Transfemoral TAVI in a High-Risk Patient with Porcelain Aorta and Severe Subrenal Abdominal Aortic Stenosis: A Case Report
Abstract
1. Introduction
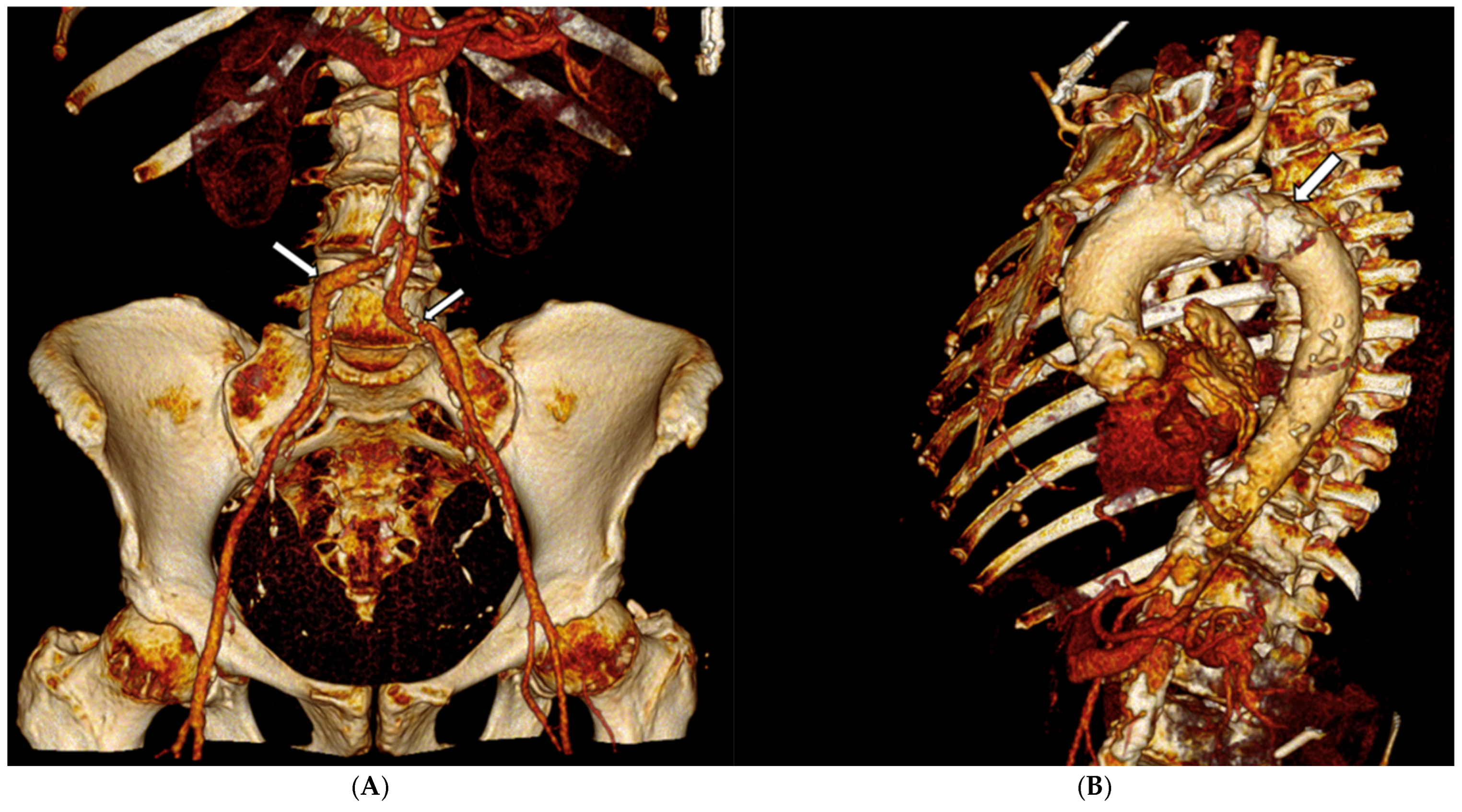
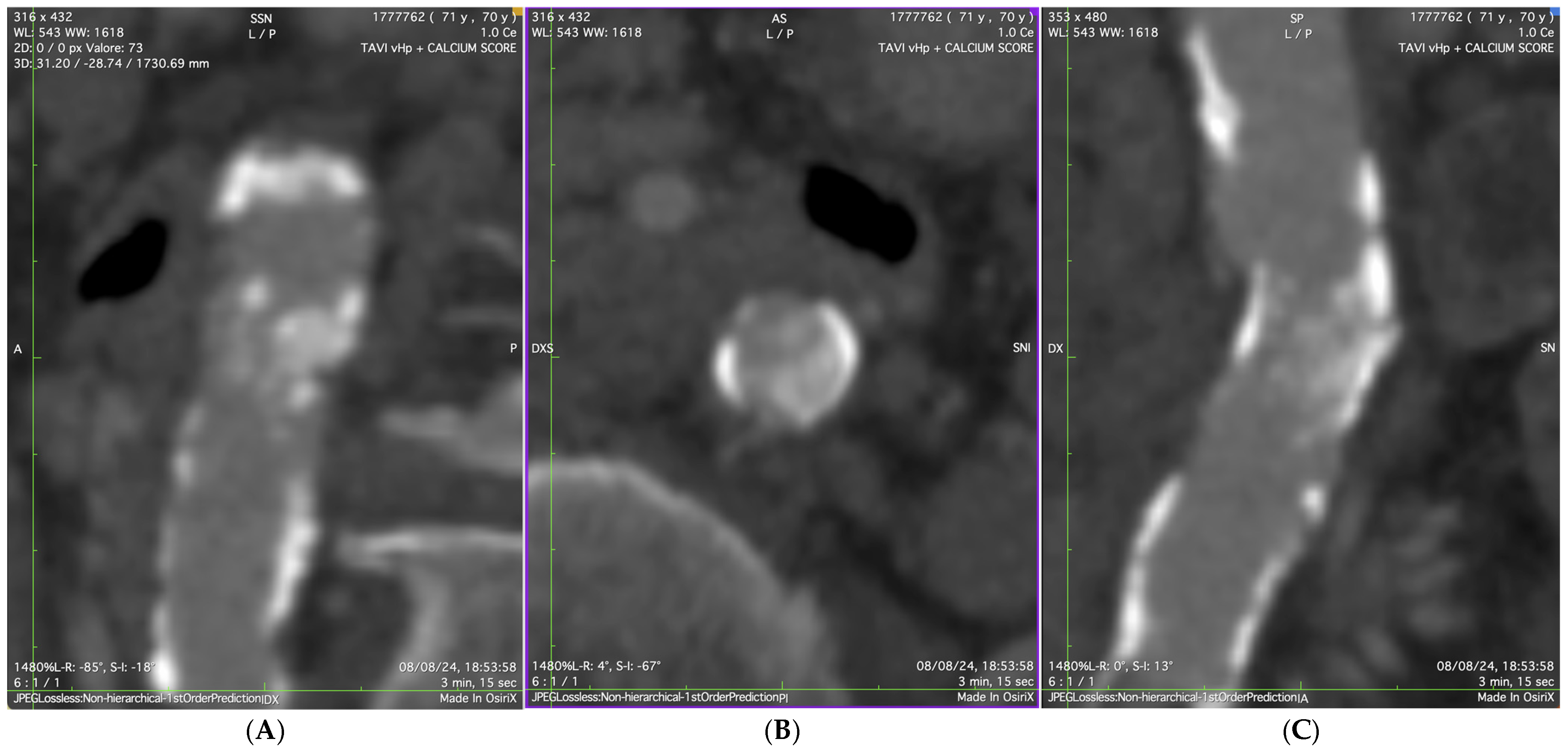
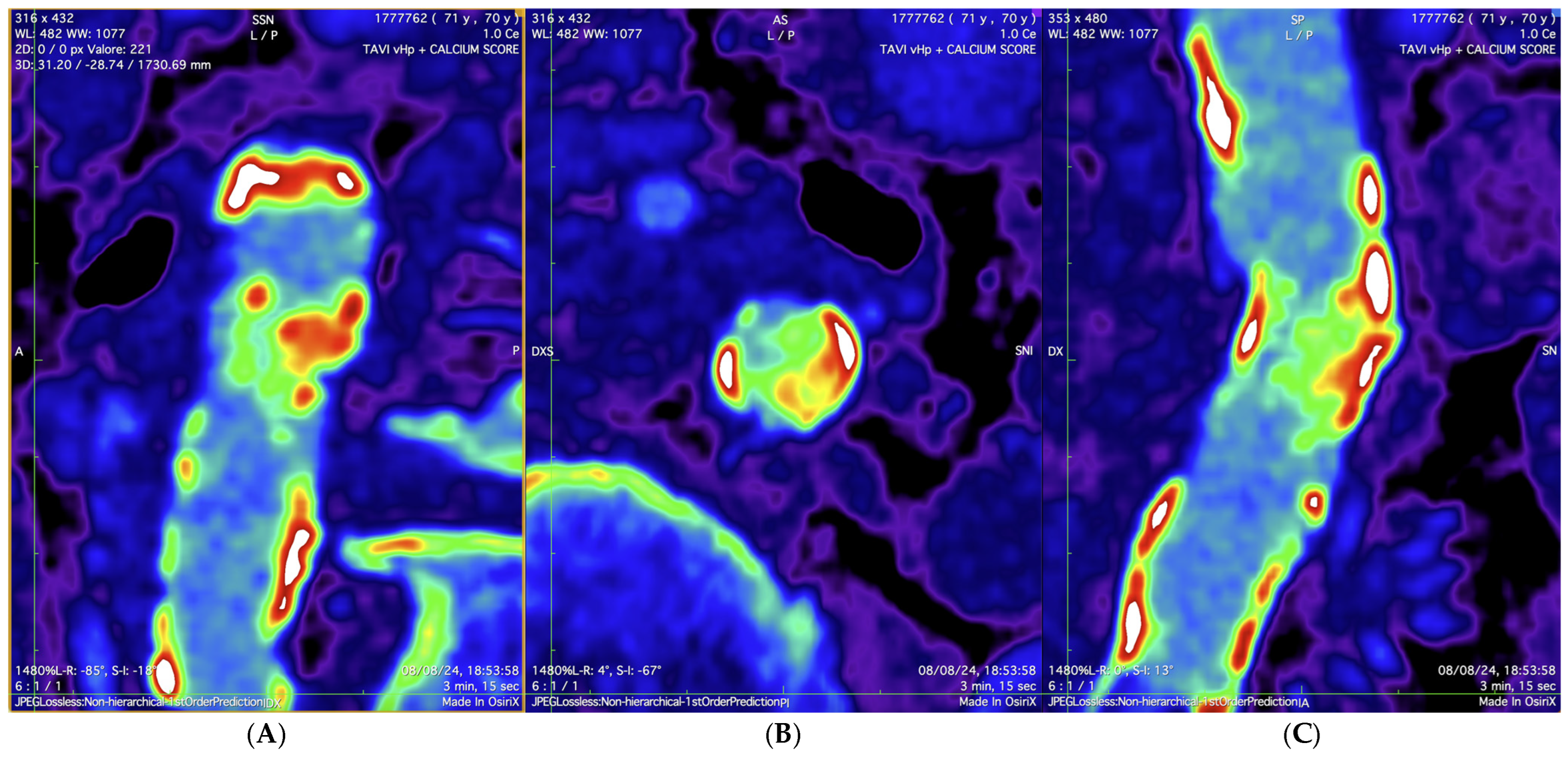
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yadgir, S.; Johnson, C.O.; Aboyans, V.; Adebayo, O.M.; Adedoyin, R.A.; Afarideh, M.; Alahdab, F.; Alashi, A.; Alipour, V.; Arabloo, J.; et al. Global, Regional, and National Burden of Calcific Aortic Valve and Degenerative Mitral Valve Diseases, 1990–2017. Circulation 2020, 141, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Gennari, M.; Severgnini, G.; Biancari, F.; Bonomi, A.; De Marco, F.; Polvani, G.; Agrifoglio, M. Clinical outcomes after transcatheter aortic valve implantation in nonagenarian patients: A retrospective population-based cohort study. Ann. Epidemiol. 2025, 102, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Siontis, G.C.; Praz, F.; Pilgrim, T.; Mavridis, D.; Verma, S.; Salanti, G.; Søndergaard, L.; Jüni, P.; Windecker, S. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of severe aortic stenosis: A meta-analysis of randomized trials. Eur. Heart J. 2016, 37, 3503–3512. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Head, S.J.; Van Mieghem, N.M.; Kodali, S.; Kirtane, A.J.; Xu, K.; Smith, C.; Serruys, P.W.; Kappetein, A.P.; Leon, M.B. Clinical Outcomes After Transcatheter Aortic Valve Replacement Using Valve Academic Research Consortium Definitions. J. Am. Coll. Cardiol. 2012, 59, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Demirci, G.; Aslan, S.; Şahin, A.A.; Demir, A.R.; Erata, Y.E.; Türkmen, I.; Kanyılmaz, M.; Türkvatan, A.; Ertürk, M. Anatomical Predictors of Access-Related Vascular Complications Following Transfemoral Transcatheter Aortic Valve Replacement. Catheter. Cardiovasc. Interv. 2025, 105, 1077–1085. [Google Scholar] [CrossRef]
- Koren, O.; Patel, V.; Tamir, Y.; Koseki, K.; Kaewkes, D.; Sanders, T.; Naami, R.; Naami, E.; Cheng, D.E.; Natanzon, S.S.; et al. Predicting the risk of iliofemoral vascular complication in complex transfemoral-TAVR using new generation transcatheter devices. Front. Cardiovasc. Med. 2023, 10, 1167212. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.J.; Soukas, P.A.; Shammas, N.; Chamberlain, J.; Pop, A.; Adams, G.; de Freitas, D.; Valle, J.; Woo, E.; Bernardo, N.L. Intravascular Lithotripsy for Treatment of Calcified, Stenotic Iliac Arteries: A Cohort Analysis from the Disrupt PAD III Study. Cardiovasc. Revasc. Med. 2020, 21, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Nardi, G.; De Backer, O.; Saia, F.; Søndergaard, L.; Ristalli, F.; Meucci, F.; Stolcova, M.; Mattesini, A.; Demola, P.; Wang, X.; et al. Peripheral intravascular lithotripsy for transcatheter aortic valve implantation: A multicentre observational study. EuroIntervention 2022, 17, e1397–e1406. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.; Grundmann, D.; Kellner, C.; Demal, T.; Waldschmidt, L.; Bhadra, O.; Ludwig, S.; Voigtländer, L.; von der Heide, I.; Nebel, N.; et al. Intravascular Lithotripsy-Assisted Transfemoral Transcatheter Aortic Valve Implantation in Patients with Severe Iliofemoral Calcifications: Expanding Transfemoral Indications. J. Clin. Med. 2024, 13, 1480. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Jabri, A.; Ravani, M.; Trianni, G.; Gasbarri, T.; Casula, M.; Berti, S. Transfemoral TAVI in a High-Risk Patient with Porcelain Aorta and Severe Subrenal Abdominal Aortic Stenosis: A Case Report. J. Cardiovasc. Dev. Dis. 2025, 12, 396. https://doi.org/10.3390/jcdd12100396
Al Jabri A, Ravani M, Trianni G, Gasbarri T, Casula M, Berti S. Transfemoral TAVI in a High-Risk Patient with Porcelain Aorta and Severe Subrenal Abdominal Aortic Stenosis: A Case Report. Journal of Cardiovascular Development and Disease. 2025; 12(10):396. https://doi.org/10.3390/jcdd12100396
Chicago/Turabian StyleAl Jabri, Anees, Marcello Ravani, Giuseppe Trianni, Tommaso Gasbarri, Marta Casula, and Sergio Berti. 2025. "Transfemoral TAVI in a High-Risk Patient with Porcelain Aorta and Severe Subrenal Abdominal Aortic Stenosis: A Case Report" Journal of Cardiovascular Development and Disease 12, no. 10: 396. https://doi.org/10.3390/jcdd12100396
APA StyleAl Jabri, A., Ravani, M., Trianni, G., Gasbarri, T., Casula, M., & Berti, S. (2025). Transfemoral TAVI in a High-Risk Patient with Porcelain Aorta and Severe Subrenal Abdominal Aortic Stenosis: A Case Report. Journal of Cardiovascular Development and Disease, 12(10), 396. https://doi.org/10.3390/jcdd12100396







