor Inhibitor, Cangrelor: Protective in Diabetics and Reliant Upon the Presence of Blood. Cardiovasc. Drugs Ther. 2015, 29, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.V.; Yang, X.M.; White, J.; Yellon, D.M.; Bell, R.M.; Downey, J.M. Cangrelor-Mediated Cardioprotection Requires Platelets and Sphingosine Phosphorylation. Cardiovasc. Drugs Ther. 2016, 30, 229–232. [Google Scholar] [CrossRef]
- Bulluck, H.; Chong, J.H.; Bryant, J.; Annathurai, A.; Chai, P.; Chan, M.; Chawla, A.; Chin, C.Y.; Chung, Y.C.; Gao, F.; et al. Effect of Cangrelor on Infarct Size in ST-Segment Elevation Myocardial Infarction Treated By Primary Percutaneous Coronary Intervention: A Randomized Controlled Trial (The PITRI Trial). Circulation 2024, 150, 91–101. [Google Scholar] [CrossRef]
- Capodanno, D.; Milluzzo, R.P.; Angiolillo, D.J. Intravenous antiplatelet therapies (glycoprotein IIb/IIIa receptor inhibitors and cangrelor) in percutaneous coronary intervention: From pharmacology to indications for clinical use. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719893274. [Google Scholar] [CrossRef]
- Dudek, D.; Siudak, Z.; Janzon, M.; Birkemeyer, R.; Aldama-Lopez, G.; Lettieri, C.; Janus, B.; Wisniewski, A.; Berti, S.; Olivari, Z.; et al. European registry on patients with ST-elevation myocardial infarction transferred for mechanical reperfusion with a special focus on early administration of abciximab—EUROTRANSFER Registry. Am. Heart J. 2008, 156, 1147–1154. [Google Scholar] [CrossRef]
- De Luca, G.; Gibson, C.M.; Bellandi, F.; Murphy, S.; Maioli, M.; Noc, M.; Zeymer, U.; Dudek, D.; Arntz, H.R.; Zorman, S.; et al. Early glycoprotein IIb-IIIa inhibitors in primary angioplasty (EGYPT) cooperation: An individual patient data meta-analysis. Heart 2008, 94, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Wohrle, J.; Neuhaus, P.; Brosteanu, O.; Sick, P.; Prondzinsky, R.; Birkemeyer, R.; Wiemer, M.; Kerber, S.; Schuehlen, H.; et al. Intracoronary compared with intravenous bolus abciximab application during primary percutaneous coronary intervention: Design and rationale of the Abciximab Intracoronary versus intravenously Drug Application in ST-Elevation Myocardial Infarction (AIDA STEMI) trial. Am. Heart J. 2010, 159, 547–554. [Google Scholar] [PubMed]
- Stone, G.W.; Maehara, A.; Witzenbichler, B.; Godlewski, J.; Parise, H.; Dambrink, J.H.; Ochala, A.; Carlton, T.W.; Cristea, E.; Wolff, S.D.; et al. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: The INFUSE-AMI randomized trial. JAMA 2012, 307, 1817–1826. [Google Scholar] [CrossRef]
- Eitel, I.; Saraei, R.; Jurczyk, D.; Fach, A.; Hambrecht, R.; Wienbergen, H.; Frerker, C.; Schmidt, T.; Allali, A.; Joost, A.; et al. Glycoprotein IIb/IIIa inhibitors in acute myocardial infarction and angiographic microvascular obstruction: The REVERSE-FLOW trial. Eur. Heart J. 2024, 45, 5058–5067. [Google Scholar] [CrossRef]
- Gavin, J.B.; Maxwell, L.; Edgar, S.G. Microvascular involvement in cardiac pathology. J. Mol. Cell Cardiol. 1998, 30, 2531–2540. [Google Scholar] [CrossRef]
- Wang, W.Z.; Anderson, G.; Fleming, J.T.; Peter, S.J.; Palestrant, A.M.; Tsuei, B.J.; Liu, Y.; Schuschke, D.A.; Morris, S.E.; Herndon, D.N. Lack of nitric oxide contributes to vasospasm during ischemia/reperfusion injury. Plast. Reconstr. Surg. 1997, 99, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Amit, G.; Cafri, C.; Yaroslavtsev, S.; Fuchs, S.; Paltiel, O.; Abu-Ful, A.; Weinstein, J.M.; Wolak, A.; Ilia, R.; Zahger, D. Intracoronary nitroprusside for the prevention of the no-reflow phenomenon after primary percutaneous coronary intervention in acute myocardial infarction. A randomized, double-blind, placebo-controlled clinical trial. Am. Heart J. 2006, 152, 887.e9–887.e14. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Fu, X.H.; Ma, X.X.; Li, Y.F.; Wang, D.W.; Liu, H.B.; Wang, H.; Guo, X.J.; Yu, H.; Yang, X.X. Intracoronary fixed dose of nitroprusside via thrombus aspiration catheter for the prevention of the no-reflow phenomenon following primary percutaneous coronary intervention in acute myocardial infarction. Exp. Ther. Med. 2013, 6, 479–484. [Google Scholar] [CrossRef]
- Nazir, S.A.; McCann, G.P.; Greenwood, J.P.; Kunadian, V.; Khan, J.N.; Mahmoud, I.Z.; Blackman, D.J.; Been, M.; Abrams, K.R.; Shipley, L. Strategies to attenuate micro-vascular obstruction during P-PCI: The randomized reperfusion facilitated by local adjunctive therapy in ST-elevation myocardial infarction trial. Eur. Heart J. 2016, 37, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lara, J.; Gracida, M.; Rivero, F.; Gutiérrez-Barrios, A.; Muntané-Carol, G.; Romaguera, R.; Fuentes, L.; Marcano, A.; Roura, G.; Ferreiro, J.L.; et al. Treatment of Slow-Flow After Primary Percutaneous Coronary Intervention with Flow-Mediated Hyperemia: The Randomized RAIN-FLOW Study. J. Am. Heart Assoc. 2023, 12, e030285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Qi, G.; Tian, W.; Chen, L.; Sun, Y. Effect of intracoronary nitroprusside in preventing no reflow phenomenon during primary percutaneous coronary intervention: A meta-analysis. J. Interv. Cardiol. 2014, 27, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Mewton, N.; Roubille, F.; Bresson, D.; Prieur, C.; Bouleti, C.; Bochaton, T.; Ivanes, F.; Dubreuil, O.; Biere, L.; Hayek, A.; et al. Effect of Colchicine on Myocardial Injury in Acute Myocardial Infarction. Circulation 2021, 144, 859–869. [Google Scholar] [CrossRef]
- Bouleti, C.; Viscogliosi, S.; Bresson, D.; Leboube, S.; Bochaton, T.; El-Jonhy, N.; Amaz, C.; Prunier, F.; Bidaux, G.; Roubille, F.; et al. Colchicine in acute myocardial infarction: Cardiovascular events at 1-year follow up. Open Heart. 2024, 11, e002474. [Google Scholar] [CrossRef]
- Madsen, J.M.; Engstrøm, T.; Obling, L.E.R.; Zhou, Y.; Nepper-Christensen, L.; Beske, R.P.; Vejlstrup, N.G.; Bang, L.E.; Hassager, C.; Folke, F.; et al. Prehospital Pulse-Dose Glucocorticoid in ST-Segment Elevation Myocardial Infarction: The PULSE-MI Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 882–891. [Google Scholar] [CrossRef]
- Broch, K.; Anstensrud, A.K.; Woxholt, S.; Sharma, K.; Tøllefsen, I.M.; Bendz, B.; Aakhus, S.; Ueland, T.; Amundsen, B.H.; Damås, J.K.; et al. Randomized Trial of Interleukin-6 Receptor Inhibition in Patients with Acute ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1845–1855. [Google Scholar] [CrossRef]
- Loubeyre, C.; Morice, M.C.; Lefevre, T.; Piechaud, J.F.; Louvard, Y.; Dumas, P. A randomized comparison of direct stenting with conventional stent implantation in selected patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2002, 39, 15–21. [Google Scholar] [CrossRef]
- Gasior, M.; Gierlotka, M.; Lekston, A.; Wilczek, K.; Zebik, T.; Hawranek, M.; Wojnar, R.; Szkodzinski, J.; Piegza, J.; Dyrbus, K.; et al. Comparison of outcomes of direct stenting versus stenting after balloon predilation in patients with acute myocardial infarction (DIRAMI). Am. J. Cardiol. 2007, 100, 798–805. [Google Scholar] [CrossRef]
- Azzalini, L.; Millan, X.; Ly, H.Q.; L’Allier, P.L.; Jolicoeur, E.M. Direct stenting versus pre-dilation in ST-elevation myocardial infarction: A systematic review and meta-analysis. J. Interv. Cardiol. 2015, 28, 119–131. [Google Scholar] [CrossRef]
- Saad, M.; Stiermaier, T.; Fuernau, G.; Pöss, J.; de Waha-Thiele, S.; Desch, S.; Thiele, H.; Eitel, I. Impact of direct stenting on myocardial injury assessed by cardiac magnetic resonance imaging and prognosis in ST-elevation myocardial infarction. Int. J. Cardiol. 2019, 283, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, B.; Li, M.; Liu, J.; Wang, L.; Liu, Y.; Wang, Z.; Wen, S. Comparing Direct Stenting with Conventional Stenting in Patients with Acute Coronary Syndromes: A Meta-Analysis of 12 Clinical Trials. Angiology 2016, 67, 317–325. [Google Scholar] [CrossRef]
- Gracida, M.; Romaguera, R.; Jacobi, F.; Gomez-Hospital, J.A.; Cequier, A. The MGuard coronary stent: Safety, efficacy, and clinical utility. Vasc. Health Risk Manag. 2015, 11, 533–539. [Google Scholar] [PubMed]
- Stone, G.W.; Abizaid, A.; Silber, S.; Dizon, J.M.; Merkely, B.; Costa, R.A.; Kornowski, R.; Abizaid, A.; Wojdyła, R.; Maehara, A.; et al. Prospective, Randomized, Multicenter Evaluation of a Polyethylene Terephthalate Micronet Mesh-Covered Stent (MGuard) in ST-Segment Elevation Myocardial Infarction: The MASTER Trial. J. Am. Coll. Cardiol. 2012, 60, 1975–1984. [Google Scholar] [CrossRef]
- Dudek, D.; Dziewierz, A.; Brener, S.J.; Abizaid, A.; Merkely, B.; Costa, R.A.; Bar, E.; Rakowski, T.; Kornowski, R.; Dressler, O.; et al. Mesh-covered embolic protection stent implantation in ST-segment-elevation myocardial infarction: Final 1-year clinical and angiographic results from the MGUARD for acute ST elevation reperfusion trial. Circ. Cardiovasc. Interv. 2015, 8, e001484. [Google Scholar] [CrossRef]
- Amoroso, G.; van Geuns, R.J.; Spaulding, C.; Manzo-Silberman, S.; Hauptmann, K.E.; Spaargaren, R.; García-García, H.M.; Serruys, P.W.; Verheye, S. Assessment of the safety and performance of the STENTYS self-expanding coronary stent in acute myocardial infarction: Results from the APPOSITION I study. EuroIntervention 2011, 7, 428–436. [Google Scholar] [CrossRef]
- van Geuns, R.J.; Tamburino, C.; Fajadet, J.; Vrolix, M.; Witzenbichler, B.; Eeckhout, E.; Spaulding, C.; Reczuch, K.; La Manna, A.; Spaargaren, R.; et al. Self-expanding versus balloon-expandable stents in acute myocardial infarction: Results from the APPOSITION II study: Self-expanding stents in ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 2012, 5, 1209–1219. [Google Scholar] [CrossRef]
- van Geuns, R.J.; Yetgin, T.; La Manna, A.; Tamburino, C.; Souteyrand, G.; Motreff, P.; Koch, K.T.; Vrolix, M.; IJsselmuiden, A.; Amoroso, G.; et al. STENTYS Self-Apposing sirolimus-eluting stent in ST-segment elevation myocardial infarction: Results from the randomised APPOSITION IV trial. EuroIntervention 2016, 11, e1267–e1274. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Grundeken, M.J.; Vos, N.S.; IJsselmuiden, A.J.J.; van Geuns, R.J.; Wessely, R.; Dengler, T.; La Manna, A.; Silvain, J.; Montalescot, G.; et al. Clinical outcomes with the STENTYS self-apposing coronary stent in patients presenting with ST-segment elevation myocardial infarction: Two-year insights from the APPOSITION III (A Post-Market registry to assess the STENTYS self-exPanding COronary Stent In AcuTe MyocardIal InfarctiON) registry. EuroIntervention 2017, 13, e572–e577. [Google Scholar] [PubMed]
- Isaaz, K.; Gerbay, A. Deferred stenting in acute ST elevation myocardial infarction. Lancet 2016, 388, 1371. [Google Scholar] [CrossRef]
- Carrick, D.; Oldroyd, K.G.; McEntegart, M.; Haig, C.; Petrie, M.C.; Eteiba, H.; Hood, S.; Owens, C.; Watkins, S.; Layland, J.; et al. A randomized trial of deferred stenting versus immediate stenting to prevent no- or slow-reflow in acute ST-segment elevation myocardial infarction (DEFER-STEMI). J. Am. Coll. Cardiol. 2014, 63, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Kelbæk, H.; Høfsten, D.E.; Køber, L.; Helqvist, S.; Kløvgaard, L.; Holmvang, L.; Jørgensen, E.; Pedersen, F.; Saunamäki, K.; De Backer, O.; et al. Deferred versus conventional stent implantation in patients with ST-segment elevation myocardial infarction (DANAMI 3-DEFER): An open-label, randomised controlled trial. Lancet 2016, 387, 2199–2206. [Google Scholar] [CrossRef]
- Nepper-Christensen, L.; Kelbæk, H.; Ahtarovski, K.A.; Høfsten, D.E.; Holmvang, L.; Pedersen, F.; Tilsted, H.H.; Aarøe, J.; Jensen, S.E.; Raungaard, B.; et al. Angiographic outcome in patients treated with deferred stenting after ST-segment elevation myocardial infarction-results from DANAMI-3-DEFER. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 742–748. [Google Scholar] [CrossRef]
- Mester, P.; Bouvaist, H.; Delarche, N.; Bouisset, F.; Abdellaoui, M.; Petiteau, P.Y.; Dubreuil, O.; Boueri, Z.; Chettibi, M.; Souteyrand, G.; et al. At least seven days delayed stenting using minimalist immediate mechanical intervention (MIMI) in ST-segment elevation myocardial infarction: The SUPER-MIMI study. EuroIntervention 2017, 13, 390–396. [Google Scholar] [CrossRef]
- Dingli, P.F.; Escaned, J. Minimalist immediate mechanical intervention in acute ST-segment elevation myocardial infarction: Is it time to redefine targets? Cardiovasc. Diagn. Ther. 2017, 7, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Cassese, S.; Belle, L.; Ndrepepa, G.; Bosson, J.L.; Fusaro, M.; Lønborg, J.; Ahtarovski, K.A.; Kelbæk, H.; Fusaro, M. Deferred vs Immediate Stenting in Primary Percutaneous Coronary Intervention: A Collaborative Meta-analysis of Randomized Trials with Cardiac Magnetic Resonance Imaging Data. Can. J. Cardiol. 2018, 34, 1573–1580. [Google Scholar] [CrossRef]
- Svilaas, T.; Vlaar, P.J.; van der Horst, I.C.; Diercks, G.F.H.; de Smet, B.J.G.L.; van den Heuvel, A.F.M.; Anthonio, R.L.; Jessurun, G.A.J.; Tan, E.S.; Suurmeijer, A.J.H.; et al. Thrombus aspiration during primary percutaneous coronary intervention. N. Engl. J. Med. 2008, 358, 557–567. [Google Scholar] [CrossRef]
- Jolly, S.S.; James, S.; Dzavik, V.; Cairns, J.A.; Mahmoud, K.D.; Zijlstra, F.; Yusuf, S.; Olivecrona, G.K.; Renlund, H.; Gao, P.; et al. Thrombus Aspiration in ST-Segment-Elevation Myocardial Infarction: An Individual Patient Meta-Analysis: Thrombectomy Trialists Collaboration. Circulation 2017, 135, 143–152. [Google Scholar] [CrossRef]
- Bianchini, E.; Lombardi, M.; Buonpane, A.; Ricchiuto, A.; Maino, A.; Laborante, R.; Anastasia, G.; D’Amario, D.; Aurigemma, C.; Romagnoli, E.; et al. Impact of thrombus aspiration on left ventricular remodeling and function in patients with ST-segment elevation myocardial infarction: A meta-analysis of randomized controlled trials. Int. J. Cardiol. 2024, 397, 131590. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 55–161. [Google Scholar] [CrossRef]
- Spirito, A.; Quagliana, A.; Coiro, M.; Melaku, G.D.; Vandenberghe, S.; Leibundgut, G.; Häner, J.; Moccetti, M.; Araco, M.; Garcia-Garcia, H.M.; et al. A prospective, first-in-human use of the NeVa mechanical thrombectomy device for patients with acute coronary syndromes. EuroIntervention 2022, 18, 242–252. [Google Scholar] [CrossRef]
- Kotronias, R.A.; Marin, F.; Emfietzoglou, M.; Langrish, J.P.; Lucking, A.J.; Channon, K.M.; Banning, A.P.; De Maria, G.L. Rationale and Design of a Randomized Controlled Pilot Trial to Assess Stent Retriever Thrombectomy for Thrombus Burden Reduction in Patients with Acute Myocardial Infarction: The RETRIEVE-AMI Study. Cardiovasc. Revasc. Med. 2023, 52, 75–85. [Google Scholar] [CrossRef]
- Kotronias, R.A.; Walsh, J.L.; Andreaggi, S.; Portolan, L.; Maino, A.; Marin, F.; Chai, J.; Sobirov, I.; Sheikh, M.; Cahill, T.J.; et al. Stent-Retriever Thrombectomy in STEMI with Large Thrombus Burden: The RETRIEVE AMI Randomized Trial. JACC Adv. 2025, 4, 101893. [Google Scholar] [CrossRef]
- Stone, G.W.; Webb, J.; Cox, D.A.; Brodie, B.R.; Qureshi, M.; Kalynych, A.; Turco, M.; Schultheiss, H.P.; Dulas, D.; Rutherford, B.D.; et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: A randomized controlled trial. JAMA 2005, 293, 1063–1072. [Google Scholar] [CrossRef]
- Gick, M.; Jander, N.; Bestehorn, H.P.; Kienzle, R.P.; Ferenc, M.; Werner, K.; Comberg, T.; Peitz, K.; Zohlnhöfer, D.; Bassignana, V.; et al. Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation. Circulation 2005, 112, 1462–1469. [Google Scholar] [CrossRef]
- Ito, N.; Nanto, S.; Doi, Y.; Kurozumi, Y.; Tonomura, D.; Natsukawa, T.; Sawano, H.; Masuda, D.; Yamashita, S.; Okada, K.; et al. Distal protection during primary coronary intervention can preserve the index of microcirculatory resistance in patients with acute anterior ST-segment elevation myocardial infarction. Circ. J. 2011, 75, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Kelbaek, H.; Terkelsen, C.J.; Helqvist, S.; Lassen, J.F.; Clemmensen, P.; Kløvgaard, L.; Kaltoft, A.; Engstrøm, T.; Bøtker, H.E.; Saunamäki, K.; et al. Randomized comparison of distal protection versus conventional treatment in primary percutaneous coronary intervention: The drug elution and distal protection in ST-elevation myocardial infarction (DEDICATION) trial. J. Am. Coll. Cardiol. 2008, 51, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Badr, S.; Ben-Dor, I.; Dvir, D.; Barbash, I.M.; Kitabata, H.; Minha, S.; Pendyala, L.K.; Loh, J.P.; Torguson, R.; Pichard, A.D.; et al. The state of the excimer laser for coronary intervention in the drug-eluting stent era. Cardiovasc. Revasc Med. 2013, 14, 93–98. [Google Scholar] [CrossRef]
- Topaz, O.; Ebersole, D.; Das, T.; Alderman, E.L.; Madyoon, H.; Vora, K.; Baker, J.D.; Hilton, D.; Dahm, J.B. Excimer laser angioplasty in acute myocardial infarction (the CARMEL multicenter trial). Am. J. Cardiol. 2004, 93, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Dörr, M.; Vogelgesang, D.; Hummel, A.; Staudt, A.; Robinson, D.M.; Felix, S.B.; Dahm, J.B. Excimer laser thrombus elimination for prevention of distal embolization and no-reflow in patients with acute ST elevation myocardial infarction: Results from the randomized LaserAMI study. Int. J. Cardiol. 2007, 116, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, K.; Shibata, N.; Takagi, K.; Mitsuhashi, H.; Morita, Y.; Kanzaki, Y.; Watanabe, N.; Yoshioka, N.; Miyazawa, H.; Imaoka, T.; et al. Excimer laser coronary angioplasty versus manual aspiration thrombectomy in patients with ST-segment elevation myocardial infarction: Analyzed by nuclear scintigraphy. Int. J. Cardiovasc. Imaging 2023, 39, 831–842. [Google Scholar] [CrossRef]
- Dahm, J.B.; Ebersole, D.; Das, T.; Madyhoon, H.; Vora, K.; Baker, J.; Hilton, D.; Topaz, O. Prevention of distal embolization and no-reflow in patients with acute myocardial infarction and total occlusion in the infarct-related vessel: A subgroup analysis of the cohort of acute revascularization in myocardial infarction with excimer laser-CARMEL multicenter study. Catheter. Cardiovasc. Interv. 2005, 64, 67–74. [Google Scholar] [PubMed]
- Karacsonyi, J.; Armstrong, E.J.; Truong, H.T.D.; Tsuda, R.; Kokkinidis, D.G.; Martinez-Parachini, J.R.; Alame, A.J.; Danek, B.A.; Karatasakis, A.; Roesle, M.; et al. Contemporary Use of Laser During Percutaneous Coronary Interventions: Insights from the Laser Veterans Affairs (LAVA) Multicenter Registry. J. Invasive Cardiol. 2018, 30, 195–201. [Google Scholar] [CrossRef]
- Van de Hoef, T.P.; Nolte, F.; Delewi, R.; Henriques, J.P.; Spaan, J.A.; Tijssen, J.G.; Siebes, M.; Wykrzykowska, J.J.; Stone, G.W.; Piek, J.J. Intracoronary hemodynamic effects of pressure-controlled intermittent coronary sinus occlusion (PICSO): Results from the First-In-Man Prepare PICSO Study. J. Interv. Cardiol. 2012, 25, 549–556. [Google Scholar] [CrossRef]
- Sehatbakhsh, S.; Mignatti, A.; Murthy, S.; Latib, A. A novel therapy in microvascular obstruction in ST-elevation myocardial infarction: Pressure-controlled intermittent coronary sinus occlusion therapy. Future Cardiol. 2023, 19, 615–623. [Google Scholar] [CrossRef]
- van de Hoef, T.P.; Nijveldt, R.; van der Ent, M.; Neunteufl, T.; Meuwissen, M.; Khattab, A.; Berger, R.; Kuijt, W.J.; Wykrzykowska, J.; Tijssen, J.G.; et al. Pressure-controlled intermittent coronary sinus occlusion (PICSO) in acute ST-segment elevation myocardial infarction: Results of the Prepare RAMSES safety and feasibility study. EuroIntervention 2015, 11, 37–44. [Google Scholar] [CrossRef]
- De Maria, G.L.; Alkhalil, M.; Borlotti, A.; Wolfrum, M.; Gaughran, L.; Dall’Armellina, E.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.K.; et al. Index of microcirculatory resistance-guided therapy with pressure-controlled intermittent coronary sinus occlusion improves coronary microvascular function and reduces infarct size in patients with ST-elevation myocardial infarction: The Oxford Acute Myocardial Infarction-Pressure-controlled Intermittent Coronary Sinus Occlusion study (OxAMI-PICSO study). EuroIntervention 2018, 14, e352–e359. [Google Scholar]
- De Maria, G.L.; Greenwood, J.P.; Zaman, A.G.; Carrié, D.; Coste, P.; Valgimigli, M.; Behan, M.; Berry, C.; Erglis, A.; Panoulas, V.F.; et al. Pressure-Controlled Intermittent Coronary Sinus Occlusion (PiCSO) in Acute Myocardial Infarction: The PiCSO-AMI-I Trial. Circ. Cardiovasc. Interv. 2024, 17, e013675. [Google Scholar] [CrossRef]
- Schafer, A.; Akin, M.; Diekmann, J.; Konig, T. Intracoronary Application of Super-Saturated Oxygen to Reduce Infarct Size Following Myocardial Infarction. J. Clin. Med. 2022, 11, 1509. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Martin, J.L.; Dixon, S.R.; Bartorelli, A.L.; Trabattoni, D.; Oemrawsingh, P.V.; Atsma, D.E.; Chang, M.; Marquardt, W.; Oh, J.K.; et al. Acute Myocardial Infarction with Hyperoxemic Therapy (AMIHOT): A prospective, randomized trial of intracoronary hyperoxemic reperfusion after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2007, 50, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Martin, J.L.; de Boer, M.J.; Margheri, M.; Bramucci, E.; Blankenship, J.C.; Metzger, D.C.; Gibbons, R.J.; Lindsay, B.S.; Weiner, B.H.; et al. Effect of supersaturated oxygen delivery on infarct size after percutaneous coronary intervention in acute myocardial infarction. Circ. Cardiovasc. Interv. 2009, 2, 366–375. [Google Scholar] [CrossRef] [PubMed]
- David, S.W.; Khan, Z.A.; Patel, N.C.; Metzger, D.C.; Wood, F.O.; Wasserman, H.S.; Lotfi, A.S.; Hanson, I.D.; Dixon, S.R.; Lalonde, T.A.; et al. Evaluation of intracoronary hyperoxemic oxygen therapy in acute anterior myocardial infarction: The IC-HOT study. Catheter. Cardiovasc. Interv. 2019, 93, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Falah, B.; Kotinkaduwa, L.N.; Schonning, M.J.; Redfors, B.; de Waha, S.; Granger, C.B.; Maehara, A.; Eitel, I.; Thiele, H.; Stone, G.W. Microvascular Obstruction in Patients with Anterior STEMI Treated with Supersaturated Oxygen. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101356. [Google Scholar] [CrossRef]
- Hanson, I.D.; David, S.W.; Dixon, S.R.; Metzger, D.C.; Généreux, P.; Maehara, A.; Xu, K.; Stone, G.W. “Optimized” delivery of intracoronary supersaturated oxygen in acute anterior myocardial infarction: A feasibility and safety study. Catheter. Cardiovasc. Interv. 2015, 86 (Suppl. 1), S51–S57. [Google Scholar] [CrossRef]
- Shin, D.; Dai, N.; Wilburn, P.; Choi, K.H.; Lee, S.H.; Kim, H.K.; Maehara, A.; Stone, G.W.; Myung Lee, J. Effect of Supersaturated Oxygen Therapy on Coronary Microcirculatory Function in Patients with Anterior STEMI. JACC Cardiovasc. Interv. 2023, 16, 2469–2471. [Google Scholar] [CrossRef]
- Kloner, R.A. Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ. Res. 2013, 113, 451–463. [Google Scholar] [CrossRef]
- Mewton, N.; Thibault, H.; Roubille, F.; Lairez, O.; Rioufol, G.; Sportouch, C.; Sanchez, I.; Bergerot, C.; Cung, T.T.; Finet, G.; et al. Postconditioning attenuates no-reflow in STEMI patients. Basic. Res. Cardiol. 2013, 108, 383. [Google Scholar] [CrossRef]
- Eitel, I.; Stiermaier, T.; Rommel, K.P.; Fuernau, G.; Sandri, M.; Mangner, N.; Linke, A.; Erbs, S.; Lurz, P.; Boudriot, E.; et al. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: The randomized LIPSIA CONDITIONING trial. Eur. Heart J. 2015, 36, 3049–3057. [Google Scholar] [CrossRef]
- Stiermaier, T.; Jensen, J.O.; Rommel, K.P.; de Waha-Thiele, S.; Fuernau, G.; Desch, S.; Thiele, H.; Eitel, I. Combined Intrahospital Remote Ischemic Perconditioning and Postconditioning Improves Clinical Outcome in ST-Elevation Myocardial Infarction. Circ. Res. 2019, 124, 1482–1491. [Google Scholar] [CrossRef]
- Engstrom, T.; Kelbaek, H.; Helqvist, S.; Høfsten, D.E.; Kløvgaard, L.; Clemmensen, P.; Holmvang, L.; Jørgensen, E.; Pedersen, F.; Saunamaki, K.; et al. Effect of Ischemic Postconditioning During Primary Percutaneous Coronary Intervention for Patients with ST-Segment Elevation Myocardial Infarction: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 490–497. [Google Scholar] [CrossRef]
- Francis, R.; Chong, J.; Ramlall, M.; Bucciarelli-Ducci, C.; Clayton, T.; Dodd, M.; Engstrøm, T.; Evans, R.; Ferreira, V.M.; Fontana, M.; et al. Effect of remote ischaemic conditioning on infarct size and remodelling in ST-segment elevation myocardial infarction patients: The CONDI-2/ERIC-PPCI CMR substudy. Basic. Res. Cardiol. 2021, 116, 59. [Google Scholar] [CrossRef] [PubMed]
- Traverse, J.H.; Swingen, C.M.; Henry, T.D.; Fox, J.; Wang, Y.L.; Chavez, I.J.; Lips, D.L.; Lesser, J.R.; Pedersen, W.R.; Burke, N.M.; et al. NHLBI-Sponsored Randomized Trial of Postconditioning During Primary Percutaneous Coronary Intervention for ST-Elevation Myocardial Infarction. Circ. Res. 2019, 124, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Noc, M.; Erlinge, D.; Neskovic, A.; Kafedzic, S.; Merkely, B.; Zima, E.; Fister, M.; Petrović, M.; Čanković, M.; Veress, G.; et al. COOL AMI EU pilot trial: A multicentre, prospective, randomised controlled trial to assess cooling as an adjunctive therapy to percutaneous intervention in patients with acute myocardial infarction. EuroIntervention 2017, 13, e531–e539. [Google Scholar] [CrossRef]
- El Farissi, M.; Pijls, N.H.J.; Good, R.; Engström, T.; Keeble, T.R.; Beleslin, B.; De Bruyne, B.; Fröbert, O.; Erlinge, D.; Teeuwen, K.; et al. A randomised trial of selective intracoronary hypothermia during primary PCI. EuroIntervention 2024, 20, e740–e749. [Google Scholar] [CrossRef]
- Kapur, N.K.; Kim, R.J.; Moses, J.W.; Stone, G.W.; Udelson, J.E.; Ben-Yehuda, O.; Redfors, B.; Issever, M.O.; Josephy, N.; Polak, S.J.; et al. Primary left ventricular unloading with delayed reperfusion in patients with anterior ST-elevation myocardial infarction: Rationale and design of the STEMI-DTU randomized pivotal trial. Am. Heart J. 2022, 254, 122–132. [Google Scholar] [CrossRef]
- Lukhna, K.; Hausenloy, D.J.; Ali, A.S.; Bajaber, A.; Calver, A.; Mutyaba, A.; Mohamed, A.A.; Kiggundu, B.; Chishala, C.; Variava, E.; et al. Remote Ischaemic Conditioning in STEMI Patients in Sub-Saharan AFRICA: Rationale and Study Design for the RIC-AFRICA Trial. Cardiovasc. Drugs Ther. 2023, 37, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Helios Health Institute GmbH. Randomized Comparison of Combined Remote Ischemic Conditioning and Local Postconditioning Compared to Standard Treatment in High-Risk ST-Elevation Myocardial Infarction Patients [Internet]. Clinicaltrials.gov. March 2025. Report No.: NCT04844931. Available online: https://clinicaltrials.gov/study/NCT04844931 (accessed on 25 October 2025).
- Zeymer, U.; Hassinger, F.; Bramlage, P.; Schafer, A.; Westermann, D.; Thiele, H.; HOT-AAMI Investigators. Hyperoxemic oxygen therapy in patients with acute anterior myocardial infarction: HOT-AAMI-design and rationale of a randomized trial. Am. Heart J. 2025, 286, 35–44. [Google Scholar] [CrossRef]
- Jeyaprakash, P.; Pathan, F.; Ozawa, K.; Robledo, K.P.; Shah, K.K.; Morton, R.L.; Yu, C.; Madronio, C.; Hallani, H.; Loh, H.; et al. Restoring microvascular circulation with diagnostic ultrasound and contrast agent: Rationale and design of the REDUCE trial. Am. Heart J. 2024, 275, 163–172. [Google Scholar] [CrossRef]
- Samir, A. Upfront Premedication for Reduction of Microvascular Obstruction and No-Reflow in Treating ST-Segment Elevation Myocardial Infarction [Internet]. Clinicaltrials.gov. August 2023. Report No.: NCT05393557. Available online: https://clinicaltrials.gov/study/NCT05393557 (accessed on 25 October 2025).
- Faraday Pharmaceuticals, Inc. A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study of Intravenous FDY 5301 in Patients with an Anterior ST-Elevation Myocardial Infarction [Internet]. Clinicaltrials.gov. September 2025. Report No.: NCT04837001. Available online: https://clinicaltrials.gov/study/NCT04837001 (accessed on 25 October 2025).
- Bo, Y. Cardioprotective Effect of Dexmedetomidine in Patients with ST-Segment Elevation Myocardial Infarction: A Double-Blind, Multicenter, Randomized, Placebo-Controlled Clinical Trial [Internet]. Clinicaltrials.gov. October 2024. Report No.: NCT04912518. Available online: https://clinicaltrials.gov/study/NCT04912518 (accessed on 25 October 2025).
- China National Center for Cardiovascular Diseases. Evaluating the Effects of Nicorandil on Microvascular Dysfunction in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention [Internet]. Clinicaltrials.gov. July 2025. Report No.: NCT06787430. Available online: https://clinicaltrials.gov/study/NCT06787430 (accessed on 25 October 2025).
| Drug | Route of Administration | Timing of Administration | Mechanism |
|---|---|---|---|
| Calcium Channel Blockers (adenosine, diltiazem, verapamil) | Intravenous or Intracoronary * | During Primary PCI | Vasoconstriction is considered a key contributor and is mediated by several receptors in epicardial conduit arteries and microvessels (such as α1-adrenergic and α2-adrenergic receptors) and by several signaling molecules (such as endothelin, serotonin and thromboxane. |
| Sodium Nitroprusside | Intracoronary | During Primary PCI | |
| Nicorandil | Intravenous | Pre-PCI, During Primary PCI, Post-PCI | |
| Epinephrine | Intracoronary | During Primary PCI | |
| Glycoprotein IIb/IIIa Inhibitors (abciximab, eptifibatide, tirofiban) | Intracoronary or Intravenous | During Primary PCI, Post-PCI ** | Reduce thrombus burden by promoting its dissolution and avoiding new thrombus formation at the site of the lesion. Antithrombotic agents can prevent and reduce distal embolization of thrombotic material. |
| Intravenous P2Y12 receptor inhibitor | Intravenous | During Primary PCI | |
| Intracoronary thrombolytic agents (urokinase, prourokinase, tenecteplase, and alteplase) | Intracoronary | During Primary PCI | |
| Oral P2Y12 Receptor Inhibitors (prasugrel, ticagrelor) | Oral | Pre-PCI |
| Mechanical Devices | Characteristics | |
|---|---|---|
| Specific Stent Platforms |  | Balloon-expandable bare-metal stent with polyethylene terephthalate (PET) micronet mesh or self-expanding stents |
| Thrombectomy |  | Catheters connected to syringe to aspirate thrombotic component from the culprit lesion using vacuum force. |
| Stent-Based Mechanical Thrombectomy Devices |  | Self-expanding nitinol stent-based devices designed to restore blood flow immediately after deployment at the lesion level. Capturing and removing atherothrombotic debris, they have the potential to minimizing distal embolization. |
| Distal Protection Devices |  | Filter with micro-holes to capture atherothrombotic debris distally to the culprit lesion while allowing blood flow maintenance. |
| Excimer laser coronary atherectomy |  | Catheters that release pulse waves by means of flexible optic fibers that reduce thrombus burden by plaque debulking and thrombus vaporization. |
| Pressure-controlled intermittent coronary sinus occlusion (PiCSO) |  | Balloon-tipped catheter that increase back-venous pressure in the coronary sinus, redistributing blood flow from the remote to the ischemic myocardium. |
| Supersaturated Oxygen |  | Intracoronary delivery of hyper-oxygenated blood. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Occhipinti, G.; Strosio, M.; Rinaldi, R.; Ruberti, A.; Brugaletta, S. Pharmacological and Interventional Prevention and Treatment of Microvascular Obstruction Following Primary PCI in STEMI. J. Cardiovasc. Dev. Dis. 2025, 12, 440. https://doi.org/10.3390/jcdd12110440
Occhipinti G, Strosio M, Rinaldi R, Ruberti A, Brugaletta S. Pharmacological and Interventional Prevention and Treatment of Microvascular Obstruction Following Primary PCI in STEMI. Journal of Cardiovascular Development and Disease. 2025; 12(11):440. https://doi.org/10.3390/jcdd12110440
Chicago/Turabian StyleOcchipinti, Giovanni, Michele Strosio, Riccardo Rinaldi, Andrea Ruberti, and Salvatore Brugaletta. 2025. "Pharmacological and Interventional Prevention and Treatment of Microvascular Obstruction Following Primary PCI in STEMI" Journal of Cardiovascular Development and Disease 12, no. 11: 440. https://doi.org/10.3390/jcdd12110440
APA StyleOcchipinti, G., Strosio, M., Rinaldi, R., Ruberti, A., & Brugaletta, S. (2025). Pharmacological and Interventional Prevention and Treatment of Microvascular Obstruction Following Primary PCI in STEMI. Journal of Cardiovascular Development and Disease, 12(11), 440. https://doi.org/10.3390/jcdd12110440






