Diagnostic Accuracy of Coronary CT Angiography in Ruling Out Significant Coronary Artery Disease in Candidates for Transcatheter Aortic Valve Replacement
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. CT Scan Protocol
2.3. Image Reconstruction and Data Analysis
3. Statistical Analysis
4. Results
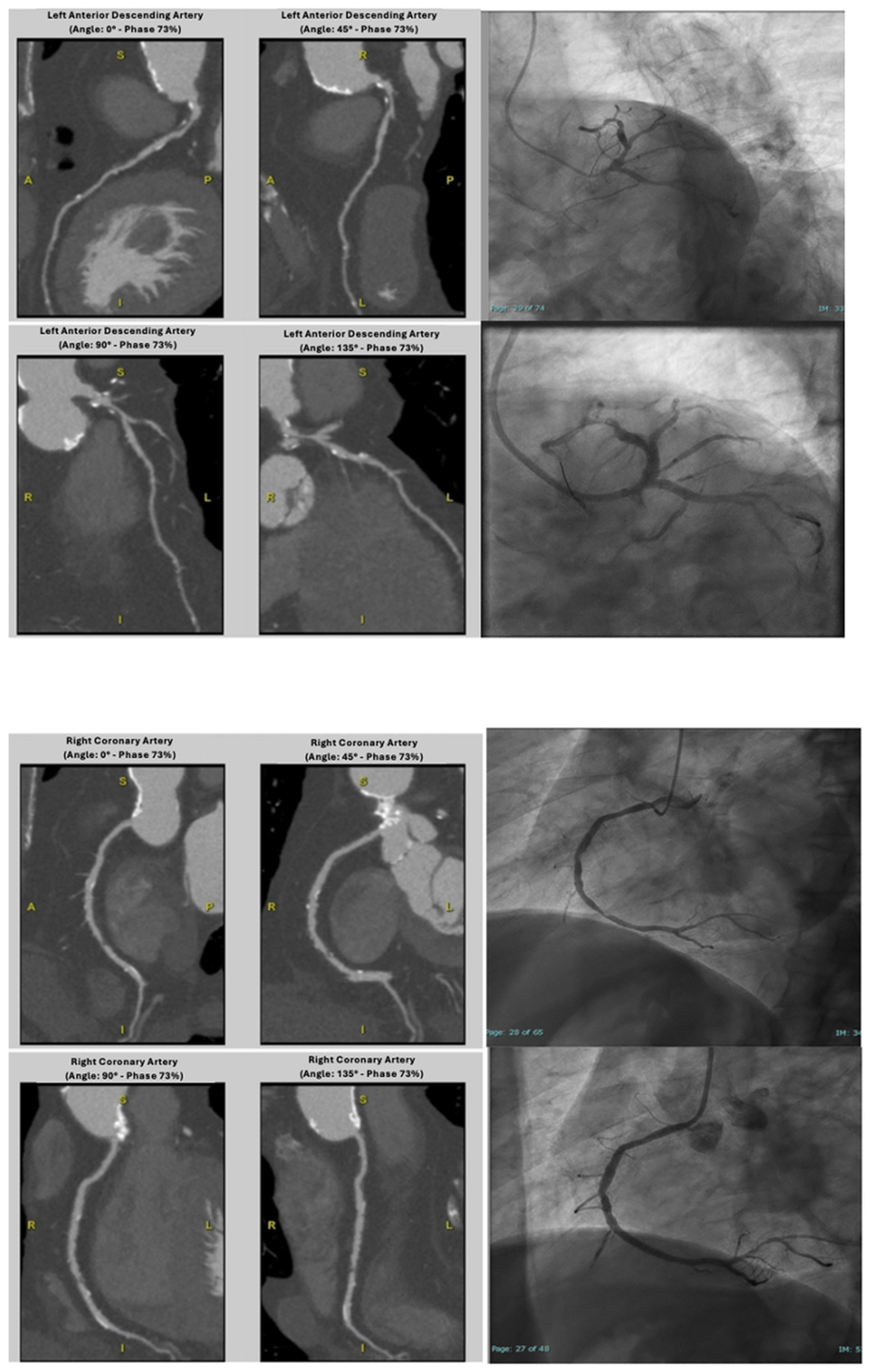
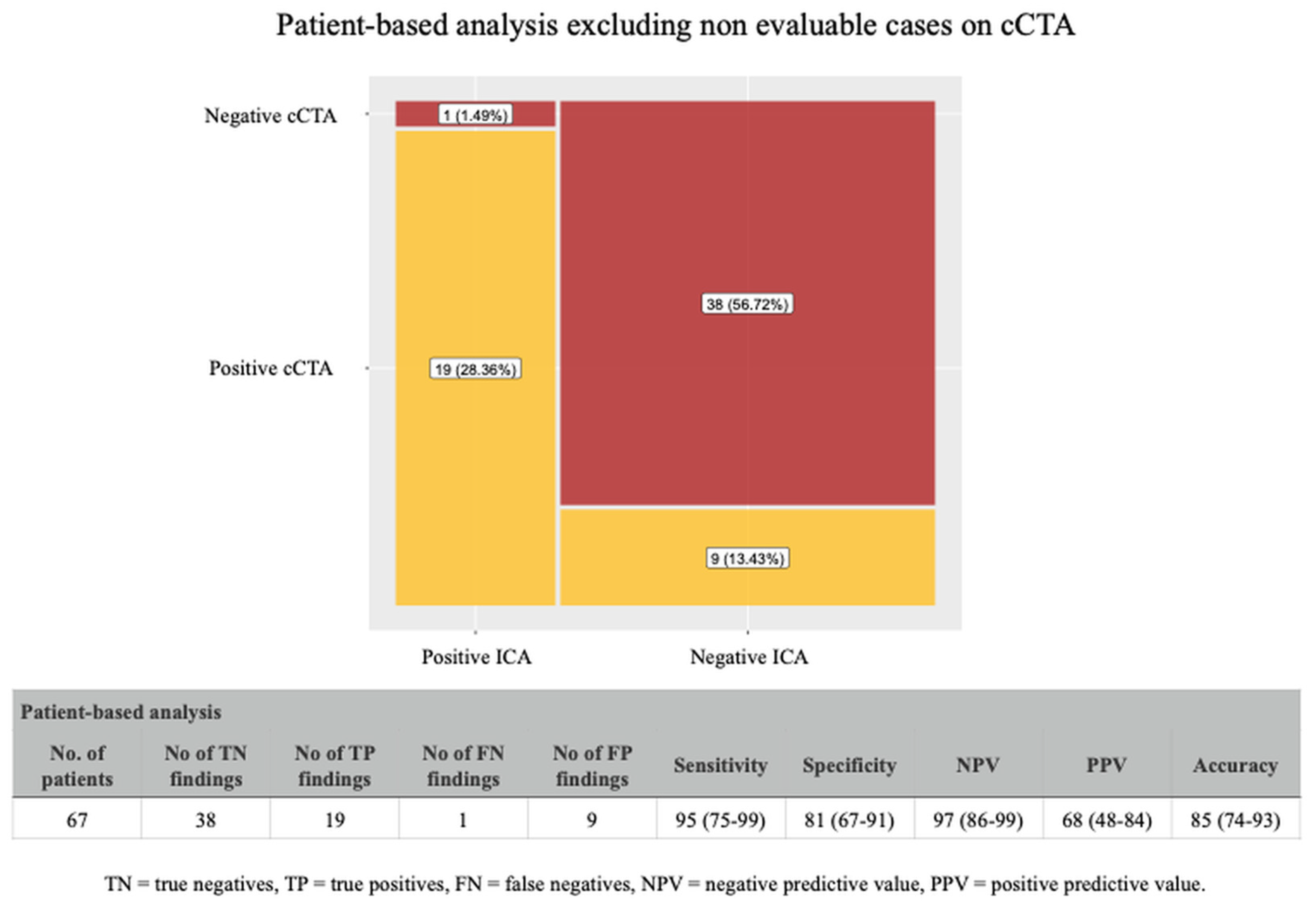
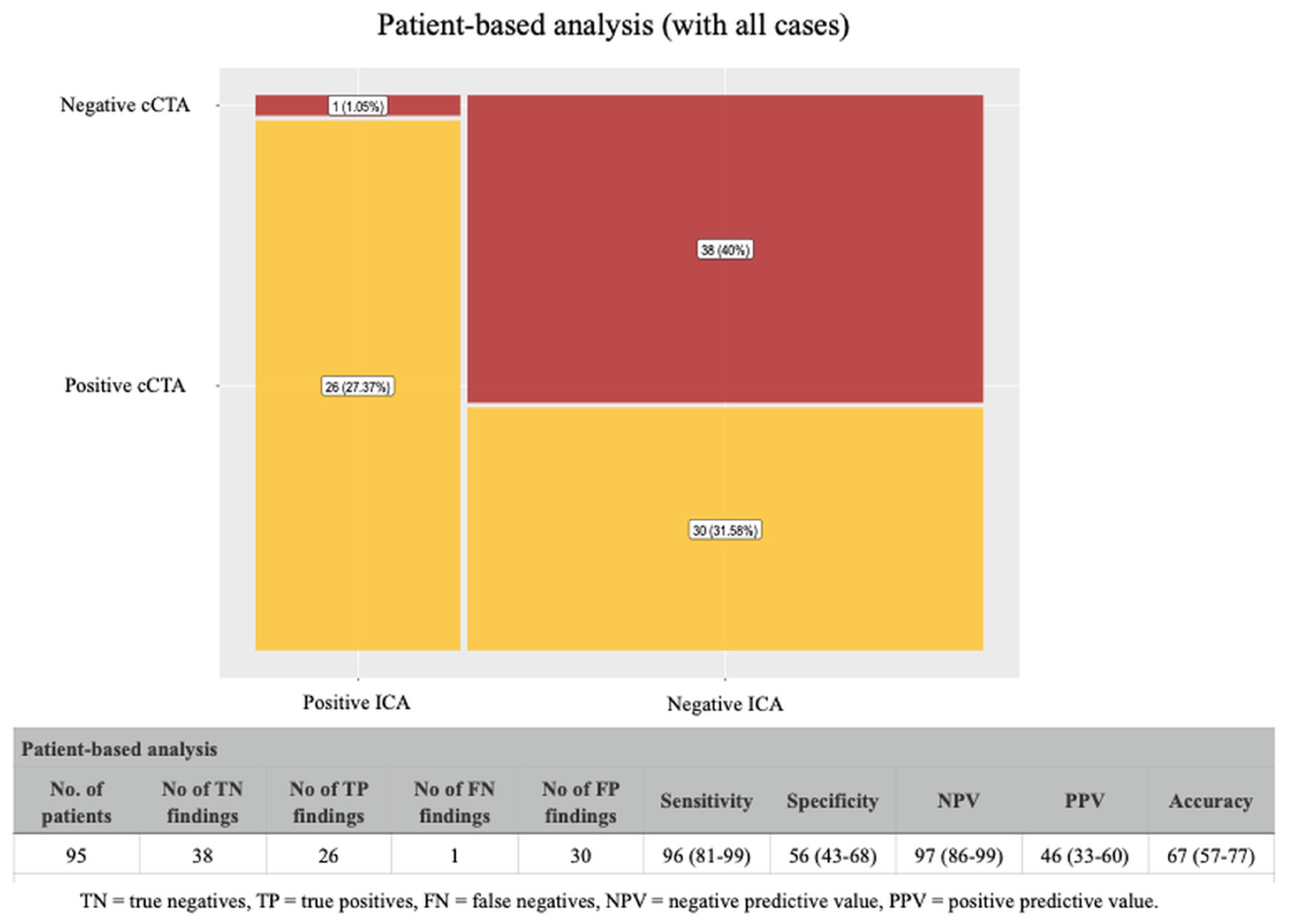
4.1. Coronary CTA Findings in Patients-Based Analysis
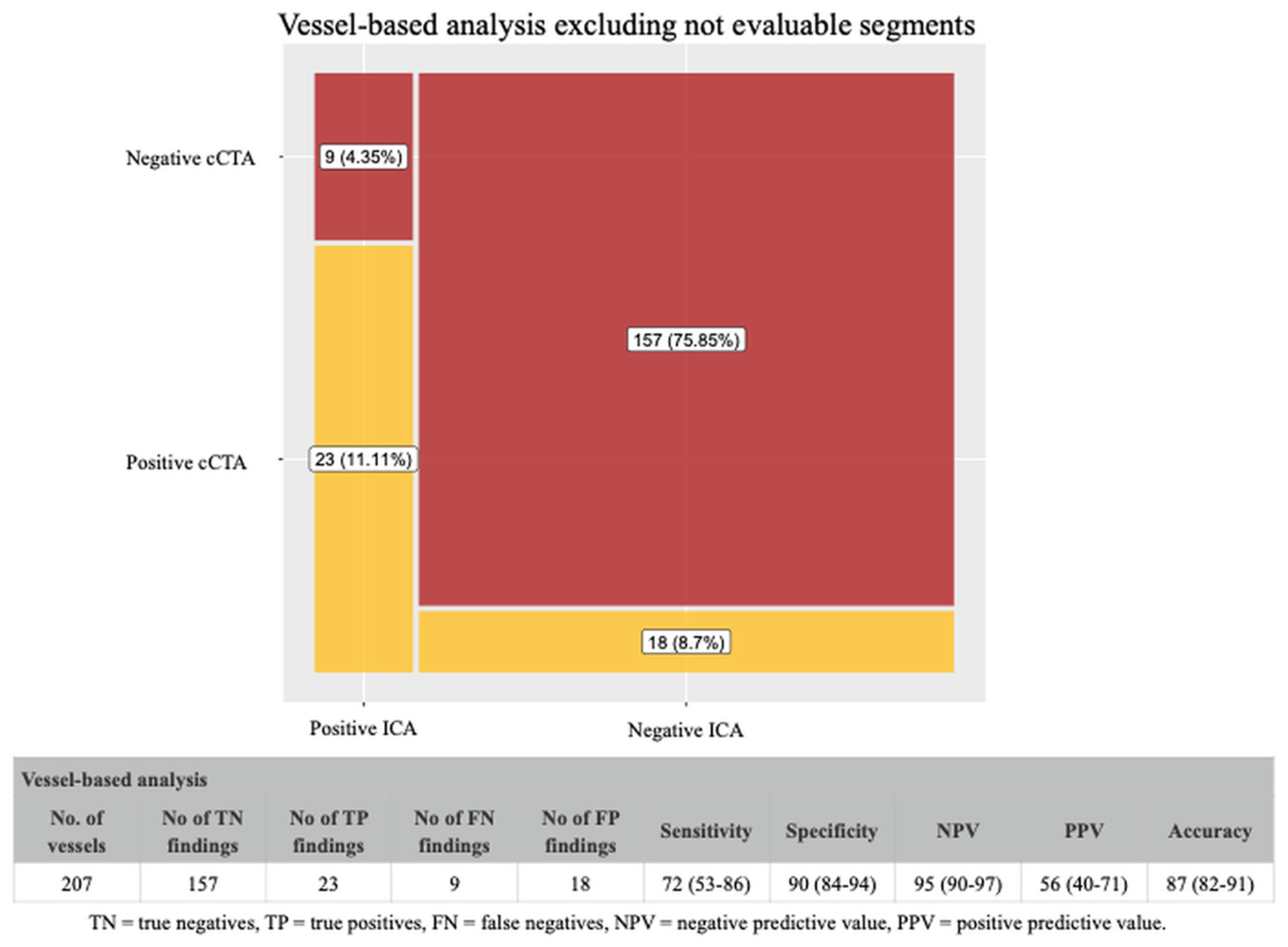
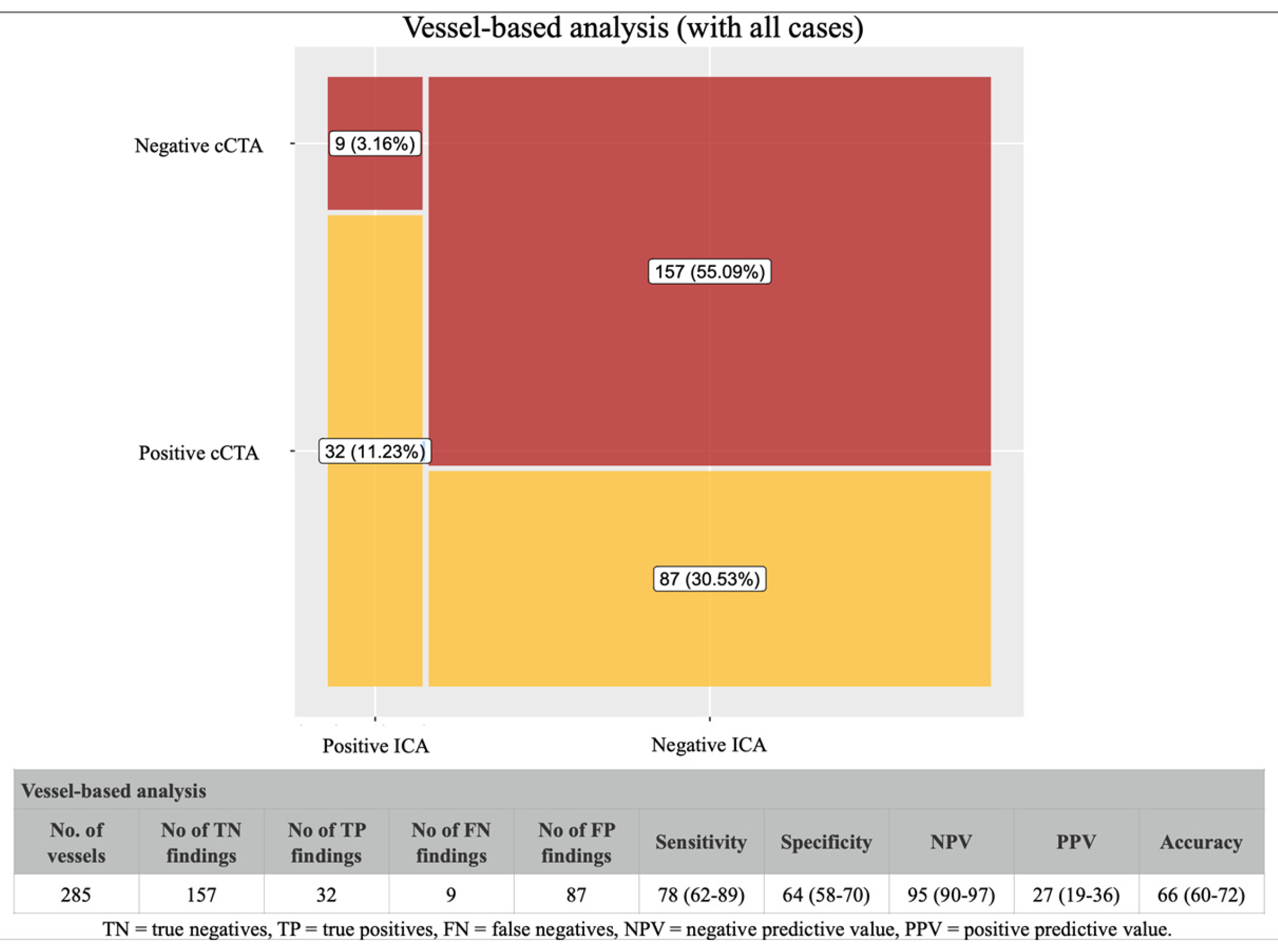
4.2. Coronary CTA Findings in Vessel-Based Analysis
4.3. Coronary CTA Findings After Sensitivity Analysis
5. Discussion
6. Study Limitations
7. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinayak, M.; Leone, P.P.; Tanner, R.; Dhulipala, V.; Camaj, A.; Makhija, R.R.K.; Hooda, A.; Kini, A.S.; Sharma, S.K.; Khera, S. Transcatheter Aortic Valve Replacement: Current Status and Future Indications. J. Clin. Med. 2024, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2021, 60, 727–800. [Google Scholar] [CrossRef] [PubMed]
- Coisne, A.; Lancellotti, P.; Habib, G.; Garbi, M.; Dahl, J.S.; Barbanti, M.; Vannan, M.A.; Vassiliou, V.S.; Dudek, D.; Chioncel, O.; et al. ACC/AHA and ESC/EACTS Guidelines for the Management of Valvular Heart Diseases: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2023, 82, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Francone, M.; Budde, R.P.J.; Bremerich, J.; Dacher, J.N.; Loewe, C.; Wolf, F.; Natale, L.; Pontone, G.; Redheuil, A.; Vliegenthart, R.; et al. CT and MR imaging prior to transcatheter aortic valve implantation: Standardisation of scanning protocols, measurements and reporting-a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur. Radiol. 2020, 30, 2627–2650. [Google Scholar] [CrossRef]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Norgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR): An Expert Consensus Document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc. Imaging 2019, 12, 1–24. [Google Scholar] [CrossRef]
- Rapp, A.H.; Hillis, L.D.; Lange, R.A.; Cigarroa, J.E. Prevalence of coronary artery disease in patients with aortic stenosis with and without angina pectoris. Am. J. Cardiol. 2001, 87, 1216–1217. [Google Scholar] [CrossRef]
- Sabbah, M.; Engstrom, T.; De Backer, O.; Sondergaard, L.; Lonborg, J. Coronary Assessment and Revascularization Before Transcutaneous Aortic Valve Implantation: An Update on Current Knowledge. Front. Cardiovasc. Med. 2021, 8, 654892. [Google Scholar] [CrossRef]
- Bleakley, C.; Monaghan, M.J. The Pivotal Role of Imaging in TAVR Procedures. Curr. Cardiol. Rep. 2018, 20, 9. [Google Scholar] [CrossRef]
- Tarantini, G.; Tang, G.; Nai Fovino, L.; Blackman, D.; Van Mieghem, N.M.; Kim, W.K.; Karam, N.; Carrilho-Ferreira, P.; Fournier, S.; Pregowski, J.; et al. Management of coronary artery disease in patients undergoing transcatheter aortic valve implantation. A clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions in collaboration with the ESC Working Group on Cardiovascular Surgery. EuroIntervention 2023, 19, 37–52. [Google Scholar] [CrossRef]
- Faroux, L.; Guimaraes, L.; Wintzer-Wehekind, J.; Junquera, L.; Ferreira-Neto, A.N.; Del Val, D.; Muntane-Carol, G.; Mohammadi, S.; Paradis, J.M.; Rodes-Cabau, J. Coronary Artery Disease and Transcatheter Aortic Valve Replacement: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 362–372. [Google Scholar] [CrossRef]
- Patterson, T.; Clayton, T.; Dodd, M.; Khawaja, Z.; Morice, M.C.; Wilson, K.; Kim, W.K.; Meneveau, N.; Hambrecht, R.; Byrne, J.; et al. ACTIVATION (PercutAneous Coronary inTervention prIor to transcatheter aortic VAlve implantaTION): A Randomized Clinical Trial. JACC Cardiovasc. Interv. 2021, 14, 1965–1974. [Google Scholar] [CrossRef]
- Annoni, A.D.; Andreini, D.; Pontone, G.; Mancini, M.E.; Formenti, A.; Mushtaq, S.; Baggiano, A.; Conte, E.; Guglielmo, M.; Muscogiuri, G.; et al. CT angiography prior to TAVI procedure using third-generation scanner with wide volume coverage: Feasibility, renal safety and diagnostic accuracy for coronary tree. Br. J. Radiol. 2018, 91, 20180196. [Google Scholar] [CrossRef]
- Strong, C.; Ferreira, A.; Teles, R.C.; Mendes, G.; Abecasis, J.; Cardoso, G.; Guerreiro, S.; Freitas, P.; Santos, A.C.; Saraiva, C.; et al. Diagnostic accuracy of computed tomography angiography for the exclusion of coronary artery disease in candidates for transcatheter aortic valve implantation. Sci. Rep. 2019, 9, 19942. [Google Scholar] [CrossRef]
- Austen, W.G.; Edwards, J.E.; Frye, R.L.; Gensini, G.G.; Gott, V.L.; Griffith, L.S.; McGoon, D.C.; Murphy, M.L.; Roe, B.B. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975, 51, 5–40. [Google Scholar] [CrossRef] [PubMed]
- Chieffo, A.; Giustino, G.; Spagnolo, P.; Panoulas, V.F.; Montorfano, M.; Latib, A.; Figini, F.; Agricola, E.; Gerli, C.; Franco, A.; et al. Routine Screening of Coronary Artery Disease With Computed Tomographic Coronary Angiography in Place of Invasive Coronary Angiography in Patients Undergoing Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2015, 8, e002025. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Yamada, Y.; Hashimoto, M.; Okamura, T.; Yamada, M.; Yashima, F.; Hayashida, K.; Fukuda, K.; Jinzaki, M. CT imaging before transcatheter aortic valve implantation (TAVI) using variable helical pitch scanning and its diagnostic performance for coronary artery disease. Eur. Radiol. 2017, 27, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Renker, M.; Schoepf, U.J.; Kim, W.K. Combined CT Coronary Artery Assessment and TAVI Planning. Diagnostics 2023, 13, 1327. [Google Scholar] [CrossRef]
- Schicchi, N.; Fogante, M.; Pirani, P.E.; Agliata, G.; Piva, T.; Tagliati, C.; Marcucci, M.; Francioso, A.; Giovagnoni, A. Third generation dual source CT with ultra-high pitch protocol for TAVI planning and coronary tree assessment: Feasibility, image quality and diagnostic performance. Eur. J. Radiol. 2020, 122, 108749. [Google Scholar] [CrossRef]
- Andreini, D.; Pontone, G.; Mushtaq, S.; Bartorelli, A.L.; Ballerini, G.; Bertella, E.; Segurini, C.; Conte, E.; Annoni, A.; Baggiano, A.; et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in 325 consecutive patients referred for transcatheter aortic valve replacement. Am. Heart J. 2014, 168, 332–339. [Google Scholar] [CrossRef]
- Rossi, A.; De Cecco, C.N.; Kennon, S.R.O.; Zou, L.; Meinel, F.G.; Toscano, W.; Segreto, S.; Achenbach, S.; Hausleiter, J.; Schoepf, U.J.; et al. CT angiography to evaluate coronary artery disease and revascularization requirement before trans-catheter aortic valve replacement. J. Cardiovasc. Comput. Tomogr. 2017, 11, 338–346. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.M.; Rybicki, F.J.; Steigner, M. CT coronary angiography: 256-slice and 320-detector row scanners. Curr. Cardiol. Rep. 2010, 12, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, J.; Xu, D.; Dong, Z.; Li, X.; Zhang, L. CTCA image quality improvement by using snapshot freeze technique under prospective and retrospective electrocardiographic gating. J. Comput. Assist. Tomogr. 2015, 39, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Sheta, H.M.; Egstrup, K.; Husic, M.; Heinsen, L.J.; Nieman, K.; Lambrechtsen, J. Impact of a motion correction algorithm on image quality in patients undergoing CT angiography: A randomized controlled trial. Clin. Imaging 2017, 42, 1–6. [Google Scholar] [CrossRef]
- Abdelkarim, A.; Roy, S.K.; Kinninger, A.; Salek, A.; Baranski, O.; Andreini, D.; Pontone, G.; Conte, E.; O’Rourke, R.; Hamilton-Craig, C.; et al. Evaluation of Image Quality for High Heart Rates for Coronary Computed Tomographic Angiography with Advancement in CT Technology: The CONVERGE Registry. J. Cardiovasc. Dev. Dis. 2023, 10, 404. [Google Scholar] [CrossRef]
- Flohr, T.; Petersilka, M.; Henning, A.; Ulzheimer, S.; Ferda, J.; Schmidt, B. Photon-counting CT review. Phys. Med. 2020, 79, 126–136. [Google Scholar] [CrossRef]
- Hagar, M.T.; Soschynski, M.; Saffar, R.; Molina-Fuentes, M.F.; Weiss, J.; Rau, A.; Schuppert, C.; Ruile, P.; Faby, S.; Schibilsky, D.; et al. Ultra-high-resolution photon-counting detector CT in evaluating coronary stent patency: A comparison to invasive coronary angiography. Eur. Radiol. 2024, 34, 4273–4283. [Google Scholar] [CrossRef]
- Hussain, K.; Lee, K.; Minga, I.; Wathen, L.; Balasubramanian, S.S.; Vyas, N.; Singh, L.; Shetty, M.; Rosenberg, J.R.; Levisay, J.P.; et al. Real-world application of CCTA with CT-FFR for coronary assessment pre-TAVI: The CT2TAVI study. Int. J. Cardiovasc. Imaging 2025, 41, 523–535. [Google Scholar] [CrossRef]
- Kodeboina, M.; Piayda, K.; Jenniskens, I.; Vyas, P.; Chen, S.; Pesigan, R.J.; Ferko, N.; Patel, B.P.; Dobrin, A.; Habib, J.; et al. Challenges and Burdens in the Coronary Artery Disease Care Pathway for Patients Undergoing Percutaneous Coronary Intervention: A Contemporary Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 5633. [Google Scholar] [CrossRef]

| Total Number of Patients | 95 |
| Age (years) | 77.7 ± 8.5 |
| Male/female | 45/50 |
| BMI (kg/m2) | 26.6 ± 4.6 |
| BSA (m2) | 1.73 ± 0.22 |
| Baseline creatinine (mg/dL) | 1.35 ± 1.05 |
| Base hemoglobin (g/dL) | 11.8 ± 1.8 |
| Heart rate (bpm) | 77.8 ± 24.6 |
| EF (%) | 49 ± 10.8 |
| EF < 50%, n (%) | 37 (39) |
| Aortic valve mean gradient (mmHg) | 38.7 ± 14.9 |
| CAC score | 1972.5 ± 1425.3 |
| Atrial fibrillation, n (%) | 25 (26.3) |
| Patients with previous coronary stenting, n (%) | 13 (13.7) |
| Stent number | 27 |
| Drug Eluting Stent (DES), n | 27 |
| Patients with previous CABG, n (%) | 9 (9.5) |
| Patients with a background history of aortic valve replacement, n (%) | 8 (8.4) |
| Cardiovascular risk factors | |
| Hypertension, n (%) | 70 (73.7) |
| Dyslipidemia, n (%) | 59 (62.1) |
| Diabetes mellitus, n (%) | 40 (42.1) |
| Current smoking, n (%) | 28 (29.5) |
| Family history of CAD, n (%) | 7 (7.4) |
| Patients with Two or More Cardiovascular Risk Factors; N (%) | 20 (74.1) |
| Male sex, n (%) | 17 (63) |
| Age ≥ 75 years, n (%) | 16 (59.3) |
| High calcium score (>1200 for women and >2000 for men), n (%) | 13 (48.1) |
| BMI ≥ 25, n (%) | 13 (48.1) |
| Cause of Non-Diagnostic Scan; N (%) | 28 (29.5) |
| Extensive coronary calcification, n (%) | 11 (39.3%) |
| Previously implanted coronary stents, n (%) | 8 (28.6) |
| Motion artifacts (e.g., atrial fibrillation), n (%) | 5 (17.9) |
| Poor contrast opacification, n (%) | 3 (10.7) |
| Image noise due to high BMI or artifacts, n (%) | 1 (3.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, C.; Campanile, A.; Izzo, C.; Paoletta, S.; Russo, V.; Chivasso, P.; Vigorito, F.; Di Maio, M.; Ciccarelli, M.; Ravera, A.; et al. Diagnostic Accuracy of Coronary CT Angiography in Ruling Out Significant Coronary Artery Disease in Candidates for Transcatheter Aortic Valve Replacement. J. Cardiovasc. Dev. Dis. 2025, 12, 395. https://doi.org/10.3390/jcdd12100395
Gallo C, Campanile A, Izzo C, Paoletta S, Russo V, Chivasso P, Vigorito F, Di Maio M, Ciccarelli M, Ravera A, et al. Diagnostic Accuracy of Coronary CT Angiography in Ruling Out Significant Coronary Artery Disease in Candidates for Transcatheter Aortic Valve Replacement. Journal of Cardiovascular Development and Disease. 2025; 12(10):395. https://doi.org/10.3390/jcdd12100395
Chicago/Turabian StyleGallo, Chiara, Alfonso Campanile, Carmine Izzo, Sonia Paoletta, Valentina Russo, Pierpaolo Chivasso, Francesco Vigorito, Marco Di Maio, Michele Ciccarelli, Amelia Ravera, and et al. 2025. "Diagnostic Accuracy of Coronary CT Angiography in Ruling Out Significant Coronary Artery Disease in Candidates for Transcatheter Aortic Valve Replacement" Journal of Cardiovascular Development and Disease 12, no. 10: 395. https://doi.org/10.3390/jcdd12100395
APA StyleGallo, C., Campanile, A., Izzo, C., Paoletta, S., Russo, V., Chivasso, P., Vigorito, F., Di Maio, M., Ciccarelli, M., Ravera, A., Attisano, T., Maraziti, G., Di Gennaro, D., Coscioni, E., Vecchione, C., & Caleo, O. (2025). Diagnostic Accuracy of Coronary CT Angiography in Ruling Out Significant Coronary Artery Disease in Candidates for Transcatheter Aortic Valve Replacement. Journal of Cardiovascular Development and Disease, 12(10), 395. https://doi.org/10.3390/jcdd12100395








