Hypertrabeculation in Olympic Athletes: Advanced LV Function Analysis by CMR
Abstract
1. Introduction
2. Methods
2.1. Cardiac Magnetic Resonance
2.2. Hypertrabeculation
2.3. Statistical Analysis
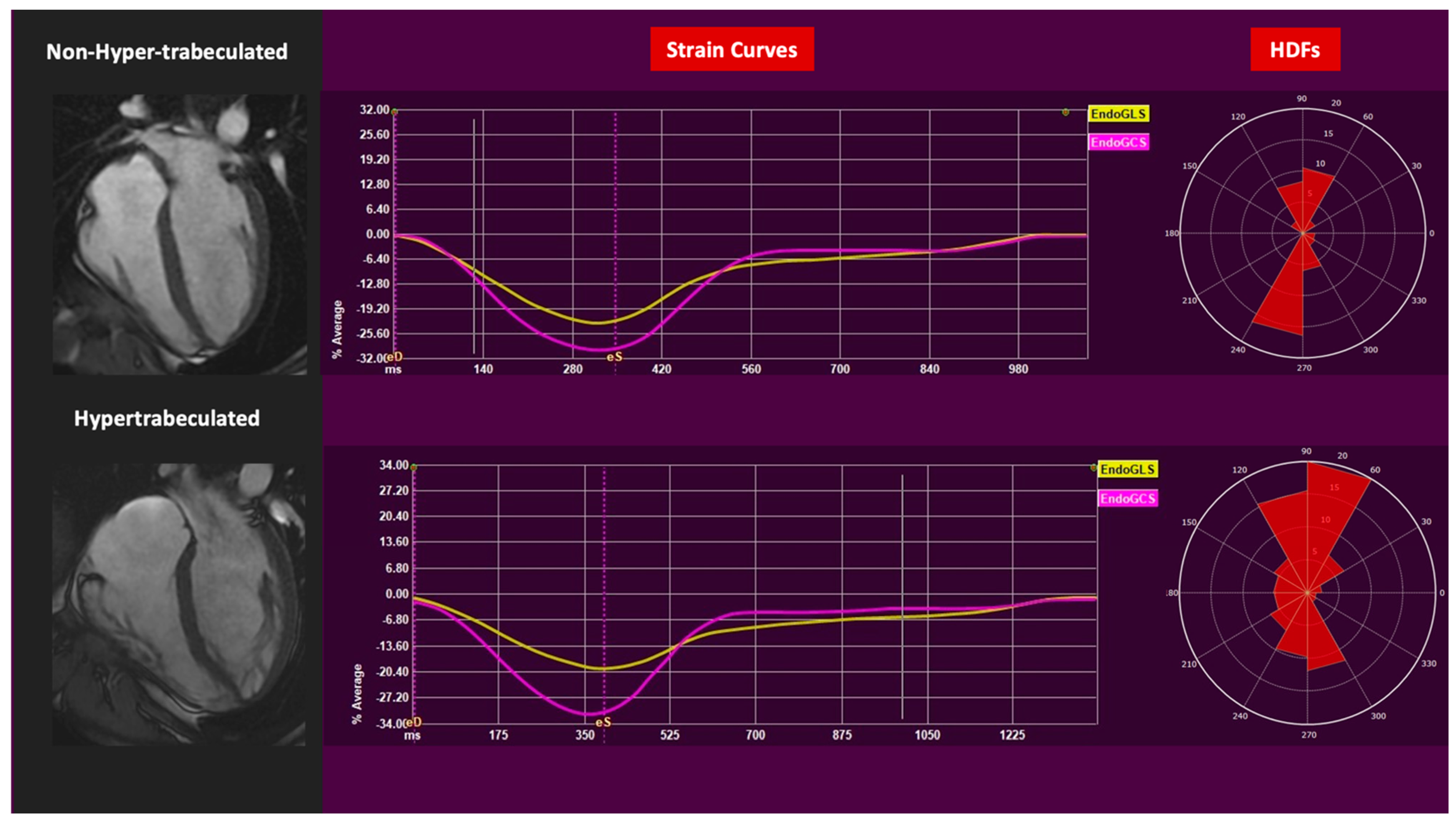
3. Results
4. Discussion
5. Limits
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gati, S.; Sharma, S. Dilated Cardiomyopathy, Left Ventricular Hypertrabeculation and Noncompaction. In IOC Manual of Sports Cardiology; Wilson, M.G., Drezner, J.A., Sharma, S., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 231–239. [Google Scholar]
- Caselli, S.; Ferreira, D.; Kanawati, E.; Di Paolo, F.; Pisicchio, C.; Jost, C.A.; Spataro, A.; Jenni, R.; Pelliccia, A. Prominent left ventricular trabeculations in competitive athletes: A proposal for risk stratification and management. Int. J. Cardiol. 2016, 223, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Jensen, B.; Aung, N.; Friedrich, M.G.; McMahon, C.J.; Mohiddin, S.A.; Pignatelli, R.H.; Ricci, F.; Anderson, R.H.; Bluemke, D.A. Excessive Trabeculation of the Left Ventricle. JACC Cardiovasc. Imaging 2023, 16, 408–425. [Google Scholar] [CrossRef] [PubMed]
- Gati, S.; Chandra, N.; Bennett, R.L.; Reed, M.; Kervio, G.; Panoulas, V.F.; Ghani, S.; Sheikh, N.; Zaidi, A.; Wilson, M.; et al. Increased left ventricular trabeculation in highly trained athletes: Do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes? Heart 2013, 99, 401–408. [Google Scholar] [CrossRef]
- D’AScenzi, F.; Pelliccia, A.; Natali, B.M.; Bonifazi, M.; Mondillo, S. Exercise-induced left-ventricular hypertrabeculation in athlete’s heart. Int. J. Cardiol. 2015, 181, 320–322. [Google Scholar] [CrossRef]
- Chin, T.K.; Perloff, J.K.; Williams, R.G.; Jue, K.; Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990, 82, 507–513. [Google Scholar] [CrossRef]
- Jenni, R.; Oechslin, E.; Schneider, J.; Jost, C.A.; A Kaufmann, P. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart 2001, 86, 666–671. [Google Scholar] [CrossRef]
- Captur, G.; Muthurangu, V.; Cook, C.; Flett, A.S.; Wilson, R.; Barison, A.; Sado, D.M.; Anderson, S.; McKenna, W.J.; Mohun, T.J.; et al. Quantification of left ventricular trabeculae using fractal analysis. J. Cardiovasc. Magn. Reson. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Stacey, R.B.; Andersen, M.M.; St. Clair, M.; Hundley, W.G.; Thohan, V. Comparison of Systolic and Diastolic Criteria for Isolated LV Noncompaction in CMR. JACC Cardiovasc. Imaging 2013, 6, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, A.; Thuny, F.; Jop, B.; Giorgi, R.; Cohen, F.; Gaubert, J.-Y.; Vidal, V.; Bartoli, J.M.; Habib, G.; Moulin, G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur. Heart J. 2010, 31, 1098–1104. [Google Scholar] [CrossRef]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Neubauer, S. Left Ventricular Non-Compaction: Insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef]
- Di Gioia, G.; Crispino, S.P.; Monosilio, S.; Maestrini, V.; Nenna, A.; Spinelli, A.; Lemme, E.; Squeo, M.R.; Pelliccia, A. Left Ventricular Trabeculation: Arrhythmogenic and Clinical Significance in Elite Athletes. J. Am. Soc. Echocardiogr. 2024, 37, 577–586. [Google Scholar] [CrossRef]
- Monosilio, S.; Prosperi, S.; Filomena, D.; Lemme, E.; Di Gioia, G.; Mango, R.; Netti, L.; Tonti, G.; Pedrizzetti, G.; Gualdi, G.; et al. Cardiac magnetic resonance feature tracking in Olympic athletes: A myocardial deformation analysis. Eur. J. Prev. Cardiol. 2025, zwaf042. [Google Scholar] [CrossRef]
- Filomena, D.; Cimino, S.; Monosilio, S.; Galea, N.; Mancuso, G.; Francone, M.; Tonti, G.; Pedrizzetti, G.; Maestrini, V.; Fedele, F.; et al. Impact of intraventricular haemodynamic forces misalignment on left ventricular remodelling after myocardial infarction. ESC Heart Fail. 2021, 9, 496–505. [Google Scholar] [CrossRef]
- Swoboda, P.P.; Erhayiem, B.; McDiarmid, A.K.; Lancaster, R.E.; Lyall, G.K.; Dobson, L.E.; Ripley, D.P.; Musa, T.A.; Garg, P.; Ferguson, C.; et al. Relationship between cardiac deformation parameters measured by cardiovascular magnetic resonance and aerobic fitness in endurance athletes. J. Cardiovasc. Magn. Reson. 2016, 18, 48. [Google Scholar] [CrossRef]
- Prosperi, S.; Monosilio, S.; Lemme, E.; Filomena, D.; Penza, M.; Birtolo, L.I.; Mango, R.; Di Gioia, G.; Gualdi, G.; Squeo, M.R.; et al. CMR native T1 and T2 mapping in Olympic athletes: The influence of sports discipline and sex. Eur. Heart J. Cardiovasc. Imag. 2024, 26, 89–95. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Camastra, G.; Monti, L.; Lombardi, M.; Pepe, A.; Castelletti, S.; Maestrini, V.; Todiere, G.; Masci, P.; di Giovine, G.; et al. Reference values of cardiac volumes, dimensions, and new functional parameters by MR: A multicenter, multivendor study. J. Magn. Reson. Imaging 2016, 45, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Pedrizzetti, G.; Claus, P.; Kilner, P.J.; Nagel, E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J. Cardiovasc. Magn. Reson. 2016, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Pedrizzetti, G.; Tanacli, R.; Lapinskas, T.; Zovatto, L.; Pieske, B.; Tonti, G.; Kelle, S. Integration between volumetric change and strain for describing the global mechanical function of the left ventricle. Med Eng. Phys. 2019, 74, 65–72. [Google Scholar] [CrossRef]
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’aNdrea, A.; D’aScenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: Recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s heart. Eur. Heart J. 2017, 39, 1949–1969. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, M.S.; Maron, B.J. Assessment of Left Ventricular Hypertrophy in a Trained Athlete: Differential Diagnosis of Physiologic Athlete’s Heart from Pathologic Hypertrophy. Prog. Cardiovasc. Dis. 2012, 54, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; De Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Cesareo, M.; Ródenas-Alesina, E.; Guala, A.; Lozano-Torres, J.; Casas, G.; Vallelonga, F.; Airale, L.; Ferreira-González, I.; Milan, A.; Rodriguez-Palomares, J.F. Echocardiography-Derived Hemodynamic Forces Are Associated with Clinical Outcomes in Patients with Non-Ischemic Dilated Cardiomyopathy. J. Clin. Med. 2024, 13, 3862. [Google Scholar] [CrossRef]
- Visoiu, I.S.; Jensen, B.; Rimbas, R.C.; Mihaila-Baldea, S.; Nicula, A.I.; Vinereanu, D. How the trabecular layer impacts on left ventricular function. J. Cardiol. 2024, 85, 17–27. [Google Scholar] [CrossRef]
| Parameters | Non-Hypertrabeculated n = 30 | Hypertrabeculated n = 30 | p Value |
|---|---|---|---|
| Age, years | 25 ± 5 | 24 ± 4 | 0.377 |
| Years of training, years | 16 ± 6 | 13 ± 5 | 0.065 |
| Hours of training per week, h | 26 ± 11 | 30 ± 11 | 0.262 |
| Sports categories | Power (23.3% n = 7), Mixed (26.6% n = 8), Endurance (50% n = 15) | Power (23.3% n = 7), Mixed (26.6% n = 8), Endurance (50% n = 15) | N.A. |
| BSA, m2 | 2 ± 0.2 | 2 ± 0.3 | 0.762 |
| Workload peak, Watt/kg | 3.4 ± 0.6 | 3.5 ± 0.7 | 0.771 |
| EDV[i], mL/BSA | 108 ± 19 | 113 ± 16 | 0.397 |
| ESV[i], mL/BSA | 47 ± 9 | 51 ± 9 | 0.118 |
| EF, % | 57 ± 4 | 55 ± 4 | 0.101 |
| Mass[i], g/BSA | 64 ± 16 | 64 ± 19 | 0.919 |
| LVMWT max, mm | 10 ± 1 | 9 ± 1 | 0.394 |
| Sphericity index, % | 43 ± 6 | 42 ± 8 | 0.419 |
| T2 mapping, ms | 51 ± 5 | 52 ± 3 | 0.833 |
| T2 weighted for edema sequences | 0% | 0% | N.A. |
| T1 native myocardial mapping, ms | 941 ± 26 | 945 ± 24 | 0.366 |
| T1 weighted for fat sequences | 0% | 0% | N.A. |
| GLS, % | −22 ± 3 | −22 ± 3 | 0.898 |
| GCS, % | −31 ± 4 | −29 ± 3 | 0.219 |
| HDF ratio entire, % | 14 (13–16) | 14 (12–17) | 0.969 |
| HDF ratio systole, % | 11 (9–14) | 11 (9–13) | 0.474 |
| HDF ratio diastole, % | 22 (19–29) | 22 (19–26) | 0.846 |
| HDF AB entire % | 21 (18–26) | 20 (19–23) | 0.465 |
| HDF AB systole % | 35 (30–40) | 32 (29–35) | 0.092 |
| HDF AB diastole % | 11 (9–13) | 12 (9–13) | 0.592 |
| HDF LS entire % | 3 (2–4) | 3 (2–4) | 0.437 |
| HDF LS systole % | 4 (3–5) | 3 (3–4) | 0.072 |
| HDF LS diastole % | 3 (2–3) | 3 (2–3) | 0.347 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinelli, A.; Monosilio, S.; Di Gioia, G.; Pedrizzetti, G.; Tonti, G.; Daniello, C.D.; Squeo, M.R.; Pelliccia, A.; Maestrini, V. Hypertrabeculation in Olympic Athletes: Advanced LV Function Analysis by CMR. J. Cardiovasc. Dev. Dis. 2025, 12, 388. https://doi.org/10.3390/jcdd12100388
Spinelli A, Monosilio S, Di Gioia G, Pedrizzetti G, Tonti G, Daniello CD, Squeo MR, Pelliccia A, Maestrini V. Hypertrabeculation in Olympic Athletes: Advanced LV Function Analysis by CMR. Journal of Cardiovascular Development and Disease. 2025; 12(10):388. https://doi.org/10.3390/jcdd12100388
Chicago/Turabian StyleSpinelli, Alessandro, Sara Monosilio, Giuseppe Di Gioia, Gianni Pedrizzetti, Giovanni Tonti, Cosimo Damiano Daniello, Maria Rosaria Squeo, Antonio Pelliccia, and Viviana Maestrini. 2025. "Hypertrabeculation in Olympic Athletes: Advanced LV Function Analysis by CMR" Journal of Cardiovascular Development and Disease 12, no. 10: 388. https://doi.org/10.3390/jcdd12100388
APA StyleSpinelli, A., Monosilio, S., Di Gioia, G., Pedrizzetti, G., Tonti, G., Daniello, C. D., Squeo, M. R., Pelliccia, A., & Maestrini, V. (2025). Hypertrabeculation in Olympic Athletes: Advanced LV Function Analysis by CMR. Journal of Cardiovascular Development and Disease, 12(10), 388. https://doi.org/10.3390/jcdd12100388








