Impact of T-AMYLO Risk Score and Red Flag Findings on Cardiovascular Outcomes in Patients with Cardiac Conduction Defects Treated with Intracardiac Device Implantation
Abstract
1. Introduction
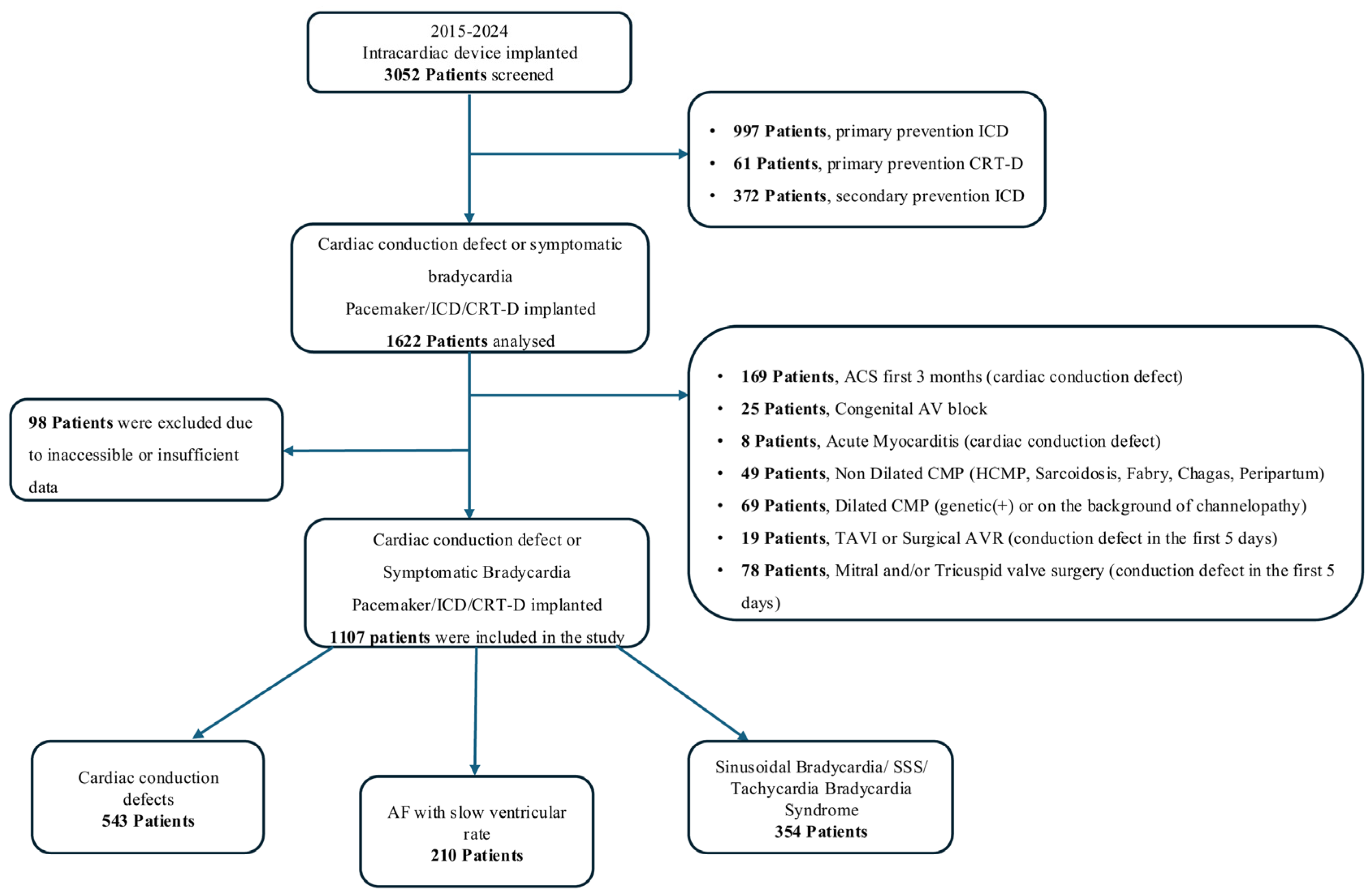
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maurer, M.S.; Elliott, P.; Comenzo, R.; Semigran, M.; Rapezzi, C. Addressing Common Questions Encountered in the Diagnosis and Management of Cardiac Amyloidosis. Circulation 2017, 135, 1357–1377. [Google Scholar] [CrossRef]
- Aimo, A.; Merlo, M.; Porcari, A.; Georgiopoulos, G.; Pagura, L.; Vergaro, G.; Sinagra, G.; Emdin, M.; Rapezzi, C. Redefining the epidemiology of cardiac amyloidosis. A systematic review and meta-analysis of screening studies. Eur. J. Heart Fail. 2022, 24, 2342–2351. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Grogan, M.; Hanna, M.; Kelly, J.W.; Maurer, M.S. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2872–2891. [Google Scholar] [CrossRef]
- González-López, E.; Gallego-Delgado, M.; Guzzo-Merello, G.; de Haro-Del Moral, F.J.; Cobo-Marcos, M.; Robles, C.; Bornstein, B.; Salas, C.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur. Heart J. 2015, 36, 2585–2594. [Google Scholar] [CrossRef]
- Scully, P.R.; Treibel, T.A.; Fontana, M.; Lloyd, G.; Mullen, M.; Pugliese, F.; Hartman, N.; Hawkins, P.N.; Menezes, L.J.; Moon, J.C. Prevalence of Cardiac Amyloidosis in Patients Referred for Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 463–464. [Google Scholar] [CrossRef]
- Saturi, G.; De Frutos, F.; Sguazzotti, M.; Gonzalez-Lopez, E.; Nardi, E.; Domínguez, F.; Ponziani, A.; Cabrera, E.; Caponetti, A.G.; Lozano, S.; et al. Predictors and outcomes of pacemaker implantation in patients with cardiac amyloidosis. Heart 2023, 110, 40–48. [Google Scholar] [CrossRef]
- Arana-Achaga, X.; Goena-Vives, C.; Villanueva-Benito, I.; Solla-Ruiz, I.; Jimenez, A.R.; Gaspar, T.I.; Urreta-Barallobre, I.; Barge-Caballero, G.; Seijas-Marcos, S.; Cabrera, E.; et al. Development and Validation of a Prediction Model and Score for Transthyretin Cardiac Amyloidosis Diagnosis: T-Amylo. JACC Cardiovasc. Imaging 2023, 16, 1567–1580. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.R.; Redfield, M.M.; Scott, C.G.; Minamisawa, M.; Grogan, M.; Dispenzieri, A.; Chareonthaitawee, P.; Shah, A.M.; Shah, S.J.; Wehbe, R.M.; et al. A Simple Score to Identify Increased Risk of Transthyretin Amyloid Cardiomyopathy in Heart Failure with Preserved Ejection Fraction. JAMA Cardiol. 2022, 7, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Boriani, G.; Braunschweig, F.; Brignole, M.; Burri, H.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520, Erratum in Eur. Heart J. 2022, 43, 1651. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients with Bradycardia and Cardiac Conduction Delay: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 932–987, Erratum in J. Am. Coll. Cardiol. 2019, 74, 1014–1016. [Google Scholar] [CrossRef]
- Mirvis, D.M.; Goldberger, A.L. Electrocardiography. In Braunwald’s Heart Disease; Mann, D.L., Zipes, D.P., Libby, P., Bonow, R.O., Eds.; Elsevier: Philadelphia, PA, USA, 2015; pp. 114–152. [Google Scholar]
- Fang, F.; Chan, J.Y.-S.; Yip, G.W.-K.; Xie, J.-M.; Zhang, Q.; Fung, J.W.-H.; Lam, Y.-Y.; Yu, C.-M. Prevalence and determinants of left ventricular systolic dyssynchrony in patients with normal ejection fraction received right ventricular apical pacing: A real-time three-dimensional echocardiographic study. Eur. J. Echocardiogr. 2010, 11, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Zhang, Q.; Chan, J.Y.-S.; Razali, O.; Azlan, H.; Chan, H.C.-K.; Sanderson, J.E.; Xie, J.-M.; Yu, C.-M. Early pacing-induced systolic dyssynchrony is a strong predictor of left ventricular adverse remodeling: Analysis from the Pacing to Avoid Cardiac Enlargement (PACE) trial. Int. J. Cardiol. 2013, 168, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Safak, E.; Ince, H.; Gkouvatsou, L.; Schultheiss, H.P.; Ortak, J.; Caglayan, E.; Oener, A.; D’Ancona, G. Pacing-induced cardiomyopathy in chronic right ventricular apical pacing: A midterm follow-up study. Eur. J. Med. Res. 2019, 24, 23. [Google Scholar] [CrossRef]
- Tayal, B.; Fruelund, P.; Sogaard, P.; Riahi, S.; Polcwiartek, C.; Atwater, B.D.; Gislason, G.; Risum, N.; Torp-Pedersen, C.; Kober, L.; et al. Incidence of heart failure after pacemaker implantation: A nationwide Danish Registry-based follow-up study. Eur. Heart J. 2019, 40, 3641–3648. [Google Scholar] [CrossRef] [PubMed]
- Kiehl, E.L.; Makki, T.; Kumar, R.; Gumber, D.; Kwon, D.H.; Rickard, J.W.; Kanj, M.; Wazni, O.M.; Saliba, W.I.; Varma, N.; et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016, 13, 2272–2278. [Google Scholar] [CrossRef]
- Yalvaç, H.E.; Murat, S.; Ak Sivrikoz, İ.; Üsküdar Teke, H.; Çilingir, O.; Çolak, E.; Çavuşoğlu, Y. The Most Predictive Red Flags for Suspecting Cardiac Amyloidosis in Patients with Heart Failure with Preserved Ejection Fraction. Turk. Kardiyol. Dern. Ars. 2024, 52, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, İ.; Oğuz, M.; Erdem, A.; Babaoğlu, M.; Uzun, M. The Need for a New Description of Red Flags in Cardiac Amyloidosis in Turkish Population. Turk. Kardiyol. Dern. Ars. 2025, 53, 80–81. [Google Scholar] [CrossRef]
- Shingu, M.; Fujimoto, W.; Onishi, T.; Kuragaichi, T.; Murai, R.; Matsuo, K.; Inoue, T.; Takaya, T.; Matsumoto, K.; Matsue, Y.; et al. A multicenter study of clinical predictors of positive pyrophosphate scintigraphy findings in the diagnosis of transthyretin amyloidosis. Int. J. Cardiol. 2025, 418, 132664. [Google Scholar] [CrossRef]
- López-Sainz, Á.; Moral, F.J.d.H.-D.; Dominguez, F.; Restrepo-Cordoba, A.; Amor-Salamanca, A.; Hernandez-Hernandez, A.; Ruiz-Guerrero, L.; Krsnik, I.; Cobo-Marcos, M.; Castro, V.; et al. Prevalence of cardiac amyloidosis among elderly patients with systolic heart failure or conduction disorders. Amyloid 2019, 26, 156–163. [Google Scholar] [CrossRef]
- Cannie, D.; Patel, K.; Protonotarios, A.; Heenan, I.; Bakalakos, A.; Syrris, P.; Menezes, L.; Elliott, P.M. Prevalence of transthyretin cardiac amyloidosis in patients with high-degree AV block. Open Heart 2024, 11, e002606. [Google Scholar] [CrossRef]
- Aaseth, E.; Christiansen, J.R. Prevalence of transthyretin amyloid cardiomyopathy in pacemaker patients. ESC Heart Fail. 2024, 11, 871–876. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Saliba, W.I.; Hanna, M.; Kanj, M.; Patel, D.R. Prevalence, incidence, and impact on mortality of conduction system disease in transthyretin cardiac amyloidosis. Am. J. Cardiol. 2020, 128, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Blanco-López, E.; Martínez-Del Río, J.; López-Calles, A.; Negreira-Caamaño, M.; Águila-Gordo, D.; Soto-Martín, P.; Soto-Pérez, M.M.; Cubides-Novoa, A.F.; Gonzalez-Barderas, M.; Sánchez-Pérez, I.; et al. Cardiac amyloidosis and red flags: Natural history and its impact in morbimortality. Med. Clin. 2025, 164, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Sanne Bøjet, L.; Bertil, L.; Anders Lehmann Dahl, P.; Jens Kæstel, S.; Tor Skibsted, C.; Steen Hvitfeldt, P. Changes of clinical characteristics, distribution of red flags and prognosis in contemporary patients with wild-type transthyretin amyloidosis cardiomyopathy. Ann. Med. 2024, 56, 2398735. [Google Scholar] [CrossRef] [PubMed]

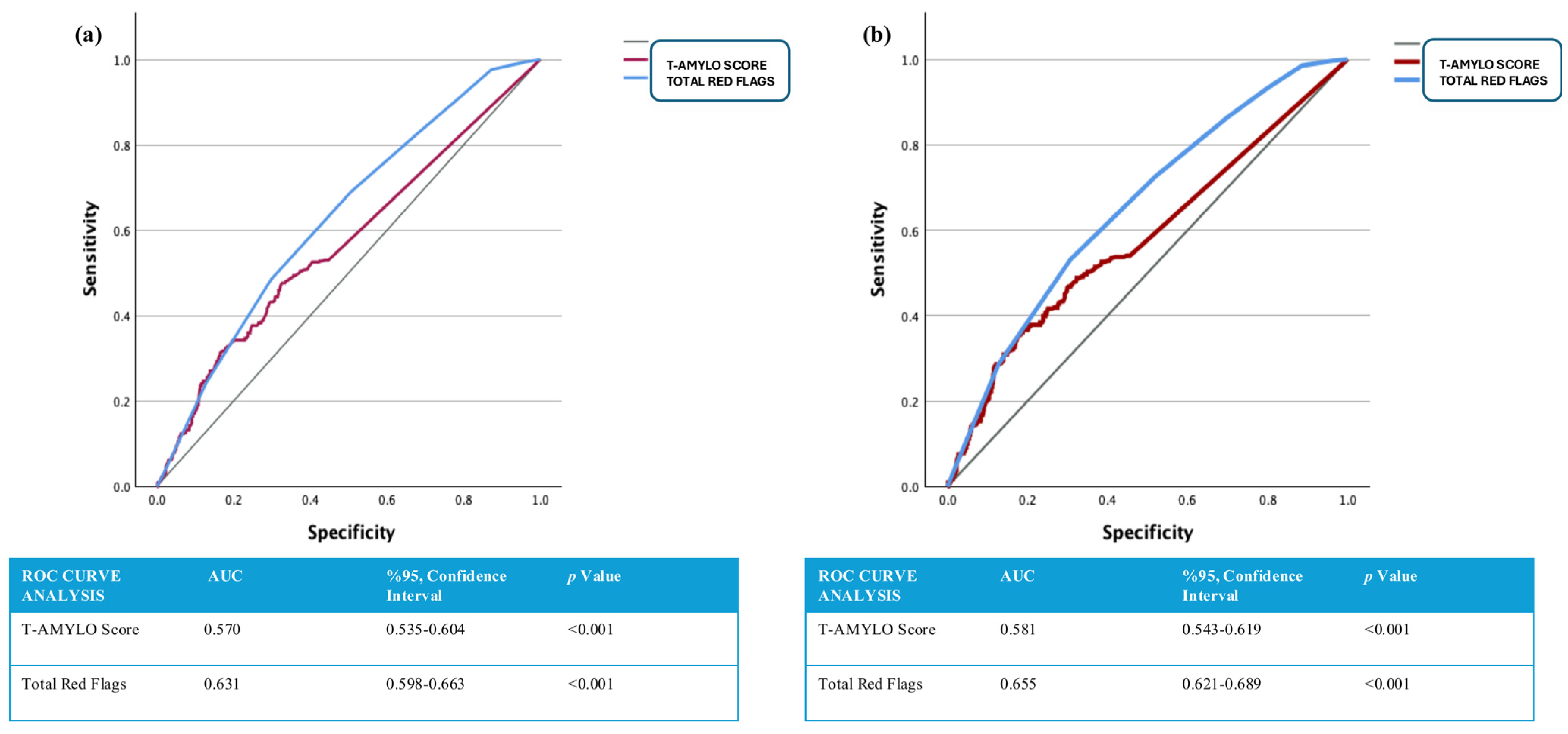
| Primary Endpoints (–) (n: 647) | Primary Endpoints (+) (n: 470) | p Value | All-Cause Mortality (–) (n: 761) | All-Cause Mortality (+) (n:346) | p Value | |
|---|---|---|---|---|---|---|
| Age, (years) * | 74 (24–97) | 82 (36–102) | <0.001 | 74 (24–97) | 84 (49–102) | <0.001 |
| Male, n (%) | 295 (46.3) | 276 (58.7) | <0.001 | 370 (48.6) | 201 (58.1) | 0.003 |
Indications for Implantation, n (%)
| 226 (35.5) 62 (9.7) 249 (39.1) 100 (15.7) | 216 (46.0) 39 (8.3) 105 (22.3) 110 (23.4) | <0.001 | 270 (35.5) 71 (9.3) 295 (38.8) 125 (16.4) | 172 (49.7) 30 (8.7) 59 (17.1) 85 (24.6) | <0.001 |
Types of Implanted CIEDs, n (%)
| 128 (20.1) 389 (61.1) 34 (5.3) 78 (12.2) 8 (1.3) | 139 (29.6) 215 (45.7) 62 (13.2) 51 (10.9) 3 (0.6) | <0.001 | 146 (19.2) 460 (60.4) 49 (6.4) 98 (8.6) 8 (0.7) | 121 (35.0) 144 (41.6) 47 (13.6) 31 (2.8) 3 (0.3) | <0.001 |
Heart Failure; n (%)
| 111 (17.4) | 163 (37.7) | <0.001 | 154 (20.2) | 120 (34.7) | <0.001 |
| DM, n (%) | 216 (33.9) | 221 (47.0) | <0.001 | 266 (35.0) | 171 (49.4) | <0.001 |
| HT, n (%) | 499 (78.3) | 423 (90.0) | <0.001 | 604 (79.4) | 318 (91.9) | <0.001 |
| Stroke, n (%) | 127 (19.9) | 155 (33.0) | <0.001 | 161 (21.2) | 121 (35.0) | <0.001 |
| CAD, n (%) | 171 (26.8) | 228 (48.5) | <0.001 | 250 (32.9) | 149 (43.1) | 0.001 |
| CKD, n (%) | 132 (20.7) | 191 (40.6) | <0.001 | 159 (20.7) | 164 (47.4) | <0.001 |
| AF, n (%) | 287 (45.1) | 256 (54.5) | 0.002 | 338 (44.4) | 205 (59.2) | <0.001 |
| Senkop-presenkop, n (%) | 481 (75.5) | 334 (71.1) | 0.097 | 569 (74.8) | 246 (71.1) | 0.199 |
| Smoker, n (%) | 156 (24.5) | 113 (24.0) | 0.860 | 191 (25.1) | 78 (22.5) | 0.358 |
Aortic Valve Disease **, n (%)
| 302 (47.4) 274 (43.0) 24 (3.8) 37 (5.8) | 155 (33.0) 247 (52.6) 20 (4.3) 48 (10.2) | <0.001 | 356 (46.8) 333 (43.8) 27 (3.5) 45 (5.9) | 101 (29.2) 188 (54.3) 17 (4.9) 40 (11.6) | <0.001 |
Medical Treatments; n (%)
| 462 (72.5) 96 (15.1) 52 (8.2) 27 (4.2) 114 (17.9) 491 (77.1) 30 (4.7) 439 (68.9) 187 (29.4) 491 (77.1) | 391 (83.2) 81 (17.2) 80 (17.0) 17 (3.6) 116 (24.7) 420 (89.4) 20 (4.3) 368 (78.3) 221 (47.0) 445 (94.7) | <0.001 0.330 <0.001 0.610 0.006 <0.001 0.710 <0.001 <0.001 <0.001 | 570 (74.9) 110 (14.5) 69 (9.1) 31 (4.1) 133 (17.5) 606 (79.6) 37 (4.3) 520 (68.3) 235 (30.9) 601 (79.0) | 283 (81.8) 67 (19.4) 63 (18.2) 13 (3.8) 97 (28.0) 305 (88.2) 13 (3.8) 287 (82.9) 173 (50.0) 335 (96.8) | 0.011 0.039 <0.001 0.801 <0.001 <0.001 0.412 <0.001 <0.001 <0.001 |
| Primary Endpoints (–) (n: 647) | Primary Endpoints (+) (n: 470) | p Value | All-Cause Mortality (–) (n:761) | All-Cause Mortality (+) (n:346) | p Value | |
|---|---|---|---|---|---|---|
Rhythm, n (%)
| 336 (52.7) 96 (15.1) 205 (32.2) | 161 (34.3) 90 (19.1) 219 (46.6) | <0.001 | 396 (52.0) 120 (15.8) 245 (32.2) | 101 (29.2) 66 (19.1) 179 (51.7) | <0.001 |
| PR, msn * | 200 (106–360) | 200 (138–400) | 0.048 | 200 (106–340) | 210 (138–400) | 0.007 |
| QRS, msn * | 130 (62–190) | 140 (72–225) | <0.001 | 130 (62–190) | 140 (72–200) | <0.001 |
| QTc, msn * | 430 (328–552) | 435 (341–543) | 0.025 | 427 (328–537) | 435 (367–557) | <0.001 |
LBBB, n (%)
| 207 (42.9) | 221 (63.1) | <0.001 | 249 (43.2) | 179 (69.6) | <0.001 |
RBBB, n (%)
| 57 (11.8) | 29 (8.3) | 0.171 | 65 (11.3) | 21 (8.2) | 0.109 |
| Fragmented QRS, n (%) | 150 (31.2) | 85 (25.4) | 0.070 | 172 (30.1) | 63 (25.2) | 0.201 |
| Mobitz Type 2 AV Block, n (%) | 178 (27.9) | 105 (22.3) | 0.032 | 205 (26.9) | 78 (22.5) | 0.120 |
| AV Complete Block, n (%) | 181 (28.1) | 180 (38.3) | <0.001 | 213 (28.0) | 148 (42.8) | <0.001 |
| Low Voltage **, n (%) | 179 (28.1) | 165 (35.1) | 0.013 | 215 (28.3) | 129 (37.3) | 0.003 |
| LVEF, (%) * | 60 (20–65) | 58 (18–65) | 0.001 | 60 (20–65) | 60 (18–65) | 0.002 |
| LA, mm * | 43 (26–64) | 45 (21–67) | <0.001 | 44 (22–65) | 45 (32–66) | <0.001 |
| LVEDd, mm * | 51 (34–75) | 53 (36–76) | <0.001 | 51 (34–75) | 54 (36–76) | <0.001 |
| IVS, mm * | 11 (6–20) | 12 (7–21) | 0.003 | 11 (6–20) | 12 (7–21) | 0.013 |
| PW, mm * | 11 (7–17) | 11 (6–20) | 0.077 | 11 (6–17) | 11 (7–20) | 0.121 |
| TAPSE, mm * | 20 (10–28) | 19 (11–28) | <0.001 | 20 (11–28) | 19 (10–28) | <0.001 |
| sPAP, mmHg * | 35 (20–95) | 35 (20–100) | <0.001 | 35 (20–90) | 38 (20–100) | <0.001 |
| LVDD, n (%) | 444 (69.7) | 356 (75.7) | 0.054 | 535 (70.3) | 257 (74.3) | 0.174 |
| Primary Endpoints (–) (n: 647) | Primary Endpoints (+) (n: 470) | p Value | All-Cause Mortality (–) (n: 761) | All-Cause Mortality (+) (n: 346) | p Value | |
|---|---|---|---|---|---|---|
| Hb, g/dL * | 13.2 (6.1–19) | 12.4 (5.2–18.3) | <0.001 | 13.2 (6.1–19.0) | 12.2 (5.2–18.3) | <0.001 |
| HCT, (%) * | 39.1 (17.9–56.0) | 37.0 (16.2–52.6) | <0.001 | 38.9 (17.9–56.0) | 36.3 (16.2–52.6) | <0.001 |
| PLT, 103 mm3 * | 220.0 (29.6–535.0) | 204.5 (42.0–676.0) | <0.001 | 218.0 (29.6–535.0) | 201.0 (42.0–636.0) | <0.001 |
| WBC, 103 mm3 * | 7.2 (3.2–17.1) | 7.4 (2.3–15.6) | 0.220 | 7.1 (3.2–17.2) | 7.5 (2.3–15.6) | 0.033 |
| Lymphocyte, 103 mm3 * | 1.9 (0.2–11.2) | 1.5 (0.2–4.5) | <0.001 | 1.9 (0.2–11.2) | 1.4 (0.2–4.5) | <0.001 |
| Monocyte, 103 mm3 * | 0.57 (0.10–2.0) | 0.60 (0.10–2.2) | 0.010 | 0.56 (0.10–2.00) | 0.60 (0.10–2.20) | <0.001 |
| Neutrophil, 103 mm3 * | 4.3 (0.3–11.3) | 4.7 (1.5–10.8) | <0.001 | 4.3 (0.3–11.4) | 4.9 (1.5–10.8) | <0.001 |
| Creatinine, mg/dL * | 0.9 (0.5–5.6) | 1.1 (0.7–8.8) | <0.001 | 0.9 (0.5–5.6) | 1.2 (0.6–8.8) | <0.001 |
| GFR, mL/min* | 69 (12–128) | 54 (5–120) | <0.001 | 68 (12–128) | 48 (5–120) | <0.001 |
| Sodium, mmol/L * | 140 (126–150) | 140 (121–149) | 0.190 | 140 (126–150) | 140 (121–149) | 0.083 |
| Potassium, mmol/L * | 4.5 (3.3–5.9) | 4.5 (3.0–6.4) | 0.210 | 4.5 (3.3–5.9) | 4.5 (3.0–6.4) | 0.911 |
| LDL, mg/dL * | 112 (15–224) | 109 (38–274) | 0.070 | 110 (15–245) | 112 (38–270) | 0.933 |
| Troponin T, ng/mL * | 0.011 (0.001–0.288) | 0.016 (0.001–0.430) | <0.001 | 0.011 (0.001–0.340) | 0.019 (0.006–0.430) | <0.001 |
| NT-pro BNP, pg/mL * | 144.7 (7.0–12533.0) | 423.0 (3.5–18877.0) | <0.001 | 156.0 (3.5–12556.0) | 636.1 (10.5–18,877.2) | <0.001 |
| CRP, mg/L * | 3.9 (0.3–75.0) | 6.7 (1.0–106.0) | <0.001 | 3.8 (0.1–75.2) | 8.6 (0.8–106.5) | <0.001 |
| Primary Endpoints (–) (n: 647) | Primary Endpoints (+) (n: 470) | p Value | All-Cause Mortality (–) (n: 761) | All-Cause Mortality (+) (n: 346) | p Value | |
|---|---|---|---|---|---|---|
T-AMYLO Score Risk Groups, n (%) *
| 352 (55.3) 182 (28.6) 73 (11.5) 30 (4.7) | 221 (47.0) 108 (23.0) 102 (21.7) 39 (8.3) | <0.001 | 414 (54.4) 218 (28.6) 93 (12.2) 36 (4.7) | 159 (46.0) 72 (20.8) 82 (23.7) 33 (9.5) | <0.001 |
| T-AMYLO Score, n ** | 1.0 (0.0–94.1) | 2.9 (0.0–99.1) | <0.001 | 0.2 (0.0–99.1) | 4.8 (1.8–99.1) | <0.001 |
| Aortic Valve Disease, n (%) *** | 237 (37.2) | 246 (52.3) | <0.001 | 287 (37.7) | 196 (56.6) | <0.001 |
| Heart Failure, (LVEF > 40%), n (%) | 354 (55.0) | 301 (64.0) | 0.006 | 423 (55.8) | 226 (65.4) | 0.004 |
| Age > 65, n (%) | 485 (76.1) | 434 (92.3) | <0.001 | 592 (77.8) | 327 (94.5) | <0.001 |
| CTS, n (%) | 106 (16.6) | 83 (17.7) | 0.656 | 123 (16.2) | 66 (19.1) | 0.233 |
| Peripheral Neuropathy, n (%) | 50 (7.8) | 91 (19.4) | <0.001 | 63 (8.3) | 78 (22.5) | <0.001 |
| Autonomic Dysfunction, n (%) | 115 (18.1) | 96 (20.4) | 0.321 | 142 (18.7) | 69 (19.9) | 0.615 |
| Presence of AV Blocks ****, n (%) | 413 (64.8) | 373 (79.4) | <0.001 | 495 (65.0) | 291 (84.1) | <0.001 |
| Low Voltage with (IVS > 12 mm), n (%) | 86 (13.5) | 86 (18.3) | 0.029 | 107 (14.1) | 65 (18.8) | 0.044 |
| Low Voltage with (IVS < 12 mm), n (%) | 93 (14.6) | 81 (17.2) | 0.234 | 108 (14.2) | 66 (19.1) | 0.038 |
| LVDD, n (%) | 448 (70.3) | 344 (73.2) | 0.297 | 535 (70.3) | 257 (74.3) | 0.174 |
| Pseudo Q Wave, n (%) | 27 (4.2) | 33 (7.0) | 0.043 | 31 (4.1) | 29 (8.4) | 0.003 |
| IVS > 12 mm, n (%) | 282 (44.3) | 246 (52.3) | 0.008 | 345 (45.3) | 183 (52.9) | 0.020 |
| Total Number of Red Flags ** | 4.5 (0–9) | 5.5 (1–9) | <0.001 | 5 (0–9) | 6 (1–9) | <0.001 |
| Total Follow-up Time, n (Month) ** | 70 (1–114) | 39 (3–114) | <0.001 | 70 (1–114) | 31 (3–107) | <0.001 |
| Independent Variables for All-Cause Mortality | Hazard Ratio (HR) | Lower–Upper (95% Confidence Interval (CI)) | p Value |
|---|---|---|---|
| WBC | 1.00 | 1.00–1.00 | 0.749 |
| HT | 0.92 | 0.66–1.27 | 0.618 |
| Hb | 0.96 | 0.90–1.02 | 0.198 |
| GFR | 0.98 | 0.97–0.99 | <0.001 |
| NT-pro BNP | 1.01 | 1.00–1.02 | 0.001 |
| Troponin T | 0.50 | 0.40–6.20 | 0.579 |
| CRP | 1.01 | 1.01–1.02 | <0.001 |
| Aortic Valve Disease * | 1.29 | 1.02–1.62 | 0.030 |
| Heart Failure, (LVEF > 40%) | 1.42 | 1.09–1.97 | 0.037 |
| Low Voltages on ECG | 1.10 | 0.89–1.36 | 0.367 |
| LVEF | 0.98 | 0.97–1.01 | 0.054 |
| TAPSE | 0.98 | 0.94–1.02 | 0.456 |
| AF | 1.21 | 0.96–1.52 | 0.098 |
| DM | 1.05 | 0.83–1.32 | 0.667 |
| CAD | 1.00 | 0.78–1.27 | 0.998 |
| Peripheral Neuropathy | 1.52 | 1.16–2.00 | 0.002 |
| AV Blocks ** | 1.48 | 1.08–2.03 | 0.013 |
| Complete LBBB | 1.43 | 1.13–1.80 | 0.002 |
| LVDD | 0.95 | 0.73–1.23 | 0.716 |
| IVS > 12 mm | 1.20 | 0.86–1.67 | 0.274 |
| T-AMYLO Risk Score *** | 1.00 | 1.00–1.01 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arabaci, H.O.; Arslan, S.; Kurt, C.; Hunkar, P.; Ozkan, F.; Gecit, M.H.; Arslan, S.; Yildiz, M. Impact of T-AMYLO Risk Score and Red Flag Findings on Cardiovascular Outcomes in Patients with Cardiac Conduction Defects Treated with Intracardiac Device Implantation. J. Cardiovasc. Dev. Dis. 2025, 12, 424. https://doi.org/10.3390/jcdd12110424
Arabaci HO, Arslan S, Kurt C, Hunkar P, Ozkan F, Gecit MH, Arslan S, Yildiz M. Impact of T-AMYLO Risk Score and Red Flag Findings on Cardiovascular Outcomes in Patients with Cardiac Conduction Defects Treated with Intracardiac Device Implantation. Journal of Cardiovascular Development and Disease. 2025; 12(11):424. https://doi.org/10.3390/jcdd12110424
Chicago/Turabian StyleArabaci, Hidayet Ozan, Sukru Arslan, Cem Kurt, Pelinsu Hunkar, Fatih Ozkan, Muhammet Heja Gecit, Seyma Arslan, and Mustafa Yildiz. 2025. "Impact of T-AMYLO Risk Score and Red Flag Findings on Cardiovascular Outcomes in Patients with Cardiac Conduction Defects Treated with Intracardiac Device Implantation" Journal of Cardiovascular Development and Disease 12, no. 11: 424. https://doi.org/10.3390/jcdd12110424
APA StyleArabaci, H. O., Arslan, S., Kurt, C., Hunkar, P., Ozkan, F., Gecit, M. H., Arslan, S., & Yildiz, M. (2025). Impact of T-AMYLO Risk Score and Red Flag Findings on Cardiovascular Outcomes in Patients with Cardiac Conduction Defects Treated with Intracardiac Device Implantation. Journal of Cardiovascular Development and Disease, 12(11), 424. https://doi.org/10.3390/jcdd12110424






