Effect of Acute Grape Seed Extract Supplementation on Heart Rate Recovery in Young Individuals
Abstract
1. Introduction
2. Materials and Methods
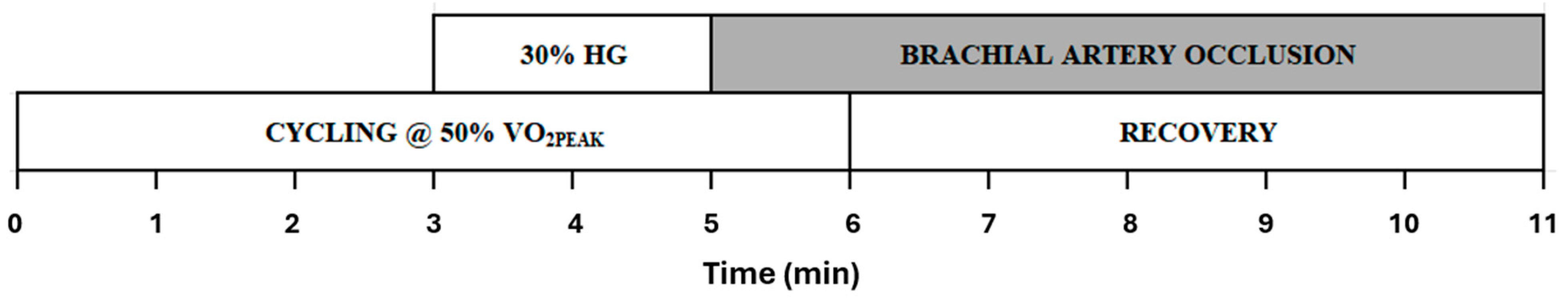
2.1. Experimental Procedures
2.2. Measurement of Hemodynamic Responses
2.3. Supplementations
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Heart Rate Recovery Kinetics and MMA
4.2. Hemodynamic Responses to GSE Supplementation
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef] [PubMed]
- Ardell, J.L.; Andresen, M.C.; Armour, J.A.; Billman, G.E.; Chen, P.S.; Foreman, R.D.; Herring, N.; O’Leary, D.S.; Sabbah, H.N.; Schultz, H.D.; et al. Translational neurocardiology: Preclinical models and cardioneural integrative aspects. J. Physiol. 2016, 594, 3877–3909. [Google Scholar] [CrossRef] [PubMed]
- Shivkumar, K.; Ajijola, O.A.; Anand, I.; Armour, J.A.; Chen, P.S.; Esler, M.; De Ferrari, G.M.; Fishbein, M.C.; Goldberger, J.J.; Harper, R.M.; et al. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J. Physiol. 2016, 594, 3911–3954. [Google Scholar] [CrossRef] [PubMed]
- Florea, V.G.; Cohn, J.N. The autonomic nervous system and heart failure. Circ. Res. 2014, 114, 1815–1826. [Google Scholar] [CrossRef]
- Zygmunt, A.; Stanczyk, J. Methods of evaluation of autonomic nervous system function. Arch. Med. Sci. 2010, 6, 11–18. [Google Scholar] [CrossRef]
- Aneni, E.; Roberson, L.L.; Shaharyar, S.; Blaha, M.J.; Agatston, A.A.; Blumenthal, R.S.; Meneghelo, R.S.; Conceicao, R.D.; Nasir, K.; Santos, R.D. Delayed heart rate recovery is strongly associated with early and late-stage prehypertension during exercise stress testing. Am. J. Hypertens. 2014, 27, 514–521. [Google Scholar] [CrossRef]
- Sydo, N.; Sydo, T.; Gonzalez Carta, K.A.; Hussain, N.; Farooq, S.; Murphy, J.G.; Merkely, B.; Lopez-Jimenez, F.; Allison, T.G. Prognostic Performance of Heart Rate Recovery on an Exercise Test in a Primary Prevention Population. J. Am. Heart Assoc. 2018, 7, e008143. [Google Scholar] [CrossRef]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G. Autonomic imbalance and metabolic syndrome: Unravelling interactions, mechanisms and outcomes. J. Hypertens. 2006, 24, 47–49. [Google Scholar] [CrossRef]
- Pecanha, T.; Bartels, R.; Brito, L.C.; Paula-Ribeiro, M.; Oliveira, R.S.; Goldberger, J.J. Methods of assessment of the post-exercise cardiac autonomic recovery: A methodological review. Int. J. Cardiol. 2017, 227, 795–802. [Google Scholar] [CrossRef]
- Greaney, J.L.; Schwartz, C.E.; Edwards, D.G.; Fadel, P.J.; Farquhar, W.B. The neural interaction between the arterial baroreflex and muscle metaboreflex is preserved in older men. Exp. Physiol. 2013, 98, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.L.; Victor, R.G.; Nerhed, C.; Wallin, B.G. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ. Res. 1985, 57, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Victor, R.G.; Seals, D.R.; Mark, A.L. Differential control of heart rate and sympathetic nerve activity during dynamic exercise. J. Clin. Investig. 1987, 79, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.J.; Huang, S.M.; De Petrocellis, L.; Bisogno, T.; Ewing, S.A.; Miller, J.D.; Zipkin, R.E.; Daddario, N.; Appendino, G.; Di Marzo, V.; et al. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J. Biol. Chem. 2003, 278, 13633–13639. [Google Scholar] [CrossRef]
- Huang, S.M.; Bisogno, T.; Trevisani, M.; Al-Hayani, A.; De Petrocellis, L.; Fezza, F.; Tognetto, M.; Petros, T.J.; Krey, J.F.; Chu, C.J.; et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 8400–8405. [Google Scholar] [CrossRef]
- Ji, R.R.; Samad, T.A.; Jin, S.X.; Schmoll, R.; Woolf, C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002, 36, 57–68. [Google Scholar] [CrossRef]
- Mannozzi, J.; Al-Hassan, M.H.; Lessanework, B.; Alvarez, A.; Senador, D.; O’Leary, D.S. Chronic ablation of TRPV1-sensitive skeletal muscle afferents attenuates the muscle metaboreflex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R385–R395. [Google Scholar] [CrossRef]
- Olah, Z.; Karai, L.; Iadarola, M.J. Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J. Biol. Chem. 2001, 276, 31163–31170. [Google Scholar] [CrossRef]
- Boyes, N.G.; Mannozzi, J.; Rapin, N.; Alvarez, A.; Al-Hassan, M.H.; Lessanework, B.; Lahti, D.S.; Olver, T.D.; O’Leary, D.S.; Tomczak, C.R. Augmented sympathoexcitation slows postexercise heart rate recovery. J. Appl. Physiol. 2023, 135, 1300–1311. [Google Scholar] [CrossRef]
- Coutsos, M.; Sala-Mercado, J.A.; Ichinose, M.; Li, Z.; Dawe, E.J.; O’Leary, D.S. Muscle metaboreflex-induced coronary vasoconstriction functionally limits increases in ventricular contractility. J. Appl. Physiol. 2010, 109, 271–278. [Google Scholar] [CrossRef]
- Coutsos, M.; Sala-Mercado, J.A.; Ichinose, M.; Li, Z.; Dawe, E.J.; O’Leary, D.S. Muscle metaboreflex-induced coronary vasoconstriction limits ventricular contractility during dynamic exercise in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1029–H1037. [Google Scholar] [CrossRef]
- O’Leary, D.S. Autonomic mechanisms of muscle metaboreflex control of heart rate. J. Appl. Physiol. 1993, 74, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.S.; Sala-Mercado, J.A.; Hammond, R.L.; Ansorge, E.J.; Kim, J.K.; Rodriguez, J.; Fano, D.; Ichinose, M. Muscle metaboreflex-induced increases in cardiac sympathetic activity vasoconstrict the coronary vasculature. J. Appl. Physiol. 2007, 103, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Sala-Mercado, J.A.; Hammond, R.L.; Kim, J.K.; Rossi, N.F.; Stephenson, L.W.; O’Leary, D.S. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H751–H757. [Google Scholar] [CrossRef] [PubMed]
- Spranger, M.D.; Kaur, J.; Sala-Mercado, J.A.; Krishnan, A.C.; Abu-Hamdah, R.; Alvarez, A.; Machado, T.M.; Augustyniak, R.A.; O’Leary, D.S. Exaggerated coronary vasoconstriction limits muscle metaboreflex-induced increases in ventricular performance in hypertension. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H68–H79. [Google Scholar] [CrossRef]
- Notay, K.; Incognito, A.V.; Millar, P.J. Acute beetroot juice supplementation on sympathetic nerve activity: A randomized, double-blind, placebo-controlled proof-of-concept study. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H59–H65. [Google Scholar] [CrossRef]
- Shrikhande, A.J.; Wang, H.; Kupina, S.A. Grape Extract, Dietary Supplement Thereof, and Processes Therefor. US Patent B2:8075929, 13 December 2011. [Google Scholar]
- Dillon, K.; Shariffi, B.; Gillum, T.; Boyer, W.; Sullivan, S.; Kim, J.K. Effects of chronic dietary grape seed extract supplementation on aortic stiffness and hemodynamic responses in obese/overweight males during submaximal exercise. Eur. J. Sport Sci. 2022, 22, 1057–1064. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, K.A.; Choi, H.M.; Park, S.K.; Stebbins, C.L. Grape Seed Extract Supplementation Attenuates the Blood Pressure Response to Exercise in Prehypertensive Men. J. Med. Food 2018, 21, 445–453. [Google Scholar] [CrossRef]
- Quiñones, M.; Guerrero, L.; Fernández-Vallinas, S.; Pons, Z.; Arola, L.; Aleixandre, A.; Muguerza, B. Involvement of nitric oxide and prostacyclin in the antihypertensive effect of low-molecular-weight procyanidin rich grape seed extract in male spontaneously hypertensive rats. J. Funct. Foods 2014, 6, 419–427. [Google Scholar] [CrossRef]
- Kim, K.A.; Stebbins, C.L.; Choi, H.M.; Nho, H.; Kim, J.K. Mechanisms Underlying Exaggerated Metaboreflex Activation in Prehypertensive Men. Med. Sci. Sports Exerc. 2015, 47, 1605–1612. [Google Scholar] [CrossRef]
- Katayama, K.; Saito, M. Muscle sympathetic nerve activity during exercise. J. Physiol. Sci. 2019, 69, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.; Shariffi, B.; Gillum, T.L.; William, B.; Sullivan, S.; Kim, J.K. Effects of combined histamine H(1) and H(2) receptor blockade on hemodynamic responses to dynamic exercise in males with high-normal blood pressure. Appl. Physiol. Nutr. Metab. 2020, 45, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Apilado, A.; Boyer, W.; Gillum, T.; Sullivan, S.; Harveson, A.; Lira, A.; Kim, J.K. Effects of acute grape seed extract supplementation on muscle metaboreflex in healthy young individuals. Sport Sci. Health 2025, 45, e70010. [Google Scholar] [CrossRef]
- Best, S.A.; Bivens, T.B.; Dean Palmer, M.; Boyd, K.N.; Melyn Galbreath, M.; Okada, Y.; Carrick-Ranson, G.; Fujimoto, N.; Shibata, S.; Hastings, J.L.; et al. Heart rate recovery after maximal exercise is blunted in hypertensive seniors. J. Appl. Physiol. 2014, 117, 1302–1307. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B.; Ahmaidi, S. Parasympathetic reactivation after repeated sprint exercise. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H133–H141. [Google Scholar] [CrossRef]
- Lauer, M.S. Heart rate recovery: What now? J. Intern. Med. 2011, 270, 597–599. [Google Scholar] [CrossRef]
- Cole, C.R.; Foody, J.M.; Blackstone, E.H.; Lauer, M.S. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann. Intern. Med. 2000, 132, 552–555. [Google Scholar] [CrossRef]
- Erdogan, D.; Gonul, E.; Icli, A.; Yucel, H.; Arslan, A.; Akcay, S.; Ozaydin, M. Effects of normal blood pressure, prehypertension, and hypertension on autonomic nervous system function. Int. J. Cardiol. 2011, 151, 50–53. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Sun, Z.; Li, L.; Zuegel, M.; Steinacker, J.M.; Schumann, U. Heart Rate Recovery and Risk of Cardiovascular Events and All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2017, 6, e005505. [Google Scholar] [CrossRef]
- Nishime, E.O.; Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Lauer, M.S. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA 2000, 284, 1392–1398. [Google Scholar] [CrossRef]




| Variables | (n = 12) |
|---|---|
| Age (yrs) | 22.2 ± 1 |
| Height (cm) | 176.3 ± 2 |
| Weight (kg) | 75.3 ± 3 |
| Body mass index (kg/m2) | 24.3 ± 1 |
| SBP (mmHg) | 101.7 ± 3.2 |
| DBP (mmHg) | 65.2 ± 2.8 |
| HR (bpm) | 68.7 ± 2 |
| Variables | (n = 12) | |
|---|---|---|
| PL | GSE | |
| HR (bpm) | 83 ± 2.4 | 80.0 ± 2.6 |
| SV (mL) | 83.4 ± 2.7 | 81.4 ± 4.0 |
| CO (L/min) | 6.9 ± 0.3 | 6.5 ± 0.3 |
| SBP (mmHg) | 105.8 ± 2.5 | 100.8 ± 3 |
| DBP (mmHg) | 65.2 ± 2.8 | 65.5 ± 2.8 |
| MAP (mmHg) | 78.7 ± 2.3 | 77.3 ± 2.3 |
| TPR (mmHg/L/min) | 11.5 ± 0.4 | 12.4 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.S.; Boyer, W.; Gillum, T.; Sullivan, S.; Yoon, I.; Bai, J.; Kim, S.-J.; Kim, J.-K. Effect of Acute Grape Seed Extract Supplementation on Heart Rate Recovery in Young Individuals. J. Cardiovasc. Dev. Dis. 2025, 12, 387. https://doi.org/10.3390/jcdd12100387
Song DS, Boyer W, Gillum T, Sullivan S, Yoon I, Bai J, Kim S-J, Kim J-K. Effect of Acute Grape Seed Extract Supplementation on Heart Rate Recovery in Young Individuals. Journal of Cardiovascular Development and Disease. 2025; 12(10):387. https://doi.org/10.3390/jcdd12100387
Chicago/Turabian StyleSong, Dae Sik, William Boyer, Trevor Gillum, Sean Sullivan, Iltark Yoon, Junbei Bai, Seung-Jae Kim, and Jong-Kyung Kim. 2025. "Effect of Acute Grape Seed Extract Supplementation on Heart Rate Recovery in Young Individuals" Journal of Cardiovascular Development and Disease 12, no. 10: 387. https://doi.org/10.3390/jcdd12100387
APA StyleSong, D. S., Boyer, W., Gillum, T., Sullivan, S., Yoon, I., Bai, J., Kim, S.-J., & Kim, J.-K. (2025). Effect of Acute Grape Seed Extract Supplementation on Heart Rate Recovery in Young Individuals. Journal of Cardiovascular Development and Disease, 12(10), 387. https://doi.org/10.3390/jcdd12100387






