Progression of Untreated Mild Aortic Valve Disease in Patients Undergoing Rheumatic Mitral Valve Surgery: A Meta-Analysis of Reconstructed Time-to-Event Data
Abstract
1. Introduction
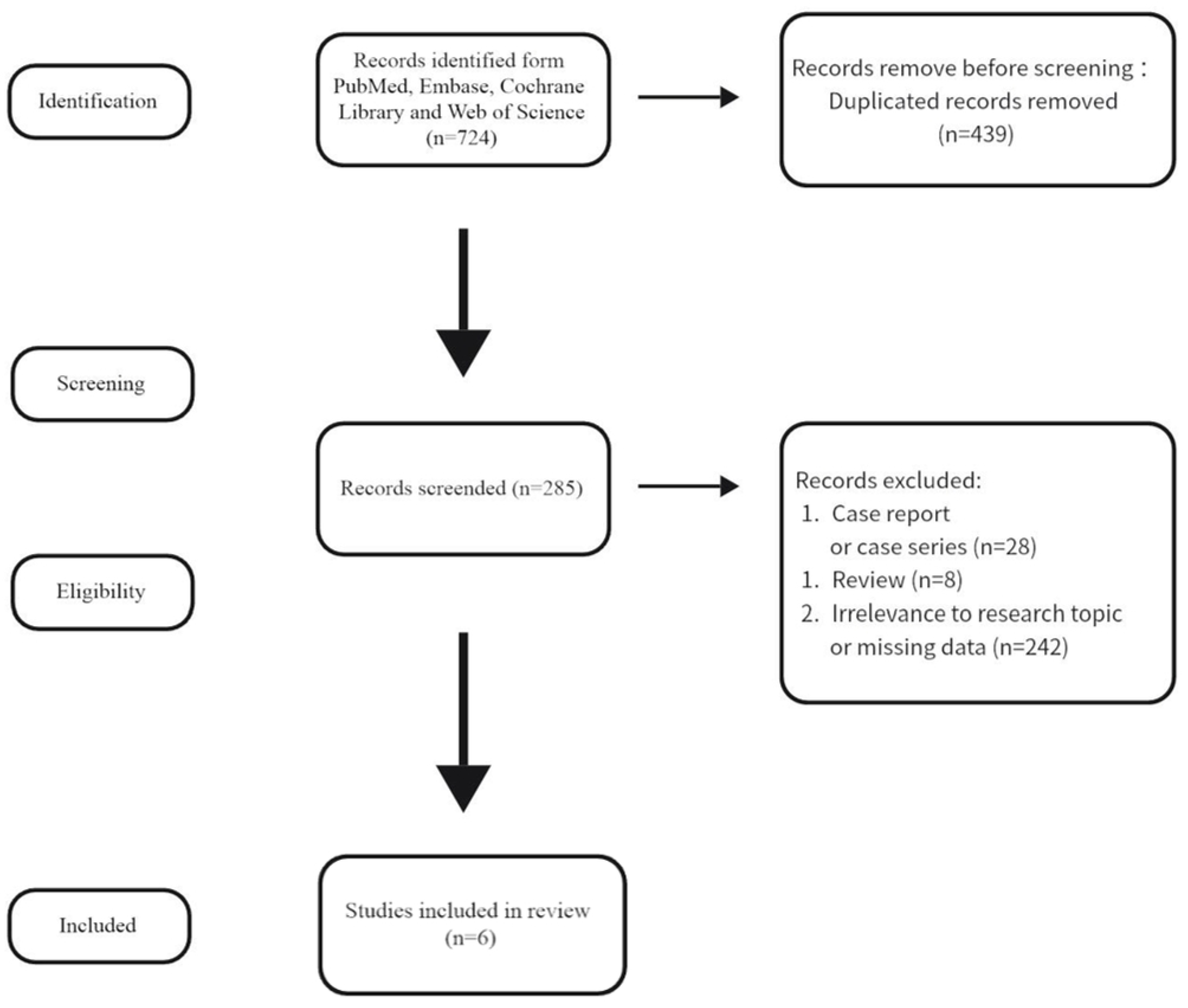
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Statistical Analyses
3. Results
3.1. Study Characteristics
3.2. Clinical Characteristics
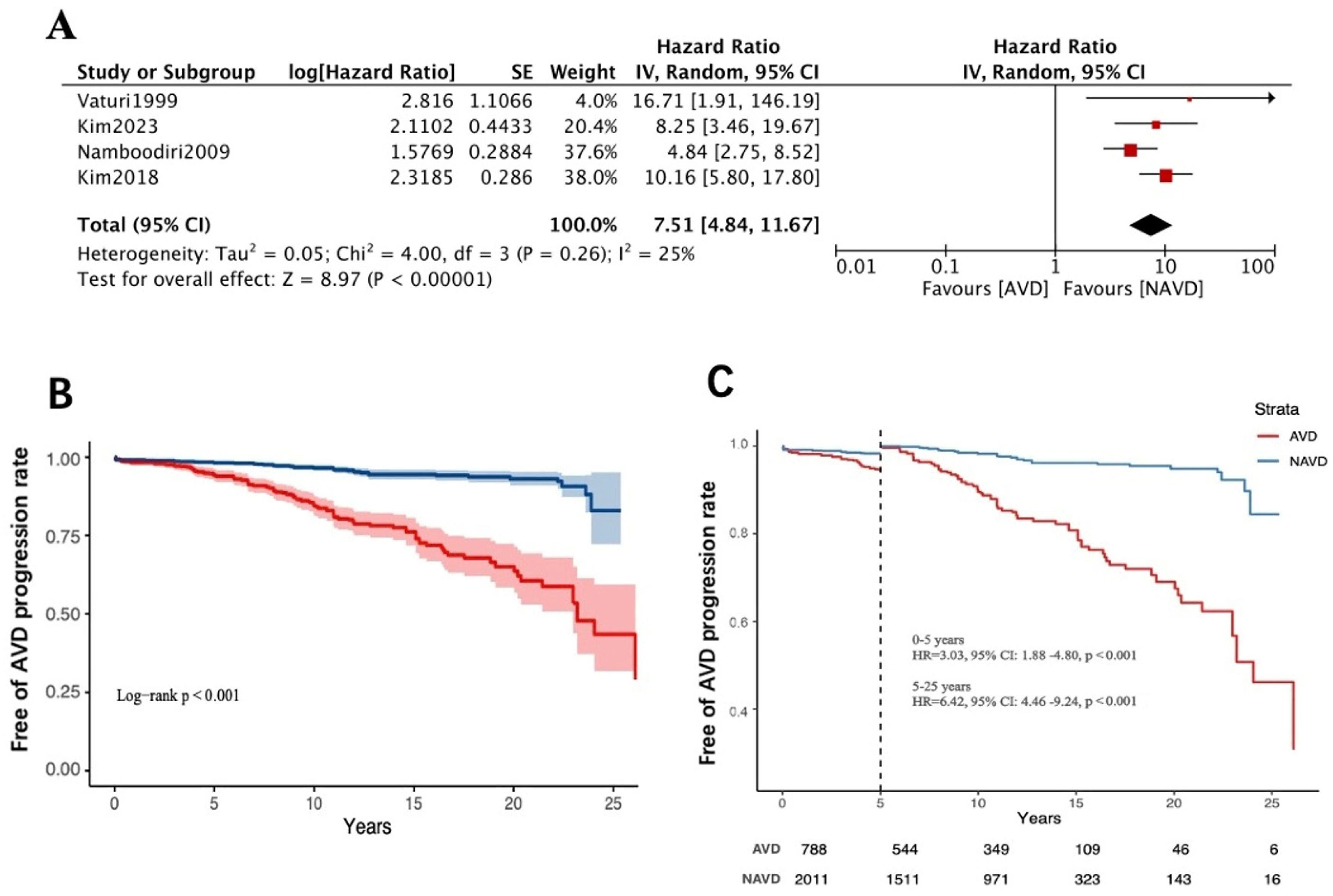
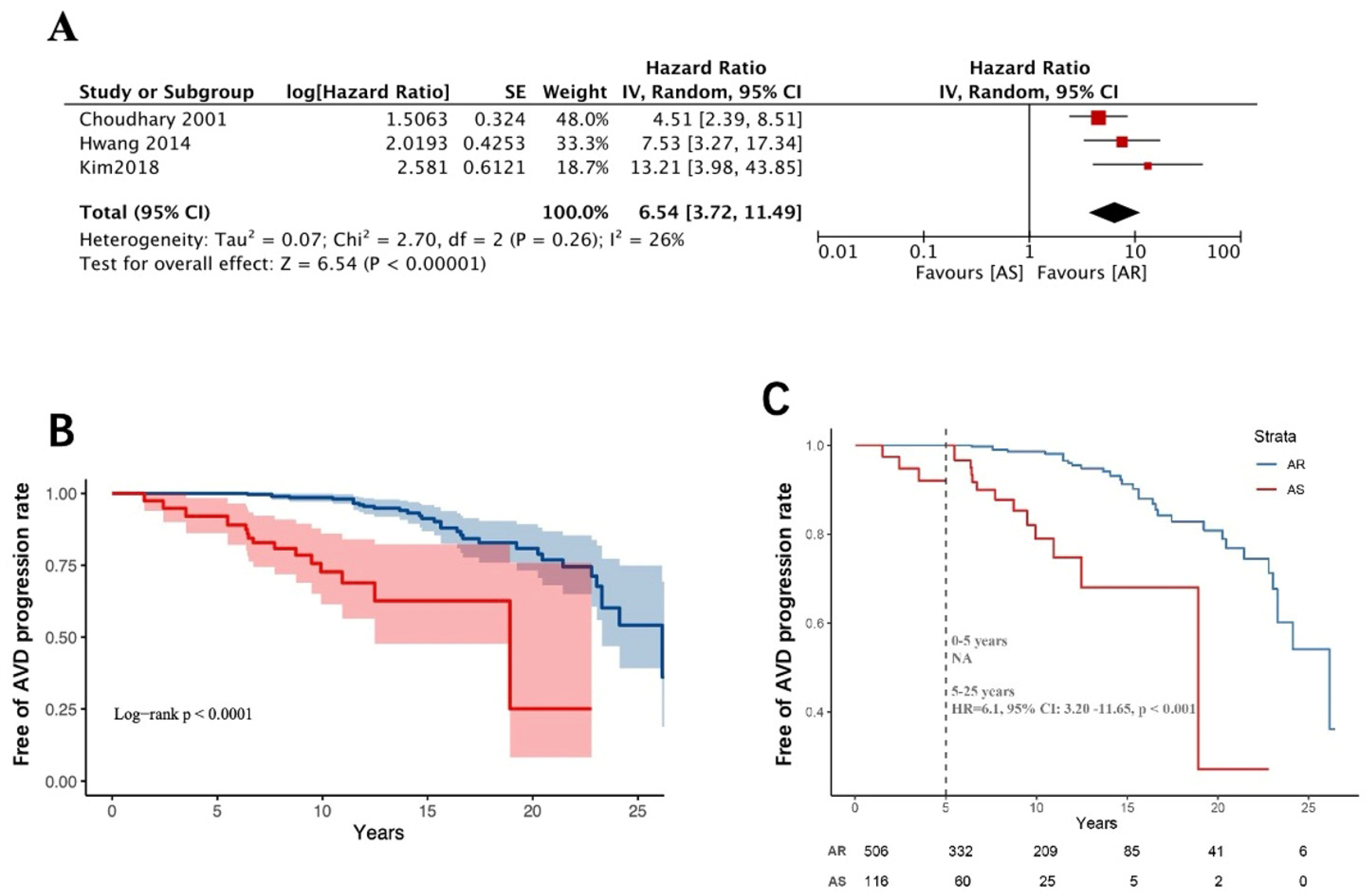
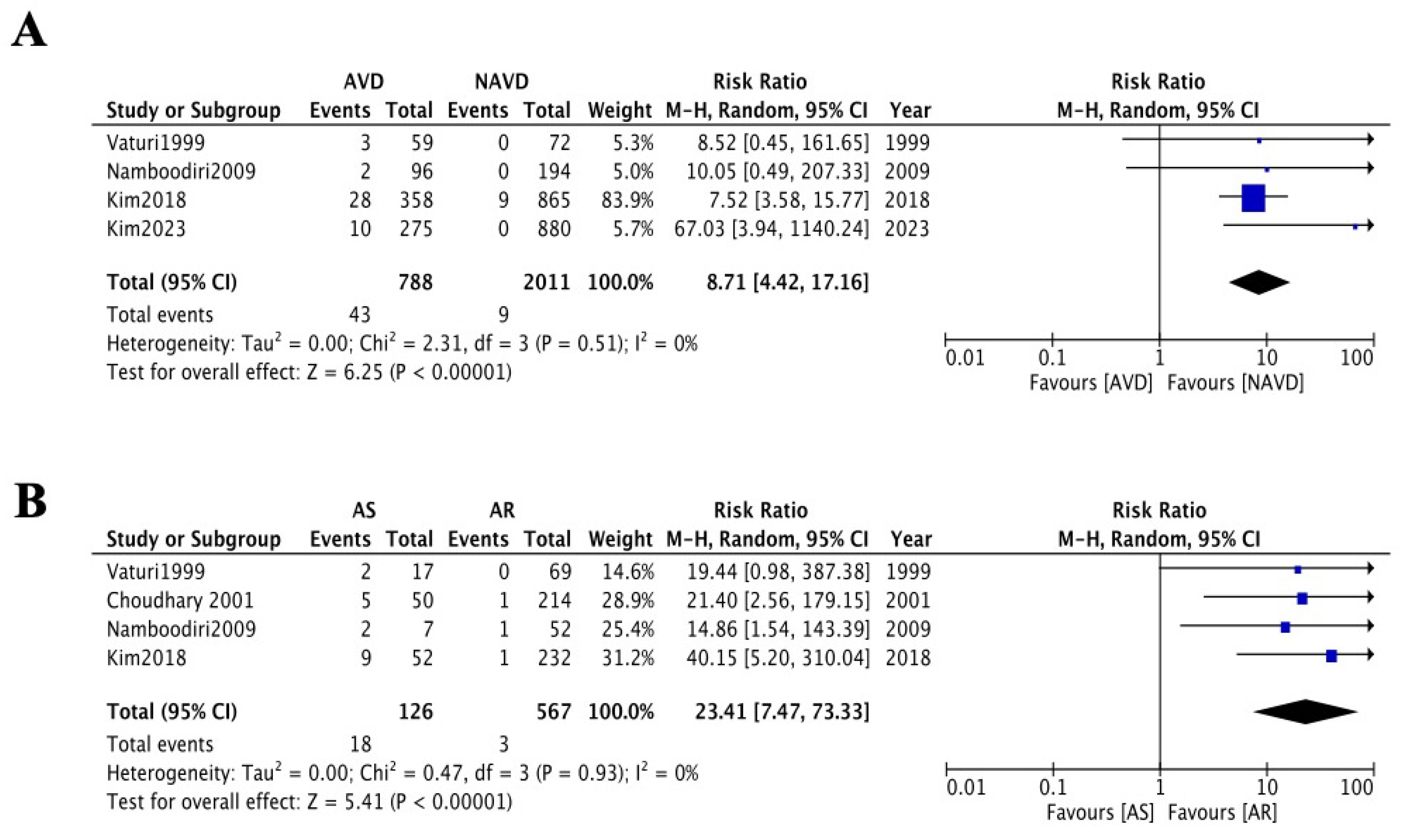
3.3. Worsening of Baseline Aortic Valve Dysfunction Above Moderate Levels
3.4. Long-Term Follow-Up of Aortic Valve-Related Reoperation Rates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| RHD | Rheumatic heart disease |
| RCT | Randomized controlled trials |
| AVD | Aortic valve disease |
| CI | Confidence intervals |
| HR | Hazard ratios |
| AVR | Aortic valve replacement |
| NAVD | Non-aortic valve disease |
| AS | Aortic stenosis |
| AR | Aortic regurgitation |
References
- Ruan, R.; Liu, X.; Zhang, Y.; Tang, M.; He, B.; Zhang, Q.W.; Shu, T. Global, Regional, and National Advances Toward the Management of Rheumatic Heart Disease Based on the Global Burden of Disease Study 2019. J. Am. Hear. Assoc. 2023, 12, e028921. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Ntsekhe, M.; Islam, S.; Rangarajan, S.; Avezum, A.; Benz, A.; Cabral, T.T.J.; Changsheng, M.; Chillo, P.; Gonzalez-Hermosillo, J.A.; et al. Mortality and Morbidity in Adults with Rheumatic Heart Disease. J. Am. Med. Assoc. 2024, 332, 133–140. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Guyton, R.A., 3rd; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, e521–e643. [Google Scholar] [CrossRef]
- Vaturi, M.; Porter, A.; Adler, Y.; Shapira, Y.; Sahar, G.; Vidne, B.; Sagie, A. The natural history of aortic valve disease after mitral valve surgery. J. Am. Coll. Cardiol. 1999, 33, 2003–2008. [Google Scholar] [CrossRef]
- Namboodiri, N.; Remash, K.; Tharakan, J.A.; Shajeem, O.; Nair, K.; Titus, T.; Ajitkumar, V.K.; Sivasankaran, S.; Krishnamoorthy, K.M.; Harikrishnan, S.P.; et al. Natural history of aortic valve disease following intervention for rheumatic mitral valve disease. J. Heart Valve Dis. 2009, 18, 61–67. [Google Scholar]
- Hwang, H.Y.; Kim, K.H.; Ahn, H. Attitude after a mild aortic valve lesion during rheumatic mitral valve surgery. J. Thorac. Cardiovasc. Surg. 2014, 147, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Choi, S.H.; Chang, B.C.; Nam, C.M.; Jang, Y.; Chung, N.; Shim, W.-H.; Cho, S.-Y.; Kim, S.-S. Is prophylactic aortic valve replacement indicated duringmitral valve surgery for mild to moderate aortic valve disease? Ann. Thorac. Surg. 2002, 74, 1115–1119. [Google Scholar] [CrossRef]
- Kim, D.J.; Joo, H.C.; Lee, S.H.; Chang, B.C.; Lee, S. Natural history of mild aortic valve disease untreated at the time of rheumatic mitral valve replacement. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Kim, W.K.; Kim, H.J.; Kim, J.B.; Jung, S.H.; Choo, S.J.; Chung, C.H.; Lee, J.W. The fate of aortic valve after rheumatic mitral valve surgery. J. Thorac. Cardiovasc. Surg. 2023, 165, 622–629.e2. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.H.Q.; Pham, T.T.T. Aortic regurgitation progression after mitral valve replacement for rheumatic heart disease. Asian Cardiovasc. Thorac. Ann. 2023, 31, 188–193. [Google Scholar] [CrossRef]
- Choudhary, S.K.; Talwar, S.; Juneja, R.; Kumar, A.S. Fate of mild aortic valve disease after mitral valve intervention. J. Thorac. Cardiovasc. Surg. 2001, 122, 583–586. [Google Scholar] [CrossRef][Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Guyton, R.A.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2438–2488. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a ComprehensiveTransthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Mathur, M.B.; Ding, P.; Riddell, C.A.; VanderWeele, T.J. Web Site and R Package for Computing E-values. Epidemiology 2018, 29, e45–e47. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: Reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2021, 21, 111. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Morgan, C.J. Landmark analysis: A primer. J. Nucl. Cardiol. 2019, 26, 391–393. [Google Scholar] [CrossRef]
- Caldonazo, T.; Hagel, S.; Doenst, T.; Kirov, H.; Sá, M.P.; Jacquemyn, X.; Tasoudis, P.; Franz, M.; Diab, M. Conservative Versus Surgical Therapy in Patients with Infective Endocarditis and Surgical Indication—Meta-Analysis of Reconstructed Time--to--Event Data. J. Am. Heart Assoc. 2024, 13, e033404. [Google Scholar] [CrossRef]
- Wilson, M.G.; Lubschez, R. Longevity in Rheumatic Fever: Based on the Experience of 1,042 Children Observed over a Period of Thirty Years. J. Am. Med. Assoc. 1948, 138, 794–798. [Google Scholar] [CrossRef]
- Bland, E.F.; Duckett Jones, T. Rheumatic fever and rheumatic heart disease; a twenty year report on 1000 patients followed since childhood. Circulation 1951, 4, 836–843. [Google Scholar] [CrossRef]
- Bernal, J.M.; Fernández-Vals, M.; Rabasa, J.M.; Gutiérrez-García, F.; Morales, C.; Revuelta, J.M. Repair of nonsevere rheumatic aortic valve disease during other valvular procedures: Is it safe? J. Thorac. Cardiovasc. Surg. 1998, 115, 1130–1135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wagner, S.; Selzer, A. Patterns of progression of aortic stenosis: A longitudinal hemodynamic study. Circulation 1982, 65, 709–712. [Google Scholar] [CrossRef]
- Horstkotte, D.; Loogen, F. The natural history of aortic valve stenosis. Eur. Heart. J. 1988, 9 (Suppl. E), 57–64. [Google Scholar] [CrossRef]
- Osnabrugge, R.L.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, A.J.; Piazza, N.; Kappetein, A.P. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef]
- Maurer, G. Aortic regurgitation. Heart 2006, 92, 994–1000. [Google Scholar] [CrossRef]
- Singh, J.P.; Evans, J.C.; Levy, D.; Larson, M.G.; Freed, L.A.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am. J. Cardiol. 1999, 83, 897–902. [Google Scholar] [CrossRef]
- Carapetis, J.R.; McDonald, M.; Wilson, N.J. Acute rheumatic fever. Lancet 2005, 366, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Ito, S. Severity of Aortic Stenosis: A Moving Target. J. Am. Coll. Cardiol. 2022, 80, 677–680. [Google Scholar] [CrossRef]
- Joseph, J.; Naqvi, S.Y.; Giri, J.; Goldberg, S. Aortic Stenosis: Pathophysiology, Diagnosis, and Therapy. Am. J. Med. 2017, 130, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Baumbach, A.; Patel, K.P.; Rudolph, T.K.; Delgado, V.; Treede, H.; Tamm, A.R. Aortic regurgitation: From mechanisms to management. EuroIntervention 2024, 20, e1062–e1075. [Google Scholar] [CrossRef] [PubMed]
- Akinseye, O.A.; Pathak, A.; Ibebuogu, U.N. Aortic Valve Regurgitation: A Comprehensive Review. Curr. Probl. Cardiol. 2018, 43, 315–334. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B. Valvular heart disease. Man. Cardiovasc. Med. 2013, 1, 238–355. [Google Scholar]
- Manzo, R.; Ilardi, F.; Nappa, D.; Mariani, A.; Angellotti, D.; Immobile Molaro, M.; Sgherzi, G.; Castiello, D.S.; Simonetti, F.; Santoro, C.; et al. Echocardiographic Evaluation of Aortic Stenosis: A Comprehensive Review. Diagnostics 2023, 13, 2527. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Study Period | Study Type | AVD/NAVD | AS/AR | Mean Follow-Up Years | Primary and Endpoints | NOS |
|---|---|---|---|---|---|---|---|---|
| Vaturi 1999 [4] | Israel | 1975–1992 | Retrospective cohort | 59/72 | —— | 13 ± 7 years | Progression of AVD and reoperation rates in long-term follow-up | 6 |
| Choudhary 2001 [11] | India | 1979–1997 | Retrospective cohort | —— | 52/232 | 10.8 ± 3.7 years | Progression of AVD and reoperation rates in long-term follow-up | 7 |
| Namboodiri 2009 [5] | India | 1994–1996 | Retrospective cohort | 96/194 | 17/69 | 11.98 ± 6.4 years | Progression of AVD and reoperation rates in long-term follow-up | 5 |
| Hwang 2014 [6] | Korea | 1992–2010 | Retrospective cohort | —— | 7/104 | 11.8 years | Reoperation rates in long-term follow-up | 7 |
| Kim 2018 [8] | Korea | 1990–2015 | Retrospective cohort | 358/865 | 66/292 | 11.2 ± 7.3 years | Progression of AVD and reoperation rates in long-term follow-up | 7 |
| Kim 2023 [9] | Korea | 1997–2015 | Retrospective cohort | 275/880 | —— | 142.1 months | Progression of AVD and reoperation rates in long-term follow-up | 7 |
| Variable | AVD | NAVD | p | RR/MD |
|---|---|---|---|---|
| Number of patients | 618 | 999 | / | / |
| Sex, female | 438 | 699 | 0.704 | 0.365 |
| Mean age, years | 50.7 ± 13.4 | 50.1 ± 12.2 | 0.355 | 1.013 (0.949, 1.081) |
| Atrial fibrillation | 556 | 912 | 0.373 | 0.986 (0.957, 1.016) |
| NYHA class ≥ 3 | 399 | 760 | <0.001 | 0.849 (0.793, 0.908) |
| Associated diseases | ||||
| Diabetes mellitus | 45 | 53 | 0.105 | 1.373 (0.935, 2.016) |
| Hypertension | 36 | 81 | 0.085 | 0.718 (0.492, 1.050) |
| Coronary artery disease | 17 | 30 | 0.772 | 0.916 (0.510, 1.647) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.; Qin, X.; Yue, H.; Liang, W.; Wu, Z. Progression of Untreated Mild Aortic Valve Disease in Patients Undergoing Rheumatic Mitral Valve Surgery: A Meta-Analysis of Reconstructed Time-to-Event Data. J. Cardiovasc. Dev. Dis. 2025, 12, 426. https://doi.org/10.3390/jcdd12110426
Luo C, Qin X, Yue H, Liang W, Wu Z. Progression of Untreated Mild Aortic Valve Disease in Patients Undergoing Rheumatic Mitral Valve Surgery: A Meta-Analysis of Reconstructed Time-to-Event Data. Journal of Cardiovascular Development and Disease. 2025; 12(11):426. https://doi.org/10.3390/jcdd12110426
Chicago/Turabian StyleLuo, Chong, Xiaoli Qin, Honghua Yue, Weitao Liang, and Zhong Wu. 2025. "Progression of Untreated Mild Aortic Valve Disease in Patients Undergoing Rheumatic Mitral Valve Surgery: A Meta-Analysis of Reconstructed Time-to-Event Data" Journal of Cardiovascular Development and Disease 12, no. 11: 426. https://doi.org/10.3390/jcdd12110426
APA StyleLuo, C., Qin, X., Yue, H., Liang, W., & Wu, Z. (2025). Progression of Untreated Mild Aortic Valve Disease in Patients Undergoing Rheumatic Mitral Valve Surgery: A Meta-Analysis of Reconstructed Time-to-Event Data. Journal of Cardiovascular Development and Disease, 12(11), 426. https://doi.org/10.3390/jcdd12110426







