Trends in Conventional Heart Failure Therapy in a Real-World Multinational ATTR-CA Cohort
Abstract
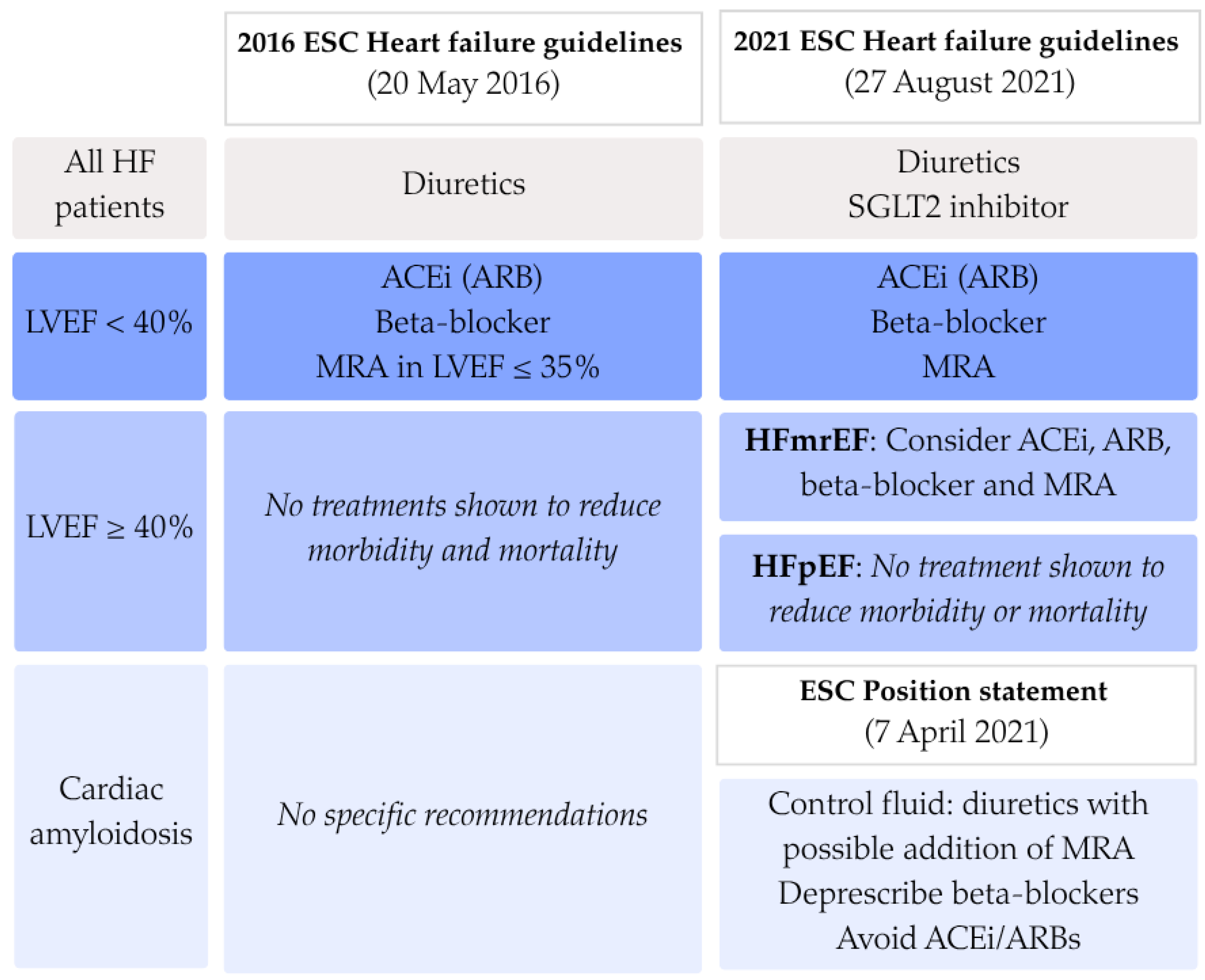
1. Introduction
2. Materials and Methods
3. Results
3.1. Prescription Patterns
3.1.1. Beta-Blockers
3.1.2. Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers
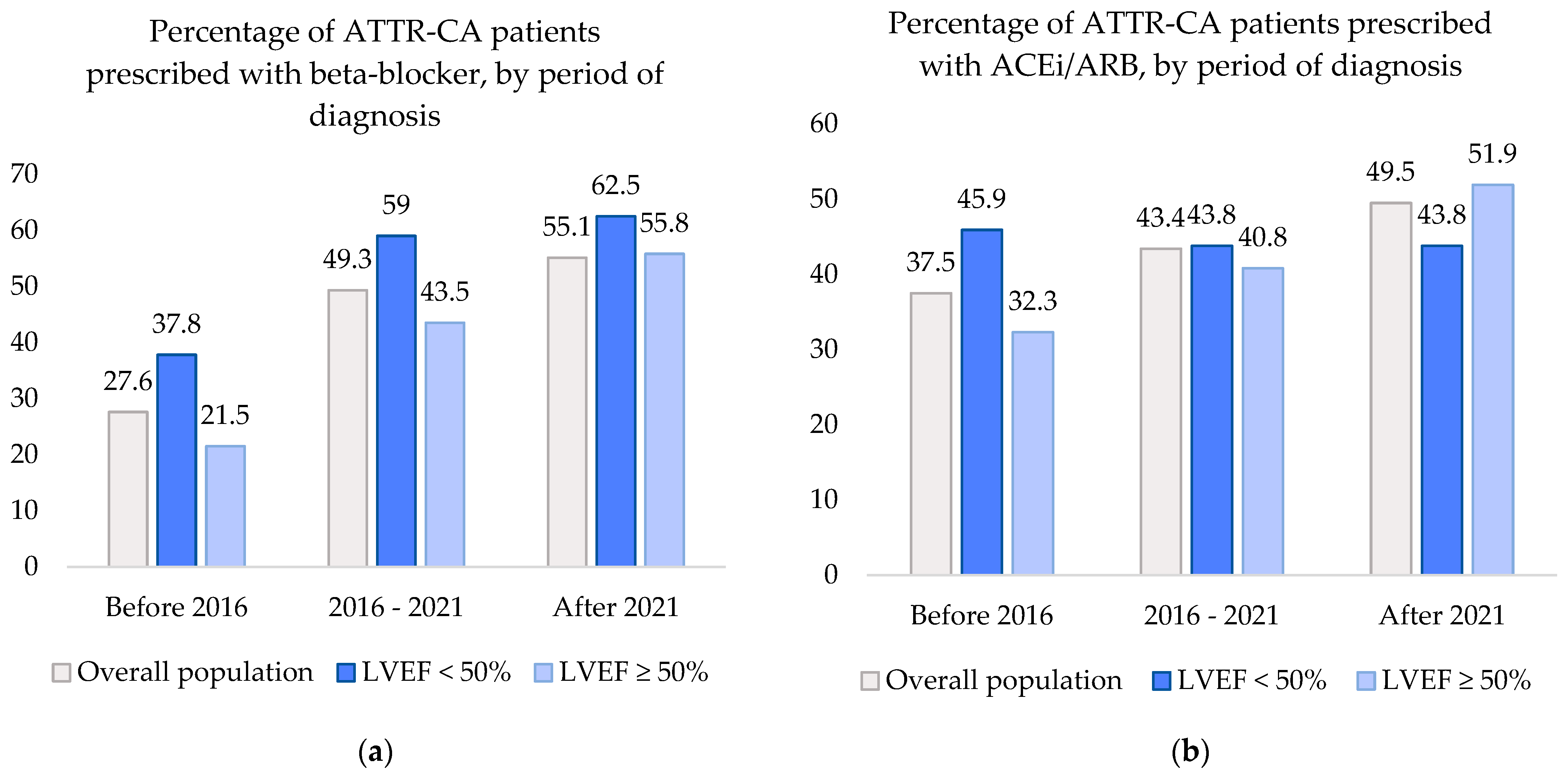
3.2. Trends in Prescription Patterns
3.2.1. Beta-Blocker and ACEi/ARB Prescription
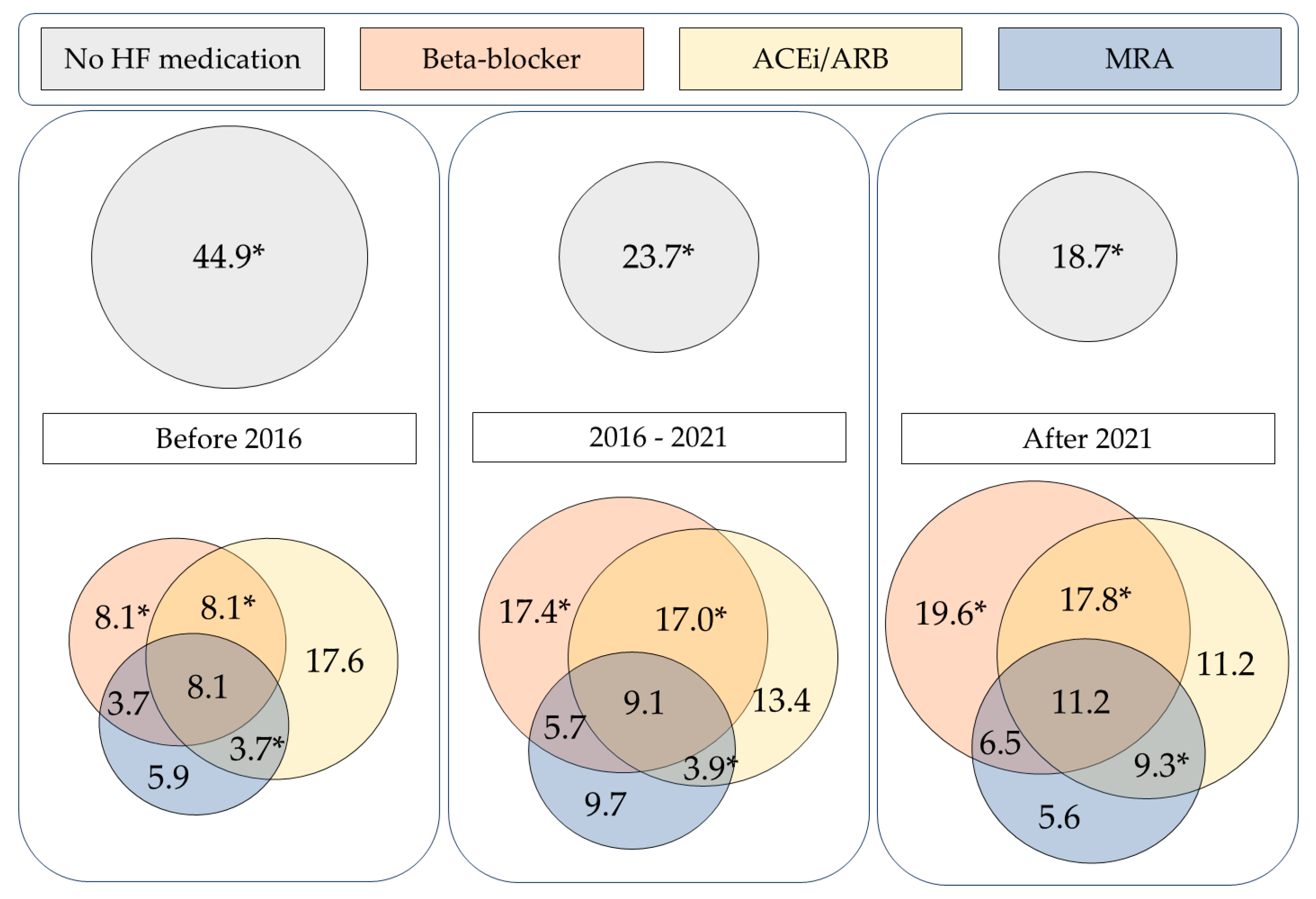
3.2.2. Combination Heart Failure Therapy
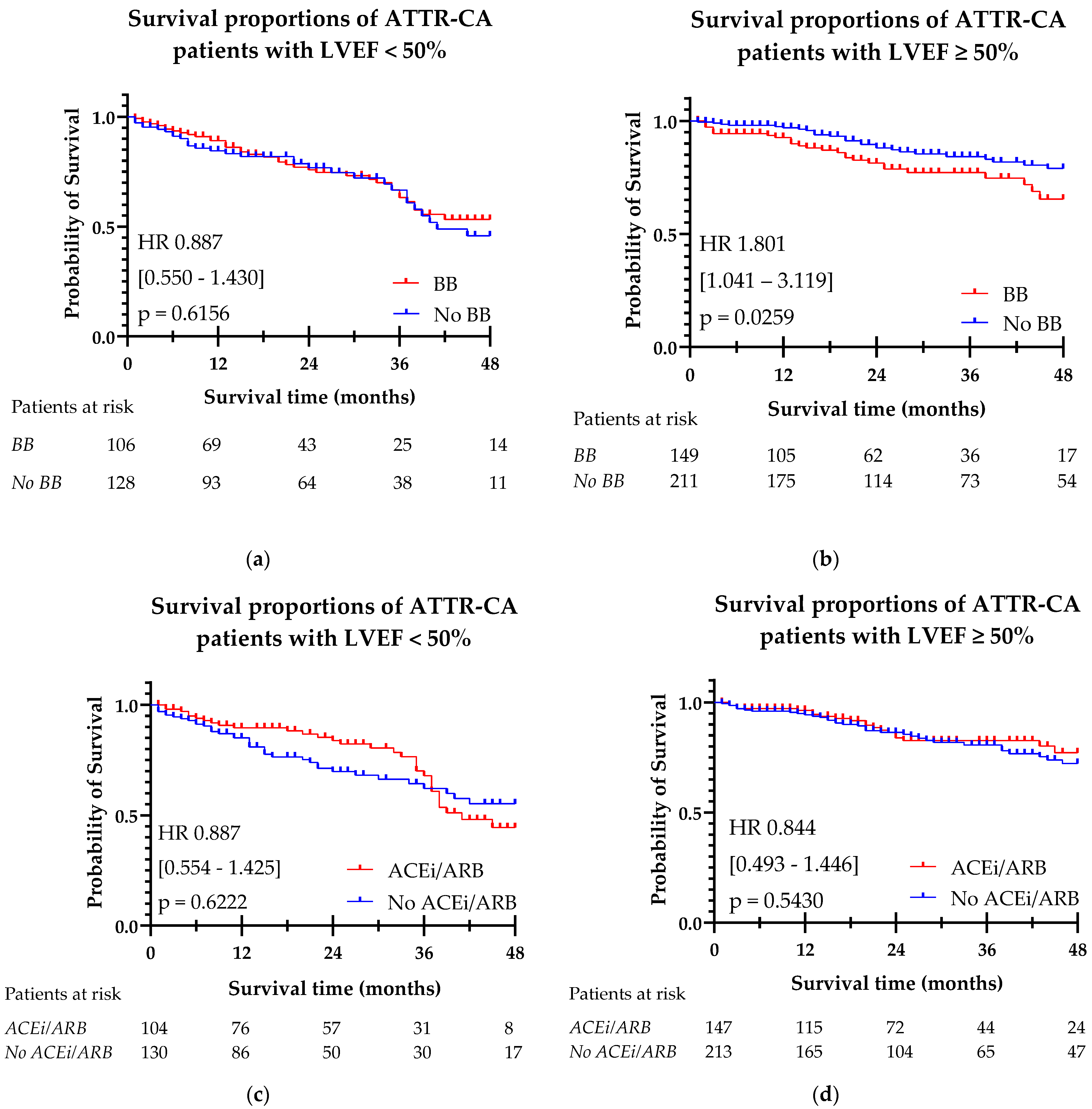
3.3. Heart Failure Medication and Survival
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gentile, L.; Coelho, T.; Dispenzieri, A.; Conceição, I.; Waddington-Cruz, M.; Kristen, A.; Wixner, J.; Diemberger, I.; Gonzalez-Moreno, J.; Cariou, E.; et al. A 15-year consolidated overview of data in over 6000 patients from the Transthyretin Amyloidosis Outcomes Survey (THAOS). Orphanet J. Rare Dis. 2023, 18, 350. [Google Scholar] [CrossRef]
- Ioannou, A.; Patel, R.K.; Razvi, Y.; Porcari, A.; Sinagra, G.; Venneri, L.; Bandera, F.; Masi, A.; Williams, G.E.; O’bEara, S.; et al. Impact of Earlier Diagnosis in Cardiac ATTR Amyloidosis Over the Course of 20 Years. Circulation 2022, 146, 1657–1670. [Google Scholar] [CrossRef]
- Teresi, L.; Trimarchi, G.; Liotta, P.; Restelli, D.; Licordari, R.; Carciotto, G.; Francesco, C.; Crea, P.; Dattilo, G.; Micari, A.; et al. Electrocardiographic Patterns and Arrhythmias in Cardiac Amyloidosis: From Diagnosis to Therapeutic Management-A Narrative Review. J. Clin. Med. 2024, 13, 5588. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Judge, D.P.; Cappelli, F.; Fontana, M.; Garcia-Pavia, P.; Gibbs, S.; Grogan, M.; Hanna, M.; Hoffman, J.; Masri, A.; et al. Efficacy and Safety of Acoramidis in Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2024, 390, 132–142. [Google Scholar] [CrossRef]
- Fontana, M.; Berk, J.L.; Gillmore, J.D.; Witteles, R.M.; Grogan, M.; Drachman, B.; Damy, T.; Garcia-Pavia, P.; Taubel, J.; Solomon, S.D.; et al. Vutrisiran in Patients with Transthyretin Amyloidosis with Cardiomyopathy. N. Engl. J. Med. 2025, 392, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.M.; Ruberg, F.L.; Ambardekar, A.V.; Brannagan, T.H.; Cheng, R.K.; Clarke, J.O.; Dember, L.M.; Frantz, J.G.; Hershberger, R.E.; Maurer, M.S.; et al. 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient With Cardiac Amyloidosis: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2023, 81, 1076–1126. [Google Scholar] [CrossRef]
- Ioannou, A.; Massa, P.; Patel, R.K.; Razvi, Y.; Porcari, A.; Rauf, M.U.; Jiang, A.; Cabras, G.; Filisetti, S.; Bolhuis, R.; et al. Conventional heart failure therapy in cardiac ATTR amyloidosis. Eur. Heart J. 2023, 44, 2893–2907. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Damy, T.; FontAna, M.; Hutchinson, M.; Lachmann, H.J.; Martinez-Naharro, A.; Quarta, C.C.; Rezk, T.; Whelan, C.J.; Gonzalez-Lopez, E.; et al. A new staging system for cardiac transthyretin amyloidosis. Eur. Heart J. 2018, 39, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Phelan, D.; Collier, P.; Thavendiranathan, P.; Popović, Z.B.; Hanna, M.; Plana, J.C.; Marwick, T.H.; Thomas, J.D. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012, 98, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Malgie, J.; Wilde, M.I.; Clephas, P.R.; Emans, M.E.; Koudstaal, S.; Schaap, J.; Mosterd, A.; van Ramshorst, J.; Wardeh, A.J.; van Wijk, S.; et al. Contemporary guideline-directed medical therapy in de novo, chronic, and worsening heart failure patients: First data from the TITRATE-HF study. Eur. J. Heart Fail. 2024, 26, 1549–1560. [Google Scholar] [CrossRef]
- Ramsell, S.; Bermudez, C.A.; Baiyee, C.A.M.T.; Rodgers, B.; Parikh, S.; Almaani, S.; Sharma, N.; LoRusso, S.; Freimer, M.; Redder, E.; et al. Beta-Adrenergic Antagonist Tolerance in Amyloid Cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 907597. [Google Scholar] [CrossRef]
- Negreira-Caamano, M.; Río, J.M.-D.; Morón-Alguacil, A.; Pérez-Díaz, P.; Piqueras-Flores, J. Starting sacubitril-valsartan is safe in patients with transthyretin cardiac amyloidosis and impaired ejection fraction. Rev. Port. Cardiol. 2023, 42, 183–186. [Google Scholar] [CrossRef]
- Foa, A.; Vaduganathan, M.; Claggett, B.L.; Pabon, M.A.; Lu, H.; Pfeffer, M.A.; Packer, M.; Vardeny, O.; Rouleau, J.L.; Lefkowitz, M.; et al. Sacubitril/Valsartan-Related Hypotension in Patients With Heart Failure and Preserved or Mildly Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2024, 83, 1731–1739. [Google Scholar] [CrossRef]
- Porcari, A.; Cappelli, F.; Nitsche, C.; Tomasoni, D.; Sinigiani, G.; Longhi, S.; Bordignon, L.; Masri, A.; Serenelli, M.; Urey, M.; et al. SGLT2 Inhibitor Therapy in Patients With Transthyretin Amyloid Cardiomyopathy. J. Am. Coll. Cardiol. 2024, 83, 2411–2422. [Google Scholar] [CrossRef]
- Sivamurugan, A.; Byer, S.H.; Grewal, U.S.; Dominic, P. Impact of SGLT2 inhibitors on the outcomes of patients with cardiac arrhythmias and transthyretin cardiac amyloidosis. Heart Rhythm. 2024, 22, 1615–1616. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.K.; Vasbinder, A.; Levy, W.C.; Goyal, P.; Griffin, J.M.; Leedy, D.J.; Maurer, M.S. Lack of Association Between Neurohormonal Blockade and Survival in Transthyretin Cardiac Amyloidosis. J. Am. Heart Assoc. 2021, 10, e022859. [Google Scholar] [CrossRef]
- Barge-Caballero, G.; Barge-Caballero, E.; López-Pérez, M.; Bilbao-Quesada, R.; González-Babarro, E.; Gómez-Otero, I.; López-López, A.; Gutiérrez-Feijoo, M.; Varela-Román, A.; González-Juanatey, C.; et al. Beta-Blocker Exposure and Survival in Patients With Transthyretin Amyloid Cardiomyopathy. Mayo Clin. Proc. 2022, 97, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Choy, C.H.; Pinney, J.; Townend, J.N.; Whelan, C.; Fontana, M.; Gillmore, J.D.; Steeds, R.P.; Moody, W.E. Effect of beta-blockade on mortality in patients with cardiac amyloidosis: A systematic review and meta-analysis. ESC Heart Fail. 2024, 11, 3901–3910. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Vergaro, G.; Castiglione, V.; Rapezzi, C.; Emdin, M. Safety and Tolerability of Neurohormonal Antagonism in Cardiac Amyloidosis. Eur. J. Intern. Med. 2020, 80, 66–72. [Google Scholar] [CrossRef] [PubMed]
| Overall Population (n = 794) | LVEF < 50% (n = 249, 38.5%) | LVEF ≥ 50% (n = 397, 61.5%) | p-Value | |
|---|---|---|---|---|
| Sex (male) | 581 (73.2%) | 200 (80.3%) | 290 (73.0%) | 0.036 |
| Age | 78 (66–83) | 80 (73–84) | 78 (64–83) | 0.001 |
| AF/flutter in history | 329 (46.7%) | 142 (62.8%) | 137 (40.5%) | <0.001 |
| CAD | 178 (22.4%) | 68 (27.3%) | 87 (22.0%) | 0.122 |
| DM | 121 (15.3%) | 49 (19.8%) | 52 (13.2%) | 0.025 |
| Hypertension | 417 (52.8%) | 128 (51.8%) | 224 (56.7%) | 0.206 |
| Stroke/TIA | 87 (13.4%) | 35 (17.7%) | 41 (13.1%) | 0.157 |
| NYHA class | <0.001 | |||
| I | 217 (30.6%) | 36 (14.9%) | 120 (33.6%) | |
| II | 336 (47.4%) | 132 (54.8%) | 174 (48.7%) | |
| III | 138 (19.5%) | 66 (27.4%) | 53 (14.8%) | |
| IV | 18 (2.5) | 7 (2.9%) | 10 (2.8%) | |
| NAC stage | <0.001 | |||
| I | 319 (59.6%) | 55 (34.8%) | 189 (65.9%) | |
| II | 124 (23.2%) | 54 (34.2%) | 60 (20.9%) | |
| III | 92 (17.2%) | 49 (31.0%) | 38 (13.2%) | |
| NT-proBNP (ng/L) | 1508 (347–4009) | 1782 (3650–6918) | 1212 (312–3265) | <0.001 |
| HS Troponin (ng/L) | 48 (28–82) | 57 (41–100) | 40 (21–68) | <0.001 |
| eGFR (mL/min/1.73 m2) | 65.0 ± 25.1 | 55.6 ± 20.8 | 67.9 ± 24.0 | <0.001 |
| CKD stage 3–5 | 319 (43.3%) | 135 (57.7%) | 147 (38.5%) | <0.001 |
| Systolic blood pressure (mmHg) | 127.5 ± 21.4 | 124.6 ± 21.8 | 129.2 ± 21.0 | 0.012 |
| Diastolic blood pressure (mmHg) | 73.8 ± 13.2 | 74.0 ± 14.3 | 73.5 ± 12.6 | 0.640 |
| Electrocardiography | ||||
| Rhythm at baseline | 0.003 | |||
| Sinus rhythm | 470 (59.9%) | 124 (50.2%) | 253 (64.9%) | |
| AF/flutter | 248 (31.6%) | 100 (40.5%) | 114 (29.2%) | |
| Paced rhythm | 62 (7.9%) | 22 (8.9%) | 21 (5.4%) | |
| Heart rate (bpm) | 74.7 ± 15.9 | 76.1 ± 18.3 | 73.1 ± 13.9 | 0.028 |
| Conduction abnormalities | <0.001 | |||
| LBBB | 125 (18.0%) | 56 (26.0%) | 59 (16.6%) | |
| RBBB | 94 (13.5%) | 32 (14.9%) | 49 (13.8%) | |
| Aspecific IV conduction delay | 46 (6.6%) | 25 (11.6%) | 16 (13.8%) | |
| Low voltages | 156 (21.2%) | 63 (28.3%) | 70 (18.8%) | 0.007 |
| Signs of LVH | 68 (9.3%) | 31 (14.1%) | 24 (6.5%) | 0.002 |
| Echocardiography | ||||
| LVEF (%) | 52.1 ± 12.2 | 39.5 ± 7.9 | 60.0 ± 6.5 | <0.001 |
| IVSd (mm) | 15.6 ± 4.3 | 17.1 ± 3.6 | 15.4 ± 4.2 | <0.001 |
| PWT (mm) | 14.1 ± 4.0 | 15.3 ± 3.5 | 14.1 ± 4.0 | <0.001 |
| LAVI (mL/m2) | 49.3 ± 24.3 | 55.9 ± 29.1 | 46.6 ± 20.0 | <0.001 |
| Indexed stroke volume (mL/m2) | 31.6 ± 11.0 | 26.8 ± 8.8 | 34.9 ± 11.2 | <0.001 |
| LV GLS (%) | −13.3 ± 4.7 | −9.7 ± 2.9 | −14.6 ± 4.2 | <0.001 |
| RELAS | 2.0 ± 0.9 | 2.0 ± 1.0 | 2.1 ± 0.8 | 0.898 |
| TAPSE | 18.2 ± 5.2 | 15.6 ± 4.3 | 19.6 ± 10.9 | <0.001 |
| RA area (cm2) | 20.8 ± 6.6 | 23.0 ± 6.6 | 19.7 ± 6.2 | <0.001 |
| E/e’ | 17.5 ± 10.2 | 19.8 ± 10.9 | 16.5 ± 9.6 | <0.001 |
| Medication | ||||
| Beta-blocker | 346 (43.7%) | 139 (56.0%) | 161 (40.8%) | <0.001 |
| ACEi/ARB | 326 (41.2%) | 109 (44.0%) | 157 (39.7%) | 0.292 |
| Diuretics | 418 (52.8%) | 180 (72.9%) | 175 (44.2%) | <0.001 |
| MRA | 204 (25.8%) | 88 (35.5%) | 87 (22.0%) | <0.001 |
| Disease-modifying treatment | 315 (44.9%) | 95 (43.4%) | 169 (47.6%) | 0.324 |
| Patients Treated with Beta-Blockers (n = 346, 43.7%) | Patients Not Treated with Beta-Blockers (n = 445, 56.3%) | p-Value | Patients Treated with ACEi/ARBs (n = 326, 41.2%) | Patients Not Treated with ACEi/ARBs (n = 465, 58.8%) | p-Value | |
|---|---|---|---|---|---|---|
| Sex (male) | 262 (75.5%) | 316 (71.0%) | 0.138 | 254 (77.9%) | 324 (69.7%) | 0.010 |
| Age | 81 (76–86) | 80 (72–84) | <0.001 | 81 (77–84) | 80 (72–85) | <0.001 |
| AF/flutter | 179 (60.3%) | 149 (36.8%) | <0.001 | 157 (54.0%) | 171 (41.6%) | 0.001 |
| CAD | 98 (28.3%) | 80 (18.0%) | <0.001 | 90 (27.6%) | 88 (18.9%) | 0.004 |
| DM | 66 (19.2%) | 55 (12.4%) | <0.001 | 58 (17.8%) | 63 (13.6%) | 0.104 |
| Hypertension | 212 (61.4%) | 203 (45.8%) | <0.001 | 233 (71.7%) | 182 (39.3%) | <0.001 |
| Stroke/TIA | 47 (17.7%) | 40 (10.4%) | 0.008 | 43 (16.6%) | 44 (11.3%) | 0.051 |
| NYHA class | <0.001 | 0.108 | ||||
| I | 69 (21.4%) | 148 (38.5%) | 81 (26.7%) | 136 (33.7%) | ||
| II | 170 (52.8%) | 163 (42.4%) | 147 (48.5%) | 186 (46.2%) | ||
| III | 73 (22.7%) | 65 (16.9%) | 64 (21.1%) | 74 (18.4%) | ||
| IV | 10 (3.1%) | 8 (2.1%) | 11 (3.1%) | 7 (1.7%) | ||
| NAC stage | <0.001 | 0.048 | ||||
| I | 109 (48.0%) | 207 (67.9) | 116 (53.5%) | 200 (63.5%) | ||
| II | 54 (23.8%) | 70 (23.0%) | 61 (28.1%) | 63 (20.0%) | ||
| III | 64 (28.2%) | 28 (9.2%) | 40 (18.4%) | 52 (16.5%) | ||
| NT-proBNP (ng/L) | 3195 (1207–6932) | 1882 (768–3892) | <0.001 | 2796 (1074–5917) | 2285 (901–5106) | <0.001 |
| HS Troponin (ng/L) | 49 (31–92) | 42 (22–67) | <0.001 | 49 (34–88) | 46 (24–82) | 0.062 |
| eGFR (mL/min/1.73 m2) | 57.8 ± 22.6 | 71.5 ± 25.3 | <0.001 | 60.8 ± 22.1 | 68.6 ± 26.6 | <0.001 |
| CKD stage 3–5 | 182 (55.8%) | 235 (33.1%) | <0.001 | 153 (50.7%) | 164 (38.0%) | <0.001 |
| Systolic blood pressure (mmHg) | 128.9 ± 23.1 | 126.3 ± 19.9 | 0.139 | 129.2 ± 21.7 | 126.3 ± 21.2 | 0.095 |
| Diastolic blood pressure (mmHg) | 73.9 ± 14.1 | 73.8 ± 16.9 | 0.819 | 74.3 ± 12.3 | 73.4 ± 13.8 | 0.399 |
| Electrocardiographic parameters | ||||||
| Rhythm at baseline | 0.097 | 0.116 | ||||
| Sinus rhythm | 189 (55.6%) | 278 (63.0%) | 183 (56.7%) | 284 (62.0%) | ||
| AF/flutter | 124 (36.5%) | 124 (28.1%) | 114 (35.3%) | 134 (29.3%) | ||
| Paced rhythm | 25 (7.4%) | 37 (8.4%) | 26 (8.0%) | 36 (7.9%) | ||
| Heart rate (bpm) | 73.8 ± 16.9 | 75.4 ± 15.0 | 0.152 | 73.5 ± 15.3 | 75.6 ± 16.2 | 0.077 |
| Conduction abnormalities | 0.012 | 0.565 | ||||
| LBBB | 55 (18.4%) | 69 (17.5%) | 52 (18.7%) | 72 (17.3%) | ||
| RBBB | 53 (17.7%) | 41 (10.4%) | 42 (15.1%) | 52 (12.5%) | ||
| Aspecific IV conduction delay | 23 (7.7%) | 22 (5.6%) | 20 (7.2%) | 25 (6.0%) | ||
| Low voltages | 75 (23.1%) | 81 (19.9%) | 0.280 | 65 (21.6%) | 91 (21.1%) | 0.876 |
| Signs of LVH | 28 (8.7%) | 40 (9.9%) | 0.585 | 31 (10.3%) | 37 (8.6%) | 0.435 |
| Echocardiographic parameters | ||||||
| LVEF (%) | 50.0 ± 11.8 | 53.8 ± 12.2 | <0.001 | 51.2 ± 11.8 | 52.2 ± 12.4 | 0.164 |
| IVSd (mm) | 16.5 ± 3.8 | 15.0 ± 4.6 | <0.001 | 16.4 ± 3.8 | 15.1 ± 4.5 | <0.001 |
| PWT (mm) | 14.8 ± 4.0 | 13.6 ± 3.9 | <0.001 | 14.6 ± 4.1 | 13.8 ±3.9 | 0.008 |
| LAVI (mL/m2) | 53.3 ± 27.9 | 46.2 ± 20.6 | 0.001 | 52.5 ± 28.3 | 46.9 ± 20.6 | 0.011 |
| Indexed stroke volume (mL/m2) | 30.7 ± 11.4 | 32.3 ± 10.4 | 0.123 | 31.9 ± 11.4 | 31.2 ± 10.5 | 0.541 |
| LV GLS (%) | −12.2 ± 4.5 | −14.0 ± 4.7 | <0.001 | −12.4 ± 4.2 | −13.8 ± 4.9 | <0.001 |
| RELAS | 2.03 ± 0.97 | 2.02 ± 0.79 | 0.900 | 2.09 ± 0.94 | 1.98 ± 0.82 | 0.191 |
| TAPSE | 17.5 ± 5.0 | 18.8 ± 5.3 | 0.003 | 18.1 ± 5.1 | 18.3 ± 5.3 | 0.554 |
| RA area (cm2) | 21.7 ± 7.1 | 20.1 ± 6.1 | 0.008 | 21.7 ± 6.4 | 20.1 ± 6.6 | 0.010 |
| E/e’ | 18.6 ± 5.3 | 16.6 ± 9.0 | 0.022 | 17.9 ± 10.2 | 17.2 ± 10.3 | 0.448 |
| Medication | ||||||
| Diuretics | 234 (68.0%) | 183 (41.1%) | <0.001 | 218 (67.3%) | 199 (42.8%) | <0.001 |
| MRA | 108 (31.2%) | 96 (21.6%) | 0.002 | 102 (31.3%) | 102 (21.9%) | 0.003 |
| Disease-modifying treatment | 115 (38.3%) | 199 (49.9%) | 0.002 | 117 (41.6%) | 197 (47.1%) | 0.152 |
| Overall Population (n = 791) | Before 2016 (n = 136) | 2016–2021 (n = 493) | After 2021 (n = 107) | p-Value | |
|---|---|---|---|---|---|
| BB and ACE | 115 (14.5%) | 11 (8.1%) | 84 (17.0%) | 19 (17.8%) | 0.030 |
| BB and MRA | 40 (5.1%) | 5 (3.7%) | 28 (5.7%) | 7 (6.5%) | 0.568 |
| ACEi/ARB and MRA | 34 (4.3%) | 5 (3.7%) | 19 (3.9%) | 10 (9.3%) | 0.042 |
| BB, ACEi/ARB, and MRA | 68 (8.6%) | 11 (8.1%) | 45 (9.1%) | 12 (11.2%) | 0.698 |
| No use of BB, ACEi/ARB, or MRA | 240 (30.3%) | 61 (44.9%) | 117 (23.7%) | 20 (18.7%) | <0.001 |
| Beta-Blocker | ACEi/ARB | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age at diagnosis | 1.073 (1.028–1.120) | <0.001 | 1.074 (1.028–1.121) | 0.001 |
| AF | 1.977 (0.884–4.419) | 0.097 | 1.887 (0.851–4.184) | 0.118 |
| CAD | 1.131 (0.592–2.163) | 0.709 | 1.102 (0.580–2.096) | 0.767 |
| DM | 0.528 (0.203–1.374) | 0.191 | 0.526 (0.201–1.375) | 0.190 |
| Wild-type | 0.678 (0.678–0.193) | 0.544 | 0.669 (0.193–2.321) | 0.669 |
| NAC stage | ||||
| I vs. II | 1.009 (0.395–2.580) | 0.985 | 1.115 (0.447–2.782) | 0.816 |
| I vs. III | 4.702 (2.022–910.937) | <0.001 | 4.302 (1.873–9.882) | <0.001 |
| LV GLS | 00.928 (0.851–1.013) | 0.095 | 0.933 (0.857–1.016) | 0.111 |
| Beta-blocker | 0.666 (0.352–1.262) | 0.213 | - | - |
| ACEi/ARB | - | - | 0.878 (0.490–1.575) | 0.663 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Geest, E.H.; Ajmone Marsan, N.; Laenens, D.; Debonnaire, P.J.M.R.; Claeys, M.; Pinto, F.; Brito, D.; Donal, E.; Droogmans, S.; Van de Veire, N.; et al. Trends in Conventional Heart Failure Therapy in a Real-World Multinational ATTR-CA Cohort. J. Cardiovasc. Dev. Dis. 2025, 12, 403. https://doi.org/10.3390/jcdd12100403
van der Geest EH, Ajmone Marsan N, Laenens D, Debonnaire PJMR, Claeys M, Pinto F, Brito D, Donal E, Droogmans S, Van de Veire N, et al. Trends in Conventional Heart Failure Therapy in a Real-World Multinational ATTR-CA Cohort. Journal of Cardiovascular Development and Disease. 2025; 12(10):403. https://doi.org/10.3390/jcdd12100403
Chicago/Turabian Stylevan der Geest, Eva H., Nina Ajmone Marsan, Dorien Laenens, Philippe J. M. R. Debonnaire, Mathias Claeys, Fauto Pinto, Dulce Brito, Erwan Donal, Steven Droogmans, Nico Van de Veire, and et al. 2025. "Trends in Conventional Heart Failure Therapy in a Real-World Multinational ATTR-CA Cohort" Journal of Cardiovascular Development and Disease 12, no. 10: 403. https://doi.org/10.3390/jcdd12100403
APA Stylevan der Geest, E. H., Ajmone Marsan, N., Laenens, D., Debonnaire, P. J. M. R., Claeys, M., Pinto, F., Brito, D., Donal, E., Droogmans, S., Van de Veire, N., Bertrand, P., Nabeta, T., Graziani, F., & Regeer, M. V. (2025). Trends in Conventional Heart Failure Therapy in a Real-World Multinational ATTR-CA Cohort. Journal of Cardiovascular Development and Disease, 12(10), 403. https://doi.org/10.3390/jcdd12100403










