Advances in Epicardial Biology: Insights from Development, Regeneration, and Human Cardiac Organoids
Abstract
1. Introduction
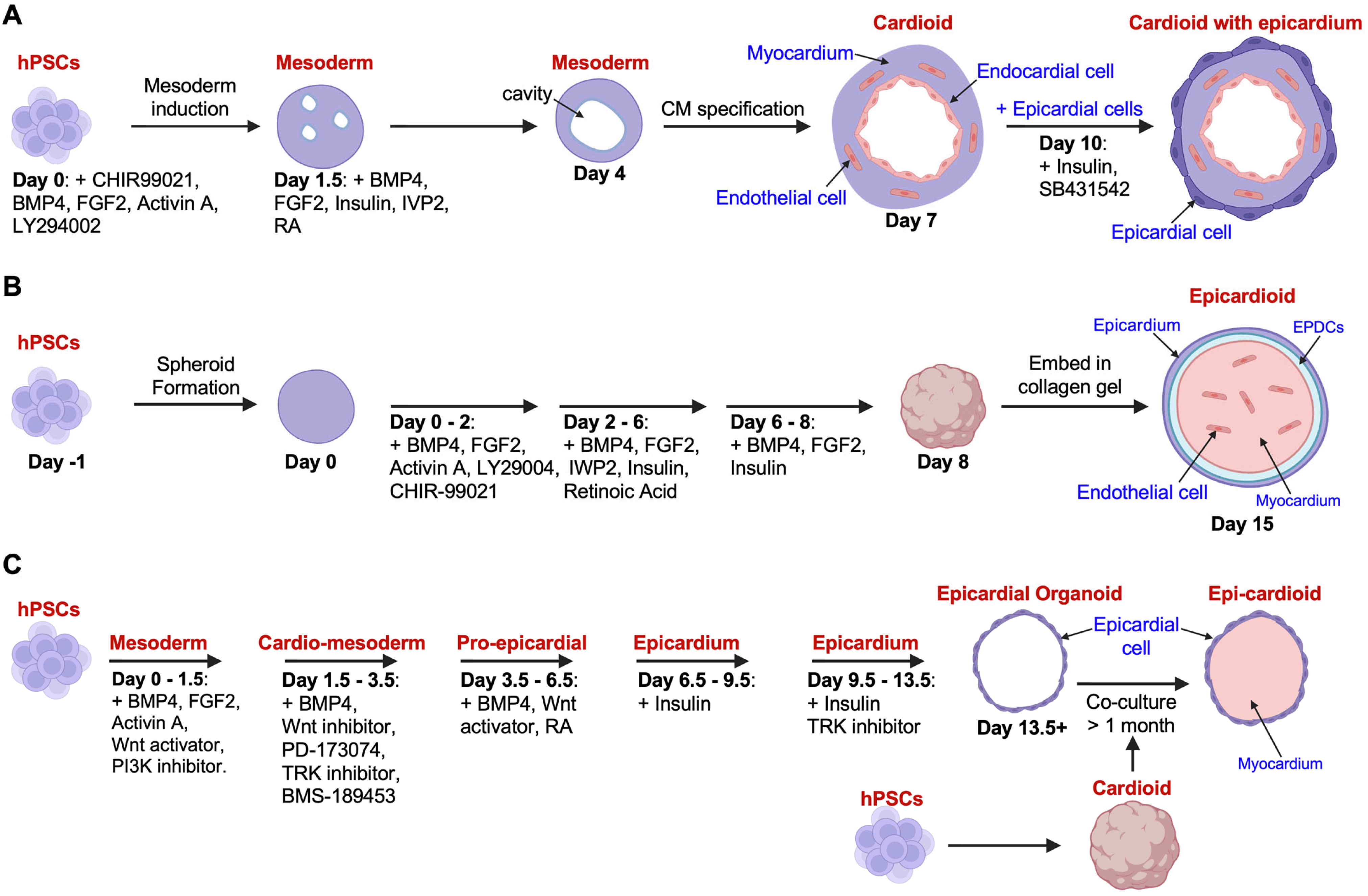
2. Modeling Epicardial Biology Using Human Cardiac Organoids
2.1. Advances in Cardiac Organoid Technology
2.2. Challenges and Future Directions in Cardioid Application
3. The Epicardial Lineage
3.1. Cellular Heterogeneity and EMT
| Consistent Conclusions | |||
|---|---|---|---|
| Cell Fates | Species (Context) | Fate Mapping Approaches and Reagents | References |
| Fibroblast | Chick (Development) | Dye labelling, retroviral labelling, and cell transplantation | [50,51,52,53,54,55] |
| Zebrafish (Post injury) | Transplantation of wt1+ cells | [11] | |
| Mouse (Development) | Tbx18Cre; R26RlacZ | [56] | |
| Tbx18Cre; Rosa26mT/mG | [57] | ||
| Wt1GFPCre; R26RmT/mG | [58] | ||
| Tcf21iCre; R26RYFP or R26RtdT | [59] | ||
| ScxGFPCre; R26RlacZ Sema3DGFPCre; R26RlacZ | [60] | ||
| Mouse (Post injury) | Wt1CreERT2; R26RmT/mG | [13] | |
| Wt1GFPCre; R26R | [61] | ||
| Thymosin β4 treatment Wt1CreERT2; R26RmT/mG | [62] | ||
| Human (in vitro) | Culture of epicardial-like cells derived from hiPSCs (TGFβ1 and BFGF Treatment) | [63] | |
| Culture of primary epicardial cells from human adults (spontaneous differentiation) | [64] | ||
| Culture of epicardial cells derived from H13 hESCs (TGFβ1 and BFGF Treatment) | [65] | ||
| Smooth muscle cell (SMC) | Chick (Development) | Dye labelling, retroviral labelling, and cell transplantation | [50,51,52,53,54,55] |
| Mouse (Development) | Wt1Cre; Rosa26fsLz or Z/Red Wt1CreERT2; Rosa26fsLz or Z/Red | [66] | |
| Tbx18Cre; R26RlacZ | [56] | ||
| Tbx18Cre; Rosa26mT/mG | [57] | ||
| ScxGFPCre; R26RlacZ Sema3DGFPCre; R26RlacZ | [60] | ||
| Mouse (Post Injury) | Wt1CreERT2; R26RmT/mG | [13] | |
| Wt1GFPCre; R26R | [61] | ||
| Thymosin β4 treatment Wt1CreERT2; R26RmT/mG | [62] | ||
| VEGFA modRNA treatment Wt1CreERT2; R26RmT/mG | [67] | ||
| Human (in vitro) | Culture of epicardial-like cells derived from hiPSCs (TGFβ1 and BFGF Treatment) | [63] | |
| Culture of primary epicardial cells from human adults (TGFβ1 or BMP2 Treatment) | [64] | ||
| Culture of epicardial cells derived from H13 hESCs (TGFβ1 and BFGF Treatment) | [65] | ||
| Culture of hPSC-derived epicardial cells (PDGF-BB and TGFβ1 treatment) | [68] | ||
| Pericyte | Mouse (Development) | Tbx18Cre; R26RlacZ | [56] |
| Mouse (Post injury) | Wt1CreERT2; R26RmT/mG | [13] | |
| Wt1CreERT2; R26RYFP | [69] | ||
| Human (in vitro) | Culture of hiPSC-derived epicardial cells (SMAD3 promotes pericyte specification) | [70] | |
| Perivascular cell (pericyte or SMC, not specified) | Zebrafish (Development) | tcf21:CreER; gata5:RnG | [6] |
| Zebrafish (Post Injury) | tcf21:CreER; gata5:RnG | [6] | |
| Transplantation of wt1+ cells | [11] | ||
| ptx3CreERt2; ubi:Switch | [42] | ||
| Adipocyte | Zebrafish (Development) | tcf21:CreER; ubi:Switch | [71] |
| Mouse (Development) | Tbx18Cre; R26RYFP | [72] | |
| Mouse (Post Injury) | Wt1CreERT2; R26RmT/mG or R26RRFP | [73] | |
| Wt1CreERT2; R26RtdT | [74] | ||
| Inconsistent Findings | |||
| Cell Fates (Notes) | Species | Fate Mapping Approaches and Reagents (Notes) | References |
| Cardiomyocyte (not observed in zebrafish studies) | Mouse (Development) | Wt1Cre; Rosa26fsLz or Z/Red Wt1CreERT2; Rosa26fsLz or Z/Red (The Wt1 transgenic lines are not epicardial cell-specific) | [66] |
| Tbx18Cre; R26RlacZ (The Cre line is not epicardial cell-specific) | [56] | ||
| ScxGFPCre; R26RlacZ Sema3DGFPCre; R26RlacZ (Very rare) | [60] | ||
| Mouse (Post injury) | Wt1GFPCre; R26R | [61] | |
| Wt1CreERT2; R26RmT/mG (only upon thymosin β4 treatment) | [62] | ||
| Salamanders (Post injury) | Microinjection of Cre recombinase to label epicardial cells (Labeling specificity could affect the conclusion) | [75] | |
| Human (in vitro) | Culture of hPSC-derived cardioid organoid | [27] | |
| Endocardial cell (There are limited studies. No mention of this fate in other studies.) | Chick (Development) | Dye labelling, retroviral labelling, and cell transplantation | [51,53,54] |
| Mouse (Development) | ScxGFPCre; R26RlacZ Sema3DGFPCre; R26RlacZ | [60] | |
| Endothelial cell (Has not been reported in zebrafish) | Chick (Development) | Dye labelling, retroviral labelling, and cell transplantation | [50,52,53,54,55] |
| Mouse (Development) | Wt1Cre; Rosa26fsLz or Z/Red Wt1CreERT2; Rosa26fsLz or Z/Red (The Cre or CreER line is not epicardial cell-specific) | [66] | |
| ScxGFPCre; R26RlacZ Sema3DGFPCre; R26RlacZ (Tracing of pro-epicardial cells) | [60] | ||
| Mouse (Post Injury) | Wt1GFPCre; R26R | [61] | |
| VEGFA modRNA treatment Wt1CreERT2; R26RmT/mG | [67] | ||
| Human (in vitro) | Culture of epicardial cells derived from hPSCs (VEGF Treatment) | [76] | |
3.2. Contribution to Cardiomyocytes
3.3. Contribution to Non-Cardiomyocytes
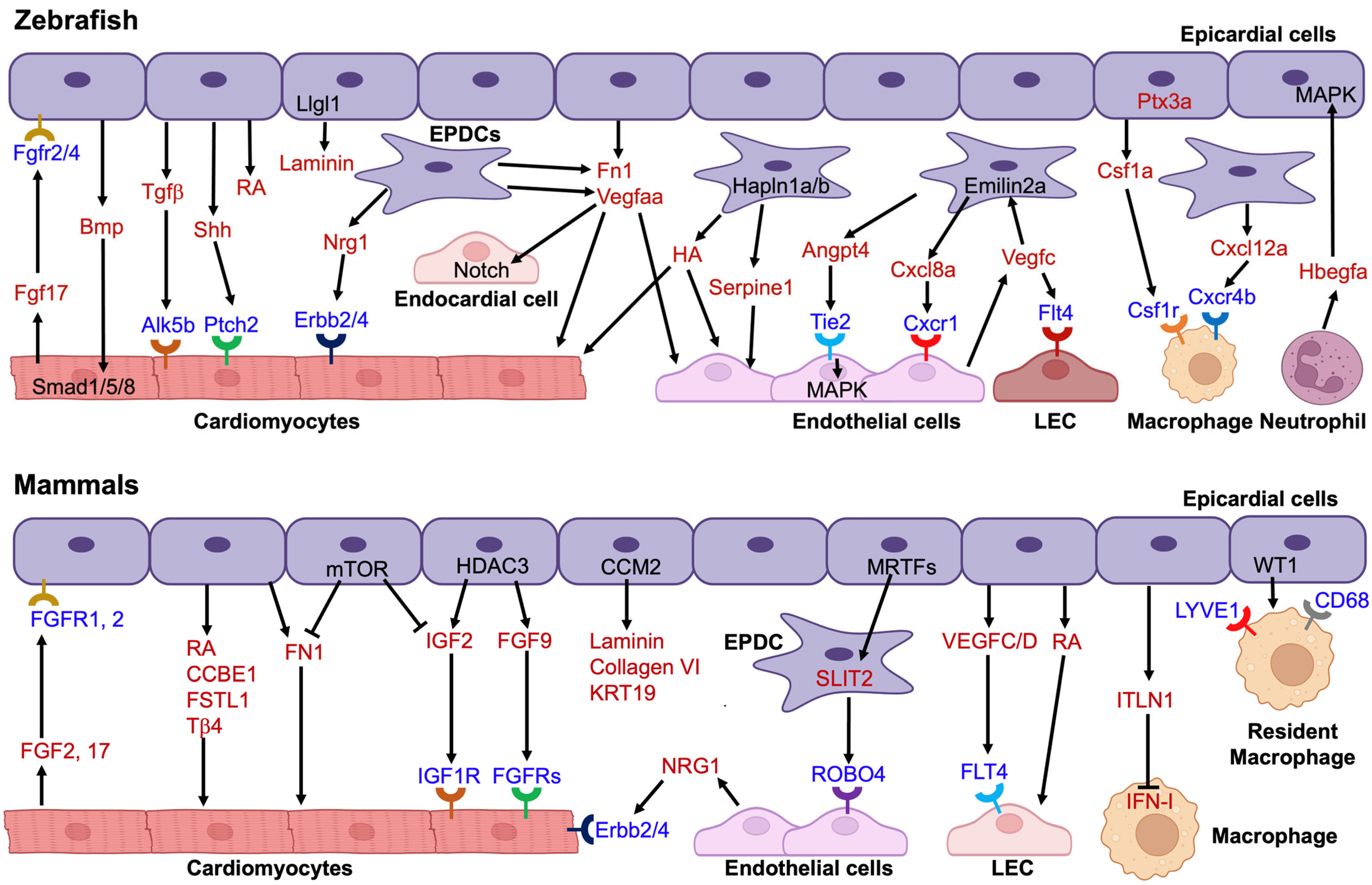
4. Paracrine Signaling and Cellular Crosstalk
4.1. Epicardial-Endothelial Interactions
4.2. Crosstalk with Cardiomyocytes
4.3. Crosstalk with Immune Cells
5. Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, J.; Poss, K.D. The epicardium as a hub for heart regeneration. Nat. Rev. Cardiol. 2018, 15, 631–647. [Google Scholar] [CrossRef]
- Cao, Y.; Duca, S.; Cao, J. Epicardium in Heart Development. Cold Spring Harb. Perspect. Biol. 2020, 12, a037192. [Google Scholar] [CrossRef]
- Simoes, F.C.; Riley, P.R. The ontogeny, activation and function of the epicardium during heart development and regeneration. Development 2018, 145, dev155994. [Google Scholar] [CrossRef]
- Schlueter, J.; Brand, T. Epicardial progenitor cells in cardiac development and regeneration. J. Cardiovasc. Transl. Res. 2012, 5, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Cao, J. Covering and Re-Covering the Heart: Development and Regeneration of the Epicardium. J. Cardiovasc. Dev. Dis. 2018, 6, 3. [Google Scholar] [CrossRef]
- Kikuchi, K.; Gupta, V.; Wang, J.; Holdway, J.E.; Wills, A.A.; Fang, Y.; Poss, K.D. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development 2011, 138, 2895–2902. [Google Scholar] [CrossRef]
- Gemberling, M.; Karra, R.; Dickson, A.L.; Poss, K.D. Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. eLife 2015, 4, e05871. [Google Scholar] [CrossRef]
- Lepilina, A.; Coon, A.N.; Kikuchi, K.; Holdway, J.E.; Roberts, R.W.; Burns, C.G.; Poss, K.D. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 2006, 127, 607–619. [Google Scholar] [CrossRef]
- Wang, J.; Karra, R.; Dickson, A.L.; Poss, K.D. Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Dev. Biol. 2013, 382, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Karra, R.; Foglia, M.J.; Choi, W.Y.; Belliveau, C.; DeBenedittis, P.; Poss, K.D. Vegfaa instructs cardiac muscle hyperplasia in adult zebrafish. Proc. Natl. Acad. Sci. USA 2018, 115, 8805–8810. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rosa, J.M.; Peralta, M.; Mercader, N. Pan-epicardial lineage tracing reveals that epicardium derived cells give rise to myofibroblasts and perivascular cells during zebrafish heart regeneration. Dev. Biol. 2012, 370, 173–186. [Google Scholar] [CrossRef]
- Wang, J.; Cao, J.; Dickson, A.L.; Poss, K.D. Epicardial regeneration is guided by cardiac outflow tract and Hedgehog signalling. Nature 2015, 522, 226–230. [Google Scholar] [CrossRef]
- Zhou, B.; Honor, L.B.; He, H.; Ma, Q.; Oh, J.H.; Butterfield, C.; Lin, R.Z.; Melero-Martin, J.M.; Dolmatova, E.; Duffy, H.S.; et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J. Clin. Investig. 2011, 121, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Serpooshan, V.; Hurtado, C.; Diez-Cunado, M.; Zhao, M.; Maruyama, S.; Zhu, W.; Fajardo, G.; Noseda, M.; Nakamura, K.; et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 2015, 525, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Quijada, P.; Misra, A.; Velasquez, L.S.; Burke, R.M.; Lighthouse, J.K.; Mickelsen, D.M.; Dirkx, R.A., Jr.; Small, E.M. Pre-existing fibroblasts of epicardial origin are the primary source of pathological fibrosis in cardiac ischemia and aging. J. Mol. Cell. Cardiol. 2019, 129, 92–104. [Google Scholar] [CrossRef]
- Cai, W.; Tan, J.; Yan, J.; Zhang, L.; Cai, X.; Wang, H.; Liu, F.; Ye, M.; Cai, C.L. Limited Regeneration Potential with Minimal Epicardial Progenitor Conversions in the Neonatal Mouse Heart after Injury. Cell Rep. 2019, 28, 190–201.e3. [Google Scholar] [CrossRef]
- Bargehr, J.; Ong, L.P.; Colzani, M.; Davaapil, H.; Hofsteen, P.; Bhandari, S.; Gambardella, L.; Le Novere, N.; Iyer, D.; Sampaziotis, F.; et al. Epicardial cells derived from human embryonic stem cells augment cardiomyocyte-driven heart regeneration. Nat. Biotechnol. 2019, 37, 895–906. [Google Scholar] [CrossRef]
- Meier, A.B.; Zawada, D.; De Angelis, M.T.; Martens, L.D.; Santamaria, G.; Zengerle, S.; Nowak-Imialek, M.; Kornherr, J.; Zhang, F.; Tian, Q.; et al. Epicardioid single-cell genomics uncovers principles of human epicardium biology in heart development and disease. Nat. Biotechnol. 2023, 41, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.A.; Dias, T.P.; Cabral, J.M.S.; Pinto-do, O.P.; Diogo, M.M. Human multilineage pro-epicardium/foregut organoids support the development of an epicardium/myocardium organoid. Nat. Commun. 2022, 13, 6981. [Google Scholar] [CrossRef]
- Hofbauer, P.; Jahnel, S.M.; Papai, N.; Giesshammer, M.; Deyett, A.; Schmidt, C.; Penc, M.; Tavernini, K.; Grdseloff, N.; Meledeth, C.; et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell 2021, 184, 3299–3317.e22. [Google Scholar] [CrossRef]
- Zimmermann, W.H.; Schneiderbanger, K.; Schubert, P.; Didie, M.; Munzel, F.; Heubach, J.F.; Kostin, S.; Neuhuber, W.L.; Eschenhagen, T. Tissue engineering of a differentiated cardiac muscle construct. Circ. Res. 2002, 90, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.J.; Titmarsh, D.M.; Koenig, X.; Parker, B.L.; Ryall, J.G.; Quaife-Ryan, G.A.; Voges, H.K.; Hodson, M.P.; Ferguson, C.; Drowley, L.; et al. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc. Natl. Acad. Sci. USA 2017, 114, E8372–E8381. [Google Scholar] [CrossRef] [PubMed]
- Voges, H.K.; Mills, R.J.; Elliott, D.A.; Parton, R.G.; Porrello, E.R.; Hudson, J.E. Development of a human cardiac organoid injury model reveals innate regenerative potential. Development 2017, 144, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Drakhlis, L.; Biswanath, S.; Farr, C.M.; Lupanow, V.; Teske, J.; Ritzenhoff, K.; Franke, A.; Manstein, F.; Bolesani, E.; Kempf, H.; et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol. 2021, 39, 737–746. [Google Scholar] [CrossRef]
- Rossi, G.; Broguiere, N.; Miyamoto, M.; Boni, A.; Guiet, R.; Girgin, M.; Kelly, R.G.; Kwon, C.; Lutolf, M.P. Capturing Cardiogenesis in Gastruloids. Cell Stem Cell 2021, 28, 230–240.e6. [Google Scholar] [CrossRef]
- Wang, F.; Zou, X.; Zheng, H.; Kong, T.; Pei, D. Human epicardial organoids from pluripotent stem cells resemble fetal stage with potential cardiomyocyte- transdifferentiation. Cell Biosci. 2025, 15, 4. [Google Scholar] [CrossRef]
- Thomas, D.; Choi, S.; Alamana, C.; Parker, K.K.; Wu, J.C. Cellular and Engineered Organoids for Cardiovascular Models. Circ. Res. 2022, 130, 1780–1802. [Google Scholar] [CrossRef]
- Hunter, C.R.; Hudson, J.E. Macrophage Containing Cardiac Organoids for Studying Inflammatory Programmes Driving Cardiovascular Disease. Curr. Cardiol. Rep. 2025, 27, 97. [Google Scholar] [CrossRef]
- Landau, S.; Zhao, Y.; Hamidzada, H.; Kent, G.M.; Okhovatian, S.; Lu, R.X.Z.; Liu, C.; Wagner, K.T.; Cheung, K.; Shawky, S.A.; et al. Primitive macrophages enable long-term vascularization of human heart-on-a-chip platforms. Cell Stem Cell 2024, 31, 1222–1238.e10. [Google Scholar] [CrossRef]
- Lock, R.I.; Graney, P.L.; Tavakol, D.N.; Nash, T.R.; Kim, Y.; Sanchez, E., Jr.; Morsink, M.; Ning, D.; Chen, C.; Fleischer, S.; et al. Macrophages enhance contractile force in iPSC-derived human engineered cardiac tissue. Cell Rep. 2024, 43, 114302. [Google Scholar] [CrossRef] [PubMed]
- Rockel, A.; Brunnbauer, T.; Wagner, N.; Gerull, B.; Ergün, S.; Wörsdörfer, P. Tissue resident macrophages innately develop in a human iPSC-derived cardiac organoid model. bioRxiv 2025, 2025.2005.2020.654824. [Google Scholar] [CrossRef]
- Tian, Y.; Lucena-Cacace, A.; Tani, K.; Elvandari, A.P.; Allendes Osorio, R.S.; Narita, M.; Matsumura, Y.; Paixao, I.C.; Miyoshi, Y.; Inagaki, A.; et al. Generation of mature epicardium derived from human-induced pluripotent stem cells via inhibition of mTOR signaling. Nat. Commun. 2025, 16, 5902. [Google Scholar] [CrossRef]
- Tan, Y.; Coyle, R.C.; Barrs, R.W.; Silver, S.E.; Li, M.; Richards, D.J.; Lin, Y.; Jiang, Y.; Wang, H.; Menick, D.R.; et al. Nanowired human cardiac organoid transplantation enables highly efficient and effective recovery of infarcted hearts. Sci. Adv. 2023, 9, eadf2898. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Gherghe, C.; Liu, D.; Hamlett, E.; Srikantha, L.; Rodgers, L.; Regan, J.N.; Rojas, M.; Willis, M.; Leask, A.; et al. Wnt1/betacatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J. 2012, 31, 429–442. [Google Scholar] [CrossRef]
- Smith, C.L.; Baek, S.T.; Sung, C.Y.; Tallquist, M.D. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ. Res. 2011, 108, e15–e26. [Google Scholar] [CrossRef]
- Sridurongrit, S.; Larsson, J.; Schwartz, R.; Ruiz-Lozano, P.; Kaartinen, V. Signaling via the Tgf-beta type I receptor Alk5 in heart development. Dev. Biol. 2008, 322, 208–218. [Google Scholar] [CrossRef]
- Smart, N.; Riley, P.R. The epicardium as a candidate for heart regeneration. Future Cardiol. 2012, 8, 53–69. [Google Scholar] [CrossRef]
- Quijada, P.; Trembley, M.A.; Small, E.M. The Role of the Epicardium During Heart Development and Repair. Circ. Res. 2020, 126, 377–394. [Google Scholar] [CrossRef]
- Cao, J.; Navis, A.; Cox, B.D.; Dickson, A.L.; Gemberling, M.; Karra, R.; Bagnat, M.; Poss, K.D. Single epicardial cell transcriptome sequencing identifies Caveolin 1 as an essential factor in zebrafish heart regeneration. Development 2016, 143, 232–243. [Google Scholar] [CrossRef]
- Weinberger, M.; Simoes, F.C.; Patient, R.; Sauka-Spengler, T.; Riley, P.R. Functional Heterogeneity within the Developing Zebrafish Epicardium. Dev. Cell 2020, 52, 574–590.e6. [Google Scholar] [CrossRef]
- Xia, Y.; Duca, S.; Perder, B.; Dundar, F.; Zumbo, P.; Qiu, M.; Yao, J.; Cao, Y.; Harrison, M.R.M.; Zangi, L.; et al. Activation of a transient progenitor state in the epicardium is required for zebrafish heart regeneration. Nat. Commun. 2022, 13, 7704. [Google Scholar] [CrossRef]
- Sun, J.; Peterson, E.A.; Chen, X.; Wang, J. hapln1a(+) cells guide coronary growth during heart morphogenesis and regeneration. Nat. Commun. 2023, 14, 3505. [Google Scholar] [CrossRef]
- Sun, J.; Peterson, E.A.; Wang, A.Z.; Ou, J.; Smith, K.E.; Poss, K.D.; Wang, J. hapln1 Defines an Epicardial Cell Subpopulation Required for Cardiomyocyte Expansion During Heart Morphogenesis and Regeneration. Circulation 2022, 146, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Rodriguez-Parks, A.; Kim, C.; Silaban, I.M.; Xia, Y.; Sun, J.; Dong, C.; Keles, S.; Wang, J.; Cao, J.; et al. Harnessing the regenerative potential of interleukin11 to enhance heart repair. Nat. Commun. 2024, 15, 9666. [Google Scholar] [CrossRef] [PubMed]
- Hesse, J.; Owenier, C.; Lautwein, T.; Zalfen, R.; Weber, J.F.; Ding, Z.; Alter, C.; Lang, A.; Grandoch, M.; Gerdes, N.; et al. Single-cell transcriptomics defines heterogeneity of epicardial cells and fibroblasts within the infarcted murine heart. eLife 2021, 10, e65921. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Weaver, O.; Ungvijanpunya, N.; Yuan, Y.; Qian, J.; Gou, Y.; Wu, J.; Shen, H.; Chen, Y.; Li, M.; Richard, S.; et al. PRMT1-p53 Pathway Controls Epicardial EMT and Invasion. Cell Rep. 2020, 31, 107739. [Google Scholar] [CrossRef]
- Astanina, E.; Doronzo, G.; Cora, D.; Neri, F.; Oliviero, S.; Genova, T.; Mussano, F.; Middonti, E.; Vallariello, E.; Cencioni, C.; et al. The TFEB-TGIF1 axis regulates EMT in mouse epicardial cells. Nat. Commun. 2022, 13, 5191. [Google Scholar] [CrossRef]
- Streef, T.J.; Groeneveld, E.J.; van Herwaarden, T.; Hjortnaes, J.; Goumans, M.J.; Smits, A.M. Single-cell analysis of human fetal epicardium reveals its cellular composition and identifies CRIP1 as a modulator of EMT. Stem Cell Rep. 2023, 18, 1421–1435. [Google Scholar] [CrossRef]
- Mikawa, T.; Gourdie, R.G. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev. Biol. 1996, 174, 221–232. [Google Scholar] [CrossRef]
- Dettman, R.W.; Pae, S.H.; Morabito, C.; Bristow, J. Inhibition of alpha4-integrin stimulates epicardial-mesenchymal transformation and alters migration and cell fate of epicardially derived mesenchyme. Dev. Biol. 2003, 257, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pomares, J.M.; Phelps, A.; Sedmerova, M.; Carmona, R.; Gonzalez-Iriarte, M.; Munoz-Chapuli, R.; Wessels, A. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: A model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs). Dev. Biol. 2002, 247, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C.; Vrancken Peeters, M.P.; Mentink, M.M.; Gourdie, R.G.; Poelmann, R.E. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ. Res. 1998, 82, 1043–1052. [Google Scholar] [CrossRef]
- Manner, J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat. Rec. 1999, 255, 212–226. [Google Scholar] [CrossRef]
- Guadix, J.A.; Carmona, R.; Munoz-Chapuli, R.; Perez-Pomares, J.M. In vivo and in vitro analysis of the vasculogenic potential of avian proepicardial and epicardial cells. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2006, 235, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.L.; Martin, J.C.; Sun, Y.; Cui, L.; Wang, L.; Ouyang, K.; Yang, L.; Bu, L.; Liang, X.; Zhang, X.; et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 2008, 454, 104–108. [Google Scholar] [CrossRef]
- Grieskamp, T.; Rudat, C.; Ludtke, T.H.; Norden, J.; Kispert, A. Notch signaling regulates smooth muscle differentiation of epicardium-derived cells. Circ. Res. 2011, 108, 813–823. [Google Scholar] [CrossRef]
- Wessels, A.; van den Hoff, M.J.; Adamo, R.F.; Phelps, A.L.; Lockhart, M.M.; Sauls, K.; Briggs, L.E.; Norris, R.A.; van Wijk, B.; Perez-Pomares, J.M.; et al. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev. Biol. 2012, 366, 111–124. [Google Scholar] [CrossRef]
- Acharya, A.; Baek, S.T.; Huang, G.; Eskiocak, B.; Goetsch, S.; Sung, C.Y.; Banfi, S.; Sauer, M.F.; Olsen, G.S.; Duffield, J.S.; et al. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 2012, 139, 2139–2149. [Google Scholar] [CrossRef]
- Katz, T.C.; Singh, M.K.; Degenhardt, K.; Rivera-Feliciano, J.; Johnson, R.L.; Epstein, J.A.; Tabin, C.J. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev. Cell 2012, 22, 639–650. [Google Scholar] [CrossRef]
- van Wijk, B.; Gunst, Q.D.; Moorman, A.F.; van den Hoff, M.J. Cardiac regeneration from activated epicardium. PLoS ONE 2012, 7, e44692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Honor, L.B.; Ma, Q.; Oh, J.H.; Lin, R.Z.; Melero-Martin, J.M.; von Gise, A.; Zhou, P.; Hu, T.; He, L.; et al. Thymosin beta 4 treatment after myocardial infarction does not reprogram epicardial cells into cardiomyocytes. J. Mol. Cell. Cardiol. 2012, 52, 43–47. [Google Scholar] [CrossRef]
- Witty, A.D.; Mihic, A.; Tam, R.Y.; Fisher, S.A.; Mikryukov, A.; Shoichet, M.S.; Li, R.K.; Kattman, S.J.; Keller, G. Generation of the epicardial lineage from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1026–1035. [Google Scholar] [CrossRef]
- van Tuyn, J.; Atsma, D.E.; Winter, E.M.; van der Velde-van Dijke, I.; Pijnappels, D.A.; Bax, N.A.; Knaan-Shanzer, S.; Gittenberger-de Groot, A.C.; Poelmann, R.E.; van der Laarse, A.; et al. Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells 2007, 25, 271–278. [Google Scholar] [CrossRef]
- Bao, X.; Lian, X.; Qian, T.; Bhute, V.J.; Han, T.; Palecek, S.P. Directed differentiation and long-term maintenance of epicardial cells derived from human pluripotent stem cells under fully defined conditions. Nat. Protoc. 2017, 12, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ma, Q.; Rajagopal, S.; Wu, S.M.; Domian, I.; Rivera-Feliciano, J.; Jiang, D.; von Gise, A.; Ikeda, S.; Chien, K.R.; et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 2008, 454, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Zangi, L.; Lui, K.O.; von Gise, A.; Ma, Q.; Ebina, W.; Ptaszek, L.M.; Spater, D.; Xu, H.; Tabebordbar, M.; Gorbatov, R.; et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013, 31, 898–907. [Google Scholar] [CrossRef]
- Iyer, D.; Gambardella, L.; Bernard, W.G.; Serrano, F.; Mascetti, V.L.; Pedersen, R.A.; Talasila, A.; Sinha, S. Robust derivation of epicardium and its differentiated smooth muscle cell progeny from human pluripotent stem cells. Development 2015, 142, 1528–1541. [Google Scholar] [CrossRef]
- Dube, K.N.; Thomas, T.M.; Munshaw, S.; Rohling, M.; Riley, P.R.; Smart, N. Recapitulation of developmental mechanisms to revascularize the ischemic heart. JCI Insight 2017, 2, e96800. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Lucena-Cacace, A.; Tian, Y.; Matsumura, Y.; Tani, K.; Nishikawa, M.; Narita, M.; Kimura, T.; Ono, K.; Yoshida, Y. SMAD3 mediates the specification of human induced pluripotent stem cell-derived epicardium into progenitors for the cardiac pericyte lineage. Stem Cell Rep. 2024, 19, 1399–1416. [Google Scholar] [CrossRef]
- Morocho-Jaramillo, P.A.; Kotlar-Goldaper, I.; Zakarauskas-Seth, B.I.; Purfurst, B.; Filosa, A.; Sawamiphak, S. The zebrafish heart harbors a thermogenic beige fat depot analog of human epicardial adipose tissue. Cell Rep. 2024, 43, 113955. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Cavallero, S.; Patterson, M.; Shen, H.; Xu, J.; Kumar, S.R.; Sucov, H.M. Adipogenesis and epicardial adipose tissue: A novel fate of the epicardium induced by mesenchymal transformation and PPARgamma activation. Proc. Natl. Acad. Sci. USA 2015, 112, 2070–2075. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, X.; Oh, J.H.; Lin, R.Z.; Duan, S.; Yu, Y.; Yang, R.; Qiu, J.; Melero-Martin, J.M.; Pu, W.T.; et al. Epicardium-to-fat transition in injured heart. Cell Res. 2014, 24, 1367–1369. [Google Scholar] [CrossRef]
- Zangi, L.; Oliveira, M.S.; Ye, L.Y.; Ma, Q.; Sultana, N.; Hadas, Y.; Chepurko, E.; Spater, D.; Zhou, B.; Chew, W.L.; et al. Insulin-Like Growth Factor 1 Receptor-Dependent Pathway Drives Epicardial Adipose Tissue Formation After Myocardial Injury. Circulation 2017, 135, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E.; Yen, C.Y.T.; Tsoi, Y.L.; Witman, N.; Elewa, A.; Joven Araus, A.; Wang, H.; Szattler, T.; Umeano, C.H.; Sohlmer, J.; et al. Epicardium-derived cells organize through tight junctions to replenish cardiac muscle in salamanders. Nat. Cell Biol. 2022, 24, 645–658. [Google Scholar] [CrossRef]
- Bao, X.; Bhute, V.J.; Han, T.; Qian, T.; Lian, X.; Palecek, S.P. Human pluripotent stem cell-derived epicardial progenitors can differentiate to endocardial-like endothelial cells. Bioeng. Transl. Med. 2017, 2, 191–201. [Google Scholar] [CrossRef]
- Rudat, C.; Kispert, A. Wt1 and epicardial fate mapping. Circ. Res. 2012, 111, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Christoffels, V.M.; Grieskamp, T.; Norden, J.; Mommersteeg, M.T.; Rudat, C.; Kispert, A. Tbx18 and the fate of epicardial progenitors. Nature 2009, 458, E8–E9. [Google Scholar] [CrossRef]
- Smart, N.; Bollini, S.; Dube, K.N.; Vieira, J.M.; Zhou, B.; Davidson, S.; Yellon, D.; Riegler, J.; Price, A.N.; Lythgoe, M.F.; et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011, 474, 640–644. [Google Scholar] [CrossRef]
- Shameem, M.; Olson, S.L.; Marron Fernandez de Velasco, E.; Kumar, A.; Singh, B.N. Cardiac Fibroblasts: Helping or Hurting. Genes. 2025, 16, 381. [Google Scholar] [CrossRef]
- Trembley, M.A.; Velasquez, L.S.; de Mesy Bentley, K.L.; Small, E.M. Myocardin-related transcription factors control the motility of epicardium-derived cells and the maturation of coronary vessels. Development 2015, 142, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Avolio, E.; Campagnolo, P.; Katare, R.; Madeddu, P. The role of cardiac pericytes in health and disease: Therapeutic targets for myocardial infarction. Nat. Rev. Cardiol. 2024, 21, 106–118. [Google Scholar] [CrossRef]
- Junghof, J.; Kogure, Y.; Yu, T.; Verdugo-Sivianes, E.M.; Narita, M.; Lucena-Cacace, A.; Yoshida, Y. CDH18 is a fetal epicardial biomarker regulating differentiation towards vascular smooth muscle cells. NPJ Regen. Med. 2022, 7, 14. [Google Scholar] [CrossRef]
- Red-Horse, K.; Ueno, H.; Weissman, I.L.; Krasnow, M.A. Coronary arteries form by developmental reprogramming of venous cells. Nature 2010, 464, 549–553. [Google Scholar] [CrossRef]
- Carmona, R.; Barrena, S.; Lopez Gambero, A.J.; Rojas, A.; Munoz-Chapuli, R. Epicardial cell lineages and the origin of the coronary endothelium. FASEB J. 2020, 34, 5223–5239. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, Z.; Lui, W.; Chen, X.; Wang, Y.; Chamberlain, A.A.; Moreno-Rodriguez, R.A.; Markwald, R.R.; O’Rourke, B.P.; Sharp, D.J.; et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell 2012, 151, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Termglinchan, V.; Shao, N.Y.; Itzhaki, I.; Liu, C.; Ma, N.; Tian, L.; Wang, V.Y.; Chang, A.C.Y.; Guo, H.; et al. A Human iPSC Double-Reporter System Enables Purification of Cardiac Lineage Subpopulations with Distinct Function and Drug Response Profiles. Cell Stem Cell 2019, 24, 802–811.e5. [Google Scholar] [CrossRef]
- Li, Y.; He, L.; Huang, X.; Bhaloo, S.I.; Zhao, H.; Zhang, S.; Pu, W.; Tian, X.; Li, Y.; Liu, Q.; et al. Genetic Lineage Tracing of Nonmyocyte Population by Dual Recombinases. Circulation 2018, 138, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Bonet, F.; Anez, S.B.; Inacio, J.M.; Futschik, M.E.; Belo, J.A. CCBE1 Is Essential for Epicardial Function during Myocardium Development. Int. J. Mol. Sci. 2022, 23, 12642. [Google Scholar] [CrossRef]
- Wasserman, A.H.; Huang, A.R.; Lewis-Israeli, Y.R.; Dooley, M.D.; Mitchell, A.L.; Venkatesan, M.; Aguirre, A. Oxytocin promotes epicardial cell activation and heart regeneration after cardiac injury. Front. Cell Dev. Biol. 2022, 10, 985298. [Google Scholar] [CrossRef]
- Wang, R.; Lu, D.; Song, R.; Du, L.; Yang, X.; Wu, S.T.; Wang, X.; Wong, J.; Xu, Z.; Zhao, Q.; et al. Epicardial CCM2 Promotes Cardiac Development and Repair Via its Regulation on Cytoskeletal Reorganization. JACC Basic. Transl. Sci. 2024, 9, 203–219. [Google Scholar] [CrossRef]
- Quijada, P.; Trembley, M.A.; Misra, A.; Myers, J.A.; Baker, C.D.; Perez-Hernandez, M.; Myers, J.R.; Dirkx, R.A., Jr.; Cohen, E.D.; Delmar, M.; et al. Coordination of endothelial cell positioning and fate specification by the epicardium. Nat. Commun. 2021, 12, 4155. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Shi, Y.; Cui, Y.; Xing, X.; Zhang, L.; Liu, D.; Zhang, Y.; Dong, J.; Jin, L.; Pang, M.; et al. Single-cell analysis reveals an Angpt4-initiated EPDC-EC-CM cellular coordination cascade during heart regeneration. Protein Cell 2023, 14, 350–368. [Google Scholar] [CrossRef]
- Knight-Schrijver, V.R.; Davaapil, H.; Bayraktar, S.; Ross, A.D.B.; Kanemaru, K.; Cranley, J.; Dabrowska, M.; Patel, M.; Polanski, K.; He, X.; et al. A single-cell comparison of adult and fetal human epicardium defines the age-associated changes in epicardial activity. Nat. Cardiovasc. Res. 2022, 1, 1215–1229. [Google Scholar] [CrossRef]
- Klotz, L.; Norman, S.; Vieira, J.M.; Masters, M.; Rohling, M.; Dube, K.N.; Bollini, S.; Matsuzaki, F.; Carr, C.A.; Riley, P.R. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 2015, 522, 62–67. [Google Scholar] [CrossRef]
- Brakenhielm, E.; Alitalo, K. Cardiac lymphatics in health and disease. Nat. Rev. Cardiol. 2019, 16, 56–68. [Google Scholar] [CrossRef]
- Karunamuni, G.; Yang, K.; Doughman, Y.Q.; Wikenheiser, J.; Bader, D.; Barnett, J.; Austin, A.; Parsons-Wingerter, P.; Watanabe, M. Expression of lymphatic markers during avian and mouse cardiogenesis. Anat. Rec. 2010, 293, 259–270. [Google Scholar] [CrossRef]
- Lioux, G.; Liu, X.; Temino, S.; Oxendine, M.; Ayala, E.; Ortega, S.; Kelly, R.G.; Oliver, G.; Torres, M. A Second Heart Field-Derived Vasculogenic Niche Contributes to Cardiac Lymphatics. Dev. Cell 2020, 52, 350–363.e6. [Google Scholar] [CrossRef]
- Travisano, S.I.; Harrison, M.R.M.; Thornton, M.E.; Grubbs, B.H.; Quertermous, T.; Lien, C.L. Single-nuclei multiomic analyses identify human cardiac lymphatic endothelial cells associated with coronary arteries in the epicardium. Cell Rep. 2023, 42, 113106. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz, E.; Cadenas, V.; Temino, S.; Oliver, G.; Torres, M. Epicardial VEGFC/D signaling is essential for coronary lymphangiogenesis. EMBO Rep. 2025, 26, 2803–2818. [Google Scholar] [CrossRef] [PubMed]
- Duca, S.; Xia, Y.; Abd Elmagid, L.; Bakis, I.; Qiu, M.; Cao, Y.; Guo, Y.; Eichenbaum, J.V.; McCain, M.L.; Kang, J.; et al. Differential vegfc expression dictates lymphatic response during zebrafish heart development and regeneration. Development 2024, 151, dev202947. [Google Scholar] [CrossRef]
- Harrison, M.R.; Feng, X.; Mo, G.; Aguayo, A.; Villafuerte, J.; Yoshida, T.; Pearson, C.A.; Schulte-Merker, S.; Lien, C.L. Late developing cardiac lymphatic vasculature supports adult zebrafish heart function and regeneration. eLife 2019, 8, e42762. [Google Scholar] [CrossRef]
- El-Sammak, H.; Yang, B.; Guenther, S.; Chen, W.; Marin-Juez, R.; Stainier, D.Y.R. A Vegfc-Emilin2a-Cxcl8a Signaling Axis Required for Zebrafish Cardiac Regeneration. Circ. Res. 2022, 130, 1014–1029. [Google Scholar] [CrossRef]
- Lavine, K.J.; Yu, K.; White, A.C.; Zhang, X.; Smith, C.; Partanen, J.; Ornitz, D.M. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev. Cell 2005, 8, 85–95. [Google Scholar] [CrossRef]
- Huang, Y.; Harrison, M.R.; Osorio, A.; Kim, J.; Baugh, A.; Duan, C.; Sucov, H.M.; Lien, C.L. Igf Signaling is Required for Cardiomyocyte Proliferation during Zebrafish Heart Development and Regeneration. PLoS ONE 2013, 8, e67266. [Google Scholar] [CrossRef]
- Chen, T.; Chang, T.C.; Kang, J.O.; Choudhary, B.; Makita, T.; Tran, C.M.; Burch, J.B.; Eid, H.; Sucov, H.M. Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Dev. Biol. 2002, 250, 198–207. [Google Scholar] [CrossRef]
- Jang, J.; Song, G.; Pettit, S.M.; Li, Q.; Song, X.; Cai, C.L.; Kaushal, S.; Li, D. Epicardial HDAC3 Promotes Myocardial Growth Through a Novel MicroRNA Pathway. Circ. Res. 2022, 131, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Pollitt, E.J.G.; Sanchez-Posada, J.; Snashall, C.M.; Derrick, C.J.; Noel, E.S. Llgl1 mediates timely epicardial emergence and establishment of an apical laminin sheath around the trabeculating cardiac ventricle. Development 2024, 151, dev202482. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.P.; Bargehr, J.; Knight-Schrijver, V.R.; Lee, J.; Colzani, M.; Bayraktar, S.; Bernard, W.G.; Marchiano, S.; Bertero, A.; Murry, C.E.; et al. Epicardially secreted fibronectin drives cardiomyocyte maturation in 3D-engineered heart tissues. Stem Cell Rep. 2023, 18, 936–951. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.J.; Guyette, J.P.; Miki, K.; Xiao, L.; Kaur, G.; Wu, T.; Zhu, L.; Hansen, K.J.; Ling, K.H.; Milan, D.J.; et al. Human iPS-derived pre-epicardial cells direct cardiomyocyte aggregation expansion and organization in vitro. Nat. Commun. 2021, 12, 4997. [Google Scholar] [CrossRef]
- Floy, M.E.; Dunn, K.K.; Mateyka, T.D.; Reichardt, I.M.; Steinberg, A.B.; Palecek, S.P. Direct coculture of human pluripotent stem cell-derived cardiac progenitor cells with epicardial cells induces cardiomyocyte proliferation and reduces sarcomere organization. J. Mol. Cell. Cardiol. 2022, 162, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Givens, S.E.; Andebrhan, A.A.; Wang, R.; Kong, X.; Rothermel, T.M.; Hosseini, S.; Xie, A.; Shameem, M.; Torniainen, A.A.; Ebrahimi-Barough, S.; et al. Developmental cues from epicardial cells simultaneously promote cardiomyocyte proliferation and electrochemical maturation. Stem Cell Rep. 2025, 20, 102572. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Lavine, K.J.; Beaudin, A.E.; Sojka, D.K.; Carrero, J.A.; Calderon, B.; Brija, T.; Gautier, E.L.; Ivanov, S.; Satpathy, A.T.; et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014, 40, 91–104. [Google Scholar] [CrossRef]
- Stevens, S.M.; von Gise, A.; VanDusen, N.; Zhou, B.; Pu, W.T. Epicardium is required for cardiac seeding by yolk sac macrophages, precursors of resident macrophages of the adult heart. Dev. Biol. 2016, 413, 153–159. [Google Scholar] [CrossRef]
- Sun, J.; Peterson, E.A.; Chen, X.; Wang, J. ptx3a(+) fibroblast/epicardial cells provide a transient macrophage niche to promote heart regeneration. Cell Rep. 2024, 43, 114092. [Google Scholar]
- Bruton, F.A.; Kaveh, A.; Ross-Stewart, K.M.; Matrone, G.; Oremek, M.E.M.; Solomonidis, E.G.; Tucker, C.S.; Mullins, J.J.; Lucas, C.D.; Brittan, M.; et al. Macrophages trigger cardiomyocyte proliferation by increasing epicardial vegfaa expression during larval zebrafish heart regeneration. Dev. Cell 2022, 57, 1512–1528.e5. [Google Scholar] [CrossRef]
- Luo, X.L.; Jiang, Y.; Li, Q.; Yu, X.J.; Ma, T.; Cao, H.; Ke, M.X.; Zhang, P.; Tan, J.L.; Gong, Y.S.; et al. hESC-Derived Epicardial Cells Promote Repair of Infarcted Hearts in Mouse and Swine. Adv. Sci. 2023, 10, e2300470. [Google Scholar] [CrossRef]
- Peterson, E.A.; Sun, J.; Chen, X.; Wang, J. Neutrophils facilitate the epicardial regenerative response after zebrafish heart injury. Dev. Biol. 2024, 508, 93–106. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, S.; Yao, A.G.C.; Xia, Y.; Cao, J. Advances in Epicardial Biology: Insights from Development, Regeneration, and Human Cardiac Organoids. J. Cardiovasc. Dev. Dis. 2025, 12, 389. https://doi.org/10.3390/jcdd12100389
Lyu S, Yao AGC, Xia Y, Cao J. Advances in Epicardial Biology: Insights from Development, Regeneration, and Human Cardiac Organoids. Journal of Cardiovascular Development and Disease. 2025; 12(10):389. https://doi.org/10.3390/jcdd12100389
Chicago/Turabian StyleLyu, Shasha, Alvin Gea Chen Yao, Yu Xia, and Jingli Cao. 2025. "Advances in Epicardial Biology: Insights from Development, Regeneration, and Human Cardiac Organoids" Journal of Cardiovascular Development and Disease 12, no. 10: 389. https://doi.org/10.3390/jcdd12100389
APA StyleLyu, S., Yao, A. G. C., Xia, Y., & Cao, J. (2025). Advances in Epicardial Biology: Insights from Development, Regeneration, and Human Cardiac Organoids. Journal of Cardiovascular Development and Disease, 12(10), 389. https://doi.org/10.3390/jcdd12100389






