Coronary Calcium Scoring as Prediction of Coronary Artery Diseases with Low-Dose Dual-Source CT
Abstract
1. Introduction
1.1. Study Population
1.2. Images Acquisition
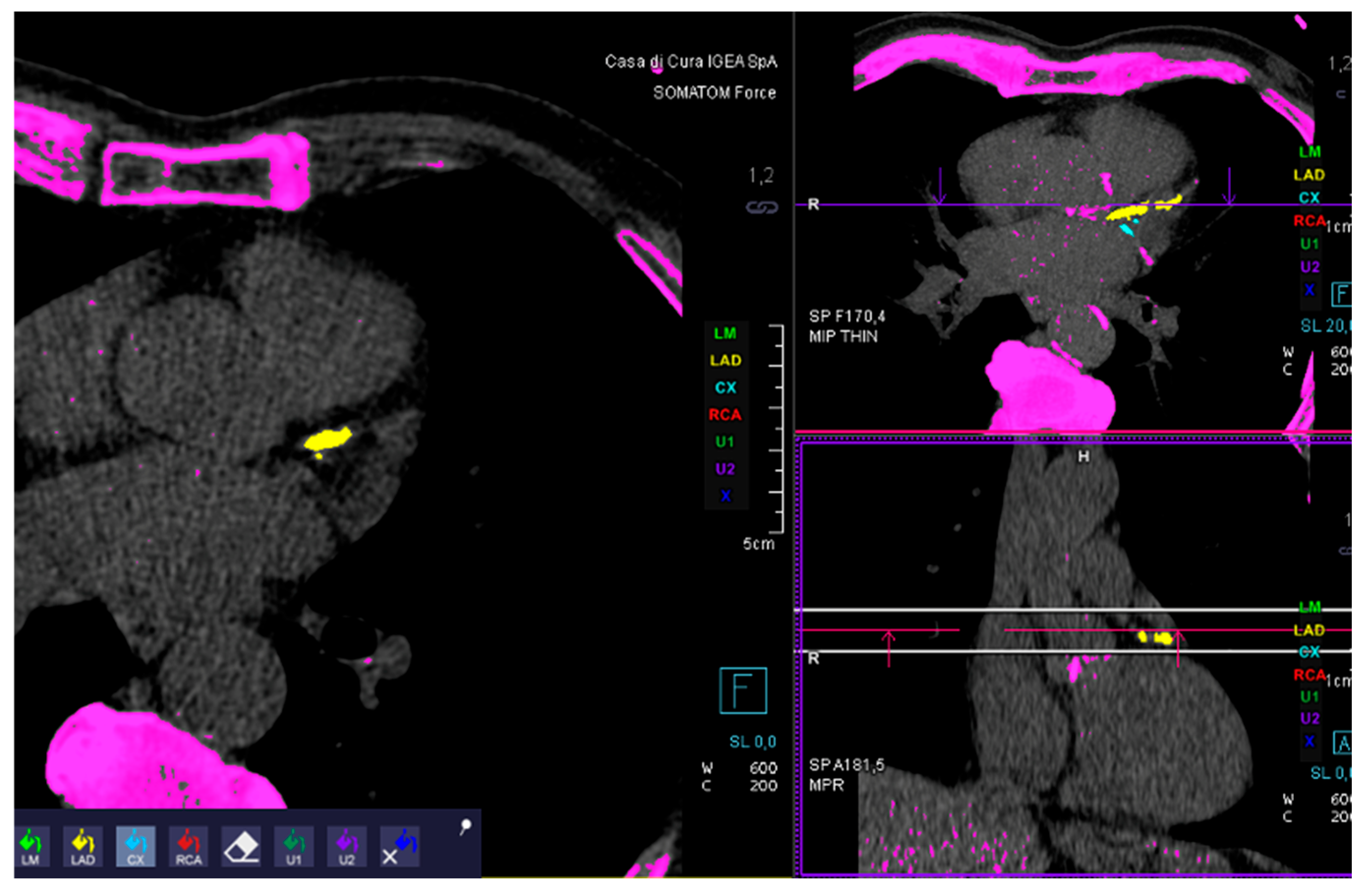
1.3. Images Analysis
1.4. Statistical Analysis
2. Results
3. Discussion
3.1. Background
3.2. Data Discussion
3.3. Limitations
3.4. Clinical Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTA | Computed Tomography Angiography |
| CCS | Coronary Calcium Score |
| CGS | Coronary Gravity Score |
| CT | Computed Tomography |
References
- Schuhbaeck, A.; Schmid, J.; Zimmer, T.; Muschiol, G.; Hell, M.M.; Marwan, M.; Achenbach, S. Influence of the coronary calcium score on the ability to rule out coronary artery stenoses by coronary CT angiography in patients with suspected coronary artery disease. J. Cardiovasc. Comput. Tomogr. 2016, 10, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Arbab-Zadeh, A.; Miller, J.M.; Rochitte, C.E.; Dewey, M.; Niinuma, H.; Gottlieb, I.; Paul, N.; Clouse, M.E.; Shapiro, E.P.; Hoe, J.; et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row ultidetector Computed Tomography Angiography) International Multicenter Study. J. Am. Coll. Cardiol. 2012, 59, 379–387. [Google Scholar] [PubMed]
- Meyer, M.; Henzler, T.; Fink, C.; Vliegenthart, R.; Barraza, J.M.; Nance, J.W.; Apfaltrer, P.; Schoenberg, S.O.; Wasser, K. Impact of coronary calcium score on the prevalence of coronary artery stenosis on dual source CT coronary angiography in caucasian patients with an intermediate risk. Acad. Radiol. 2012, 19, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.A.M.D.; de Smet, K.; de Bock, G.H.; Tio, R.A.; Oudkerk, M.; Vliegenthart, R. Diagnostic performance of coronary CT angiography for stenosis detection according to calcium score: Systematic review and meta-analysis. Eur. Radiol. 2012, 22, 2688–2698. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Jollis, J.G.; Dowe, D.; Min, J.; VCT Study Group. Diagnostic accuracy of coronary artery calcium for obstructive disease: Results from the ACCURACY trial. Int. J. Cardiol. 2013, 166, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Brodoefel, H.; Burgstahler, C.; Tsiflikas, I.; Reimann, A.; Schroeder, S.; Claussen, C.D.; Heuschmid, M.; Kopp, A.F. Dual-source CT: Effect of heart rate, heart rate variability, and calcification on image quality and diagnostic accuracy. Radiology 2008, 247, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Burgstahler, C.; Reimann, A.; Drosch, T.; Heuschmid, M.; Brodoefel, H.; Tsiflikas, I.; Häberle, E.; Uysal, I.; Wurster, D.; Claussen, C.D.; et al. Cardiac dual-source computed tomography in patients with severe coronary calcifications and a high prevalence of coronary artery disease. J. Cardiovasc. Comput. Tomogr. 2007, 1, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.A.; Maffei, E.; Martini, C.; Tarantini, G.; Di Tanna, G.L.; Berti, E.; Grilli, R.; Casolo, G.; Brambilla, V.; Cerrato, M.; et al. Coronary calcium score as gatekeeper for 64-slice computed tomography coronary angiography in patients with chest pain: Per-segment and per-patient analysis. Eur. Radiol. 2009, 19, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Sandfort, V.; Bluemke, D. CT calcium scoring. History, current status and outlook. Diagn. Interv. Imaging 2017, 98, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Task Force Members; Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; et al. ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society Cardiology. Eur. Heart J. 2013, 34, 2949–3003. [Google Scholar] [PubMed]
- Polonsky, T.S.; McClelland, R.L.; Jorgensen, N.W.; Bild, D.E.; Burke, G.L.; Guerci, A.D.; Greenland, P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA 2010, 303, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.E.; Den Ruijter, H.M.; Bots, M.L.; Moons, K.G.M. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: A systematic review. Heart 2012, 98, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Kubik, M.; et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 272–280. [Google Scholar] [PubMed]
- Greenland, P.; Blaha, M.J.; Budoff, M.J.; Erbel, R.; Watson, K.E. Coronary Calcium Score and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 72, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Budoff, M.J.; Sky, J.C.; Bommer, W.J.; Epstein, S.D.; Fisher, D.A.; Stock, E.O.; Taylor, A.J.; Wong, N.D.; DeMaria, A.N. Coronary Artery Calcium Staging to Guide Preventive Interventions. JACC Adv. 2024, 3, 101287. [Google Scholar] [CrossRef] [PubMed]
- Tehrai, M.; Almasi, A.; Pouraliakbar, H.; Sedghian, A.; Karimi, M.A.; Firouzi, A. The value of coronary artery calcium score assessed by dual-source computed tomography coronary angiography for predicting presence and severity of coronary artery disease. Pol. J. Radiol. 2014, 79, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Hanifehpour, R.; Motevalli, M.; Ghanaati, H.; Shahriari, M.; Ghasabeh, M.A. Diagnostic Accuracy of Coronary Calcium Score Less than 100 in Excluding Coronary Artery Disease. Iran. J. Radiol. 2016, 13, e16705. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.W.; Dardari, Z.A.; Blumenthal, R.S.; Dzaye, O.; Obisesan, O.H.; Iftekhar Uddin, S.M.; Nasir, K.; Blankstein, R.; Budoff, M.J.; Bødtker Mortensen, M.; et al. Very High Coronary Artery Calcium (≥1000) and Association with Cardiovascular Disease Events, Non-Cardiovascular Disease Outcomes, and Mortality: Results from MESA. Circulation 2021, 143, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- McClelland, R.L.; Jorgensen, N.W.; Budoff, M.; Blaha, M.J.; Post, W.S.; Kronmal, R.A.; Bild, D.E.; Shea, S.; Liu, K.; Watson, K.E.; et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J. Am. Coll. Cardiol. 2015, 66, 1643–1653. [Google Scholar] [PubMed]
- Cademartiri, F.; Di Cesare, E.; Francone, M.; Ballerini, G.; Ligabue, G.; Maffei, E.; Romagnoli, A.; Argiolas, G.M.; Russo, V.; Buffa, V.; et al. Italian Registry of Cardiac Computed Tomography. Radiol. Med. 2015, 120, 919–929. [Google Scholar] [CrossRef] [PubMed]

| Male | Female | Age | Smoke | Hypertension | Hypercolesterolemia |
|---|---|---|---|---|---|
| 217 | 188 | 72 ± 11 | 76% | 65% | 59% |
| CGS | 1 | 2 | 3 |
|---|---|---|---|
| No stenosis | Mild stenosis | Moderate/severe stenosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, E.; Tambè, V.; De Simoni, S.; Moltrasi, R.; Magazzeni, M.; Ciortan, E.; Bentivegna, S.; Esseridou, A.; Secchi, F. Coronary Calcium Scoring as Prediction of Coronary Artery Diseases with Low-Dose Dual-Source CT. J. Cardiovasc. Dev. Dis. 2025, 12, 425. https://doi.org/10.3390/jcdd12110425
Schwarz E, Tambè V, De Simoni S, Moltrasi R, Magazzeni M, Ciortan E, Bentivegna S, Esseridou A, Secchi F. Coronary Calcium Scoring as Prediction of Coronary Artery Diseases with Low-Dose Dual-Source CT. Journal of Cardiovascular Development and Disease. 2025; 12(11):425. https://doi.org/10.3390/jcdd12110425
Chicago/Turabian StyleSchwarz, Enrico, Valentina Tambè, Silvia De Simoni, Roberto Moltrasi, Matteo Magazzeni, Elena Ciortan, Stefano Bentivegna, Anastasia Esseridou, and Francesco Secchi. 2025. "Coronary Calcium Scoring as Prediction of Coronary Artery Diseases with Low-Dose Dual-Source CT" Journal of Cardiovascular Development and Disease 12, no. 11: 425. https://doi.org/10.3390/jcdd12110425
APA StyleSchwarz, E., Tambè, V., De Simoni, S., Moltrasi, R., Magazzeni, M., Ciortan, E., Bentivegna, S., Esseridou, A., & Secchi, F. (2025). Coronary Calcium Scoring as Prediction of Coronary Artery Diseases with Low-Dose Dual-Source CT. Journal of Cardiovascular Development and Disease, 12(11), 425. https://doi.org/10.3390/jcdd12110425






