Heart at Hand: The Role of Point-of-Care Cardiac Ultrasound in Internal Medicine
Abstract
1. Introduction
2. Materials and Methods
- -
- Publication type: influential guidelines, clinical trials randomized controlled trials, observational studies, systematic reviews and meta-analysis, and narrative reviews (published in the last 10 years to avoid outdate data and represent the actual clinical practice)
- -
- Subject matter: echocardiography and FoCUS in internal medicine.
- -
- Clinical focus: diagnosis and management of dyspnea, hypotension, heart failure, and chest pain.
- -
- Search terms: FoCUS, Bedside echocardiography, point of care cardiac echocardiography (POCUS), differential diagnosis of dyspnea, hypotension or shock, chest pain, heart failure
- -
- Bibliographic review: inclusion of studies identified through reference lists.
- -
- Practical relevance: preference for studies with direct applicability to real-world clinical practice and patient outcomes.
- -
- Irrelevance to bedside practice: studies focusing exclusively on advanced or highly specialized echocardiographic techniques without applicability to internal medicine wards.
- -
- Non-clinical content: technical or engineering papers without direct patient care implications.
- -
- Obsolete evidence: publications based on outdated technology, protocols, or training models no longer in current use.
- -
- Insufficient detail: articles lacking clear methodological description or outcome reporting, preventing meaningful clinical interpretation.
- -
- Non-peer-reviewed sources: conference abstracts, opinion pieces, or educational materials not subjected to peer review.
- -
- Population mismatch: studies conducted exclusively in pediatric, surgical, or highly selected outpatient cohorts not representative of internal medicine inpatients.
3. Learning Curve
- Is there left ventricular systolic dysfunction?
- Is the IVC dilated or collapsible?
- Is there pericardial effusion or tamponade?
- Are there signs of the right ventricular strain?
4. Accuracy
5. Echocardiography in Stable Hospitalized Patients
- a.
- Evaluation of suspected heart failure
- 1.
- Focused Cardiac Ultrasound
- 2.
- Assessment of right atrial pressure
- 3.
- Lung ultrasound evaluation
- b.
- Assessment of new cardiac murmurs
- c.
- Assessment of suspected infective endocarditis
6. Echocardiography in Internal Medicine Emergencies
- a.
- Acute dyspnea
- b.
- Hemodynamic instability
- c.
- Acute chest pain
7. Limitations
- Competency standards and accreditation: structured training programs with objective skills assessment and stepwise certification.
- Image archiving: digital storage of echocardiographic clips enables periodic audits, remote supervision, and consultation with specialists, ensuring traceability.
- Continuing education: regular refresher courses and retraining are crucial to maintain competencies and prevent quality drift.
- Audit and peer review: periodic case discussions and image reviews serve as key tools for continuous quality improvement.
- The report should be clear and transparent: the clinician must always specify the scope and depth of the examination performed and state any technical limitations. This prevents ambiguity and makes the interpretive boundaries of the findings explicit to both patients and colleagues.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IVC | Inferior vena cava |
| FoCUS | Focused cardiac ultrasound |
| RAP | Right atrial pressure |
| IE | Infective endocarditis |
| TEE | Transesophageal echocardiography |
References
- Johri, A.M.; Glass, C.; Hill, B.; Jensen, T.; Puentes, W.; Olusanya, O.; Capizzano, J.N.; Dancel, R.; Reierson, K.; Reisinger, N.; et al. The Evolution of Cardiovascular Ultrasound: A Review of Cardiac Point-of-Care Ultrasound (POCUS) Across Specialties. Am. J. Med. 2023, 136, 621–628. [Google Scholar] [CrossRef]
- Kellett, J.; Deane, B. The diagnoses and co-morbidity encountered in the hospital practice of acute internal medicine. Eur. J. Intern. Med. 2007, 18, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Trasca, L.; Popescu, M.R.; Popescu, A.C.; Balanescu, S.M. Echocardiography in the Diagnosis of Cardiomyopathies: Current Status and Future Directions. Rev. Cardiovasc. Med. 2022, 23, 280. [Google Scholar] [CrossRef]
- Cogliati, C.; Torzillo, D.; Casella, F.; Del Medico, M.; Montano, N. Bedside echocardiography in internal medicine: Which are the key questions and answers for our decision-making? Ital. J. Med. 2016, 10, 83–91. [Google Scholar] [CrossRef][Green Version]
- Topoll, A.; Berlacher, K. Enhancing Echocardiography Education in Fellowship. J. Am. Soc. Echocardiogr. 2022, 35, 1000–1001. [Google Scholar] [CrossRef]
- Mumoli, N.; Marengo, S. Clinical utility of echocardiography in internal medicine: A narrative review. Ital. J. Med. 2024, 18, 1802. [Google Scholar] [CrossRef]
- Kühl, M.; Wagner, R.; Bauder, M.; Fenik, Y.; Riessen, R.; Lammerding-Köppel, M.; Gawaz, M.; Fateh-Moghadam, S.; Weyrich, P.; Celebi, N. Student tutors for hands-on training in focused emergency echocardiography--a randomized controlled trial. BMC Med. Educ. 2012, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Jung, J.Y.; Park, J.W.; Lee, M.S.; Lee, Y.H. Operating bedside cardiac ultrasound program in emergency medicine residency: A retrospective observation study from the perspective of performance improvement. PLoS ONE 2021, 16, e0248710. [Google Scholar] [CrossRef]
- Westwood, M.; Almeida, A.G.; Barbato, E.; Delgado, V.; Dellegrottaglie, S.; Fox, K.F.; Gargani, L.; Huber, K.; Maurovich-Horvat, P.; Merino, J.L.; et al. Competency-based cardiac imaging for patient-centred care. A statement of the European Society of Cardiology (ESC). With the contribution of the European Association of Cardiovascular Imaging (EACVI), and the support of the Association of Cardiovascular Nursing & Allied Professions (ACNAP), the Association for Acute CardioVascular Care (ACVC), the European Association of Preventive Cardiology (EAPC), the European Association of Percutaneous Cardiovascular Interventions (EAPCI), the European Heart Rhythm Association (EHRA), and the Heart Failure Association (HFA) of the ESC. Eur. Heart J. Imaging Methods Pract. 2023, 1, qyad023. [Google Scholar]
- Elison, D.M.; McConnaughey, S.; Freeman, R.V.; Sheehan, F.H. Focused cardiac ultrasound training in medical students: Using an independent, simulator-based curriculum to objectively measure skill acquisition and learning curve. Echocardiography 2020, 37, 491–496. [Google Scholar] [CrossRef]
- Canty, D.; Barth, J.; Yang, Y.; Peters, N.; Palmer, A.; Royse, A.; Royse, C. Comparison of learning outcomes for teaching focused cardiac ultrasound to physicians: A supervised human model course versus an eLearning guided self- directed simulator course. J. Crit. Care 2019, 49, 38–44. [Google Scholar] [CrossRef]
- Labovitz, A.J.; Noble, V.E.; Bierig, M.; Goldstein, S.A.; Jones, R.; Kort, S.; Porter, T.R.; Spencer, K.T.; Tayal, V.S.; Wei, K. Focused cardiac ultrasound in the emergent setting: A consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J. Am. Soc. Echocardiogr. 2010, 23, 1225–1230. [Google Scholar] [CrossRef]
- Kimura, B.J. Point-of-care cardiac ultrasound techniques in the physical examination: Better at the bedside. Heart 2017, 103, 987–994. [Google Scholar] [CrossRef]
- Andersen, G.N.; Haugen, B.O.; Graven, T.; Salvesen, O.; Mjølstad, O.C.; Dalen, H. Feasibility and reliability of point-of-care pocket-sized echocardiography. Eur. J. Echocardiogr. 2011, 12, 665–670. [Google Scholar] [CrossRef]
- Michalski, B.; Kasprzak, J.D.; Szymczyk, E.; Lipiec, P. Diagnostic utility and clinical usefulness of the pocket echocardiographic device. Echocardiography 2012, 29, 1–6. [Google Scholar] [CrossRef]
- Razi, R.; Estrada, J.R.; Doll, J.; Spencer, K.T. Bedside hand carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J. Am. Soc. Echocardiogr. 2011, 24, 1319–1324. [Google Scholar] [CrossRef]
- Pastore, M.C.; Mandoli, G.E.; Aboumarie, H.S.; Santoro, C.; Bandera, F.; D’Andrea, A.; Benfari, G.; Esposito, R.; Evola, V.; Sorrentino, R.; et al. Basic and advanced echocardiography in advanced heart failure: An overview. Heart Fail. Rev. 2020, 25, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Mohamed Ali, A.; Wasim, D.; Risnes, I.; Urheim, S. Correlation between Murmurs and Echocardiographic Findings; From an Imaging Cardiologist Point of View. Curr. Probl. Cardiol. 2023, 48, 101479. [Google Scholar] [CrossRef] [PubMed]
- Vilacosta, I.; Olmos, C.; de Agustín, A.; López, J.; Islas, F.; Sarriá, C.; Ferrera, C.; Ortiz-Bautista, C.; Sánchez-Enrique, C.; Vivas, D.; et al. The diagnostic ability of echocardiography for infective endocarditis and its associated complications. Expert Rev. Cardiovasc. Ther. 2015, 13, 1225–1236. [Google Scholar] [CrossRef]
- Kuo, D.C.; Peacock, W.F. Diagnosing and managing acute heart failure in the emergency department. Clin. Exp. Emerg. Med. 2015, 2, 141–149. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Dini, F.L.; Cameli, M.; Stefanini, A.; Aboumarie, H.S.; Lisi, M.; Lindqvist, P.; Henein, M.Y. Echocardiography in the Assessment of Heart Failure Patients. Diagnostics 2024, 14, 2730. [Google Scholar] [CrossRef]
- Neskovic, A.N.; Skinner, H.; Price, S.; Via, G.; De Hert, S.; Stankovic, I.; Galderisi, M.; Donal, E.; Muraru, D.; Sloth, E.; et al. Reviewers: This document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee. Focus cardiac ultrasound core curriculum and core syllabus of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 475–481. [Google Scholar] [CrossRef]
- Neskovic, A.N.; Edvardsen, T.; Galderisi, M.; Garbi, M.; Gullace, G.; Jurcut, R.; Dalen, H.; Hagendorff, A.; Lancellotti, P.; European Association of Cardiovascular Imaging Document Reviewers; et al. Focus cardiac ultrasound: The European Association of Cardiovascular Imaging viewpoint. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 956–960. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar]
- Ruge, M.; Marhefka, G.D. IVC measurement for the noninvasive evaluation of central venous pressure. J. Echocardiogr. 2022, 20, 133–143. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Gargani, L.; Girerd, N.; Platz, E.; Pellicori, P.; Stankovic, I.; Palazzuoli, A.; Pivetta, E.; Miglioranza, M.H.; Soliman-Aboumarie, H.; Agricola, E.; et al. This document was reviewed by members of the 2020–2022 EACVI Scientific Documents Committee. Lung ultrasound in acute and chronic heart failure: A clinical consensus statement of the European Association of Cardiovascular Imaging (EACVI). Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Lichtenstein, D.A. Lung ultrasound in the critically ill. Ann. Intensive Care 2014, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A.; Mezière, G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef]
- Parkin, W.G.; Leaning, M.S. Therapeutic control of the circulation. J. Clin. Monit. Comput. 2008, 22, 391–400. [Google Scholar] [CrossRef]
- Begic, E.; Begic, Z. Accidental Heart Murmurs. Med. Arch. 2017, 71, 284–287. [Google Scholar] [CrossRef]
- Shellenberger, R.A.; Crass, S.; Jevicks, J.; Badhwar, A.; Albright, J.; Kumar, A. Bedside Physical Examination for the Diagnosis of Aortic Stenosis: A Systematic Review and Meta-analysis. CJC Open 2023, 5, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Naqvi, T.Z. Point-of-Care Ultrasound in Detection, Severity and Mechanism of Significant Valvular Heart Disease and Clinical Management. J. Clin. Med. 2023, 12, 6474. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Bai, A.D.; Steinberg, M.; Showler, A.; Burry, L.; Bhatia, R.S.; Tomlinson, G.A.; Bell, C.M.; Morris, A.M. Diagnostic Accuracy of Transthoracic Echocardiography for Infective Endocarditis Findings Using Transesophageal Echocardiography as the Reference Standard: A Meta-Analysis. J. Am. Soc. Echocardiogr. 2017, 30, 639–646. [Google Scholar] [CrossRef]
- Li, M.; Kim, J.B.; Sastry, B.K.S.; Chen, M. Infective endocarditis. Lancet 2024, 404, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Badano, L.; Tribouilloy, C.; Vilacosta, I.; Zamorano, J.L.; Galderisi, M.; Voigt, J.U.; Sicari, R.; Cosyns, B.; Fox, K.; et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur. J. Echocardiogr. 2010, 11, 202–219. [Google Scholar] [CrossRef]
- Horgan, S.J.; Mediratta, A.; Gillam, L.D. Cardiovascular Imaging in Infective Endocarditis: A Multimodality Approach. Circ. Cardiovasc. Imaging 2020, 13, e008956. [Google Scholar] [CrossRef]
- Tayal, V.S.; Kline, J.A. Emergency echocardiography to detect pericardial effusion in patients in PEA and near-PEA states. Resuscitation 2003, 59, 315–318. [Google Scholar] [CrossRef]
- Haji, D.L.; Royse, A.; Royse, C.F. Review article: Clinical impact of non-cardiologist-performed transthoracic echocardiography in emergency medicine, intensive care medicine and anaesthesia. Emerg. Med. Australas. 2013, 25, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Zanza, C.; Longhitano, Y.; Artico, M.; Cammarota, G.; Barbanera, A.; Racca, F.; Audo, A.; Ravera, E.; Migneco, A.; Piccioni, A.; et al. Bedside Cardiac Pocus in Emergency Setting: A Practice Review. Rev. Recent Clin. Trials 2020, 15, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Flamanc, T.; de Carvalho, H.; Le Bastard, Q.; Javaudin, F.; Pes, P.; Montassier, E.; Le Conte, P. Impact of an enhanced focused cardiac ultrasound on treatment changes in a population of internal medicine patients. J. Clin. Ultrasound. 2024, 52, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.M.; Bansal, M.; Sengupta, P.P. Advances in Echocardiographic Imaging in Heart Failure with Reduced and Preserved Ejection Fraction. Circ. Res. 2016, 119, 357–374. [Google Scholar] [CrossRef]
- Keikha, M.; Salehi-Marzijarani, M.; Soldoozi Nejat, R.; Sheikh Motahar Vahedi, H.; Mirrezaie, S.M. Diagnostic Accuracy of Rapid Ultrasound in Shock (RUSH) Exam; A Systematic Review and Meta-analysis. Bull. Emerg. Trauma 2018, 6, 271–278. [Google Scholar] [CrossRef]
- Oh, J.K.; Park, J.H. Role of echocardiography in acute pulmonary embolism. Korean J. Intern. Med. 2023, 38, 456–470. [Google Scholar] [CrossRef]
- Pastore, M.C.; Ilardi, F.; Stefanini, A.; Mandoli, G.E.; Palermi, S.; Bandera, F.; Benfari, G.; Esposito, R.; Lisi, M.; Pasquini, A.; et al. Bedside Ultrasound for Hemodynamic Monitoring in Cardiac Intensive Care Unit. J. Clin. Med. 2022, 11, 7538. [Google Scholar] [CrossRef]
- McConnell, M.V.; Solomon, S.D.; Rayan, M.E.; Come, P.C.; Goldhaber, S.Z.; Lee, R.T. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am. J. Cardiol. 1996, 78, 469–473. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Kennedy Hall, M.; Coffey, E.C.; Herbst, M.; Liu, R.; Pare, J.R.; Andrew Taylor, R.; Thomas, S.; Moore, C.L. The “5Es” of emergency physician-performed focused cardiac ultrasound: A protocol for rapid identification of effusion, ejection, equality, exit, and entrance. Acad. Emerg. Med. 2015, 22, 583–593. [Google Scholar] [CrossRef]
- Imazio, M.; Mayosi, B.M.; Brucato, A.; Adler, Y. Pericardial effusion triage. Int. J. Cardiol. 2010, 145, 403–404. [Google Scholar] [CrossRef]
- Koh, N.; Nieman, K. Role of cardiac imaging in acute chest pain. Br. J. Radiol. 2023, 96, 20220307. [Google Scholar] [CrossRef]
- Liu, F.; Huang, L. Usefulness of ultrasound in the management of aortic dissection. Rev. Cardiovasc. Med. 2018, 19, 103–109. [Google Scholar] [CrossRef]
- Evangelista, A.; Flachskampf, F.A.; Erbel, R.; Antonini-Canterin, F.; Vlachopoulos, C.; Rocchi, G.; Sicari, R.; Nihoyannopoulos, P.; Zamorano, J.; European Association of Echocardiography; et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur. J. Echocardiogr. 2010, 11, 645–658. [Google Scholar] [CrossRef]
- Lancellotti, P.; Price, S.; Edvardsen, T.; Cosyns, B.; Neskovic, A.N.; Dulgheru, R.; Flachskampf, F.A.; Hassager, C.; Pasquet, A.; Gargani, L.; et al. The use of echocardiography in acute cardiovascular care: Recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 3–5. [Google Scholar] [PubMed]
- Mathews, B.K.; Zwank, M. Hospital Medicine Point of Care Ultrasound Credentialing: An Example Protocol. J. Hosp. Med. 2017, 12, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Dayan, R.R.; Karni, O.; Shitrit, I.B.; Gaufberg, R.; Ilan, K.; Fuchs, L. Principles for Developing a Large-Scale Point-of-Care Ultrasound Education Program: Insights from a Tertiary University Medical Center in Israel. Perspect. Med. Educ. 2025, 14, 319–327. [Google Scholar] [CrossRef]
- Elhassan, M.G.; Grewal, S.; Nezarat, N. Point-of-Care Ultrasonography in Internal Medicine: Limitations and Pitfalls for Novice Users. Cureus 2023, 15, e43655. [Google Scholar] [CrossRef]
- Jenkins, S.; Alabed, S.; Swift, A.; Marques, G.; Ryding, A.; Sawh, C.; Wardley, J.; Shah, B.N.; Swoboda, P.; Senior, R.; et al. Diagnostic accuracy of handheld cardiac ultrasound device for assessment of left ventricular structure and function: Systematic review and meta-analysis. Heart 2021, 107, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sanchez, A.; Johnson, G.; Pucks, N.; Soni, R.N.; Lund, T.J.S.; Andrade, A.J.; Le, M.T.; Solis-McCarthy, J.; Wong, T.; Ashraf, A.; et al. Comparison of 6 handheld ultrasound devices by point-of-care ultrasound experts: A cross-sectional study. Ultrasound J. 2024, 16, 45. [Google Scholar] [CrossRef] [PubMed]

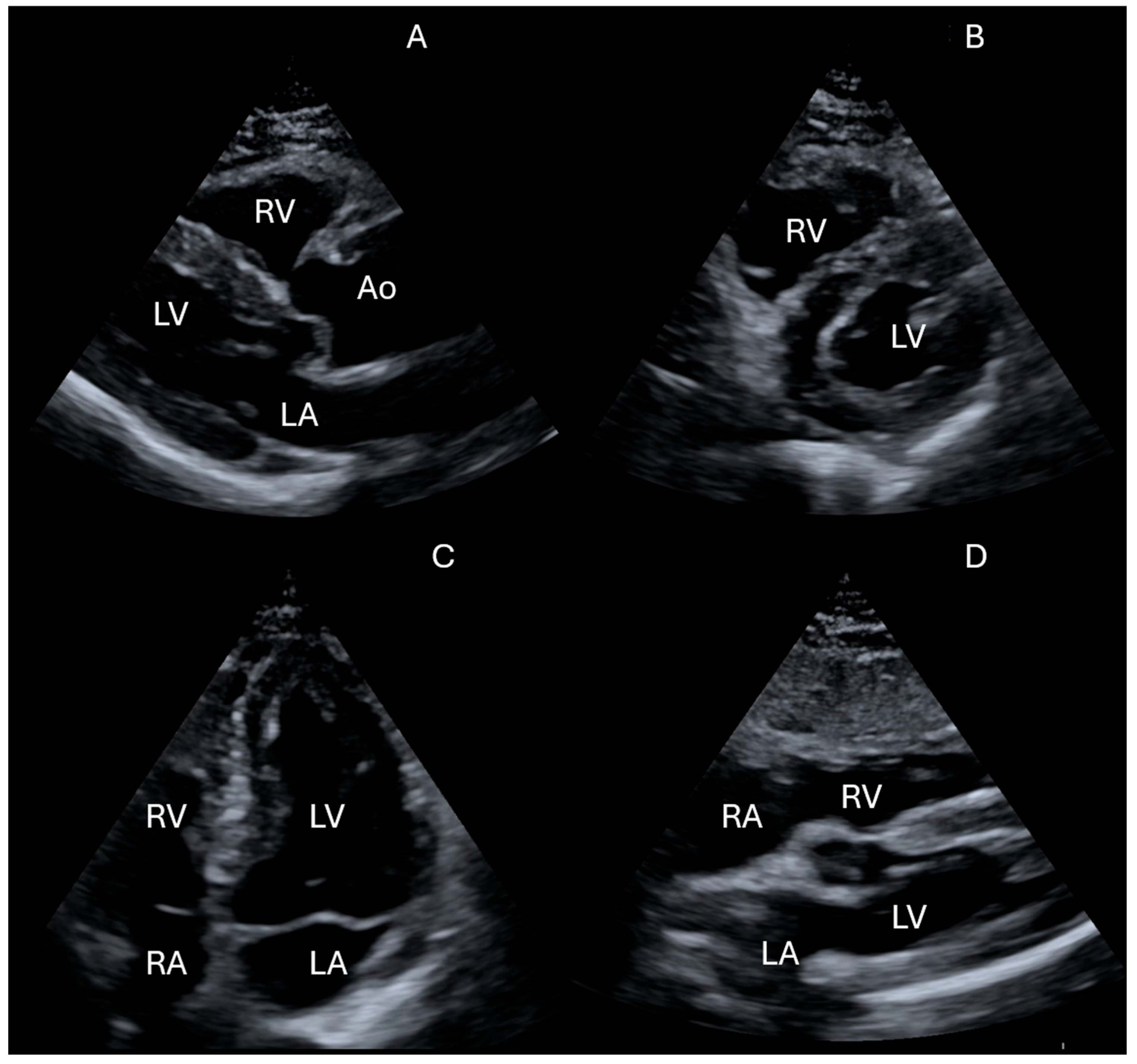

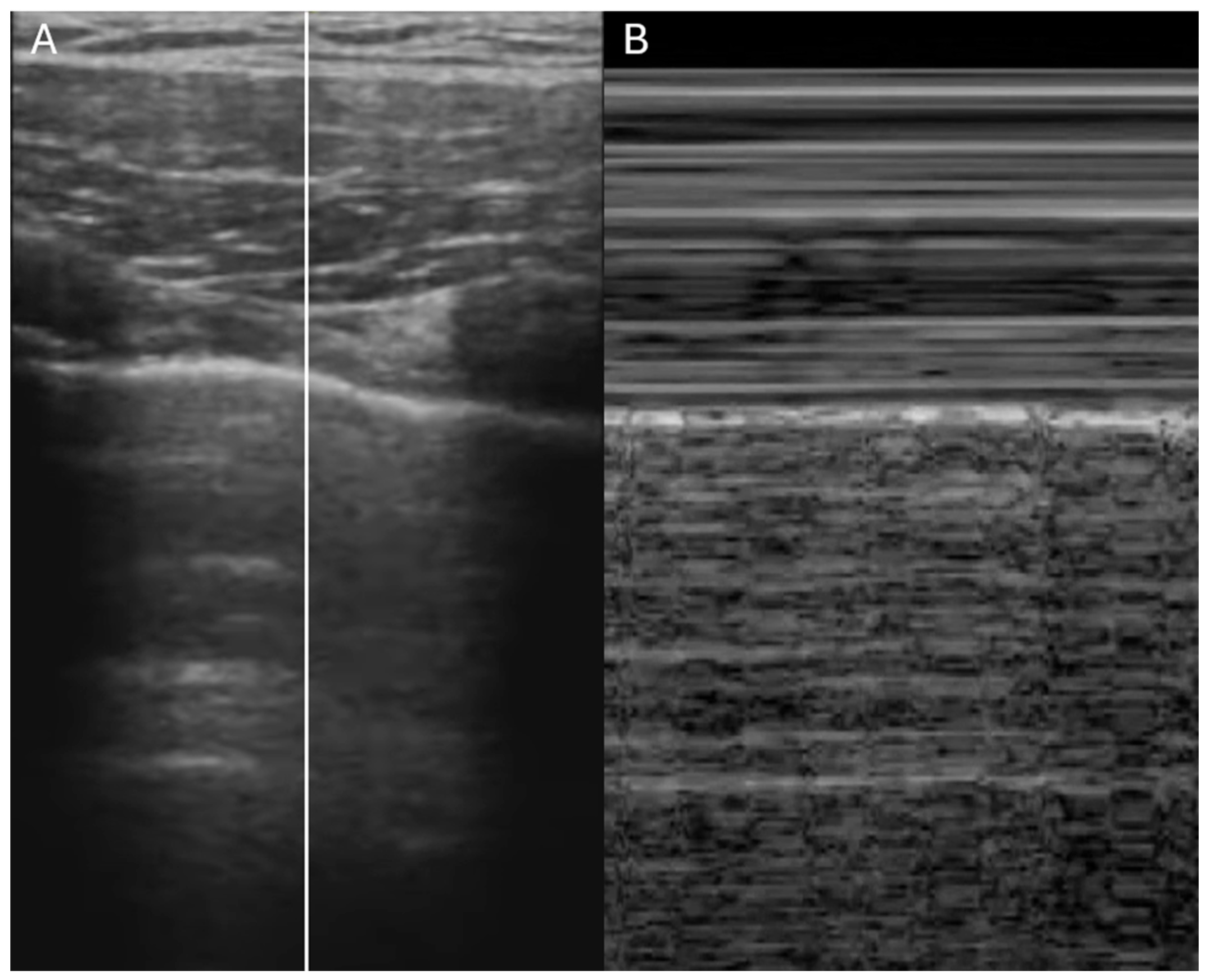
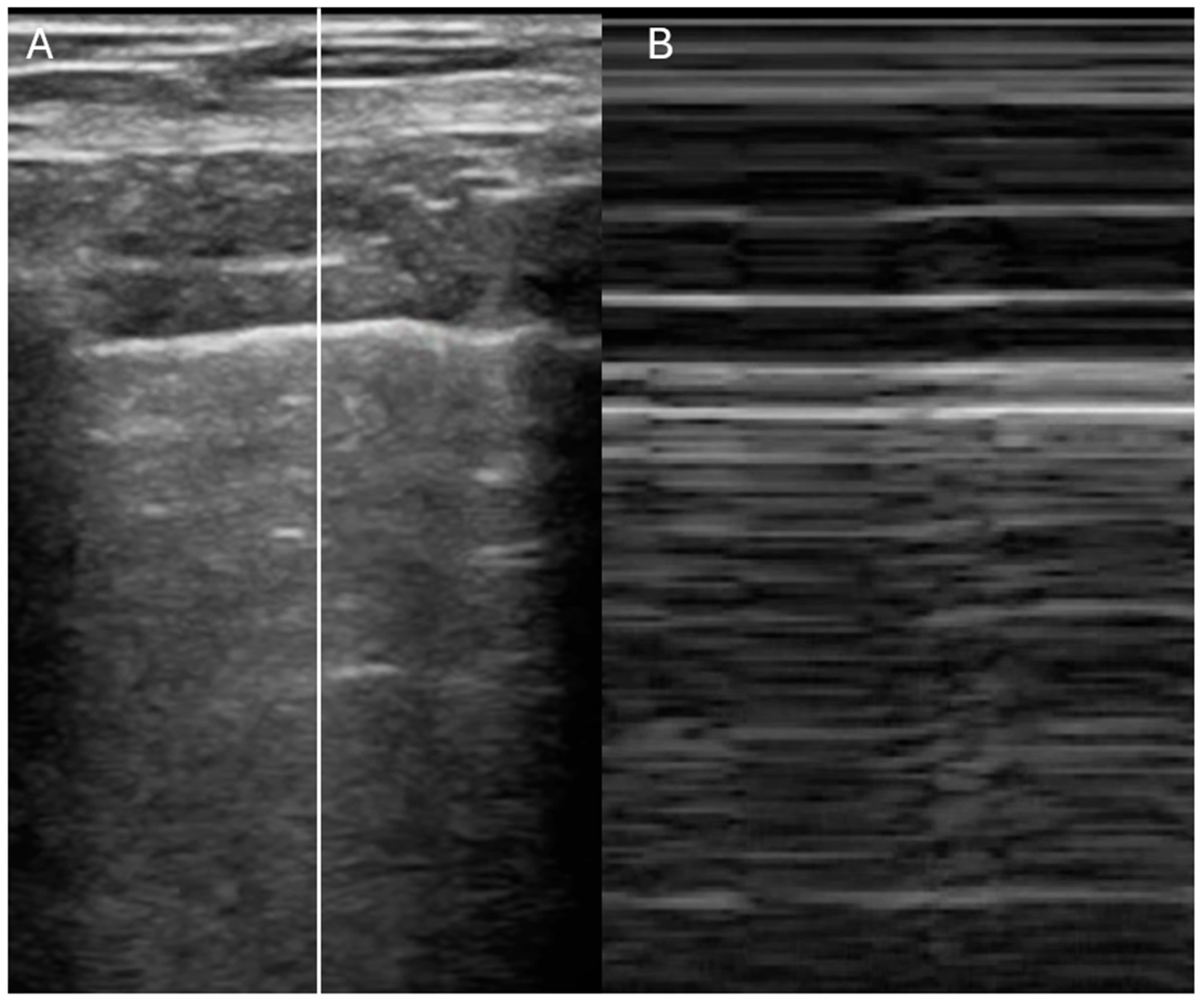
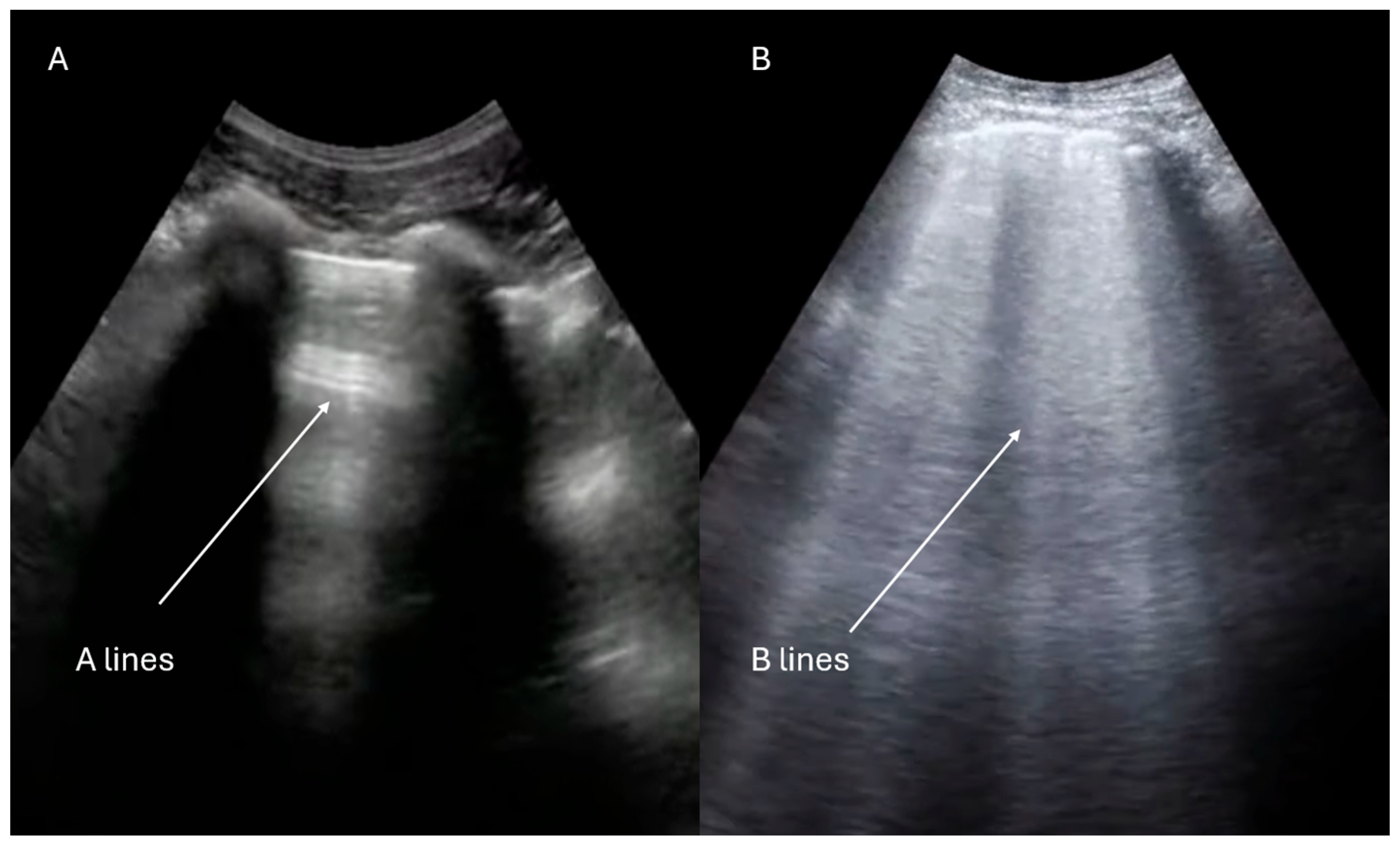


| IVC Diameter | Collapse with Sniff | Estimated RA Pressure |
|---|---|---|
| ≤2.1 cm | >50% | 0–5 mmHg |
| ≤2.1 cm | <50% | 5–10 mmHg |
| >2.1 cm | <50% | 10–20 mmHg |
| Parameter/Measurement | Basic Cardiac FoCUS/PoCUS | Advanced FoCUS | Comprehensive Echocardiography (TTE) |
|---|---|---|---|
| LV systolic function (visual) | Yes (qualitative only) | Yes (semi-quantitative; visual + basic metrics) | Yes (quantitative, biplane Simpson/3D) |
| Regional wall-motion abnormalities | Limited (gross only) | Yes (screening) | Yes (standard) |
| LV ejection fraction (numeric) | No | Limited (single-plane or eyeball estimate) | Yes (Simpson/3D) |
| RV size (base/mid/RA) | Yes (screening) | Yes (with basic metrics) | Yes (standard) |
| RV function (TAPSE/S’/FAC) | No | Yes (TAPSE/S’ optional) | Yes |
| IVC diameter & collapsibility | Yes | Yes (with trending) | Yes (plus RA pressure estimate) |
| Pericardial effusion—detection | Yes | Yes | Yes |
| Tamponade physiology (RV diastolic collapse, inflow variation) | Limited (signs only) | Yes (qualitative + basic Doppler optional) | Yes (full Doppler assessment) |
| Valvular disease—screening | Limited (gross lesions/murmur correlation) | Yes (screening + semi-quant) | Yes (comprehensive quantification) |
| Valvular quantification (continuity equation, PHT, vena contracta, PISA) | No | No | Yes |
| Diastolic function (E/A, e’, E/e’, LA vol, TRv) | No | Limited (E/A or E/e’ if available) | Yes (multi-parameter) |
| Pulmonary pressures (PASP/mean PAP) | No | Limited (TRv if feasible) | Yes (standard when feasible) |
| Cardiac output/stroke volume (LVOT VTI) | No | Limited (trend with VTI) | Yes (quantitative) |
| Aortic root/ascending aorta (screening) | Limited (root only) | Yes (screening) | Yes (standard + dimensions) |
| Aortic stenosis severity (continuity equation) | No | Limited (peak velocity if Doppler available) | Yes (Vmax, mean gradient, AVA) |
| Endocarditis features (vegetation/abscess) | No | Limited (large vegetations only) | Yes (TTE; TEE often required) |
| Intracardiac thrombus/masses | Limited (apical thrombus if obvious) | Limited (screening) | Yes (contrast/TEE/CMR as needed) |
| Lung ultrasound adjunct (B-lines, effusions, PTX signs) | Yes (integrated FoCUS) | Yes (integrated FoCUS) | Optional adjunct |
| Procedure guidance (pericardiocentesis, lines) | Yes (basic guidance) | Yes (enhanced guidance) | Yes (usually not required for full TTE) |
| Serial monitoring on ward/ED/ICU | Yes (rapid trending) | Yes (semi-quant trending) | Limited (resource-intensive) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarantini, P.; Cei, F.; Longhi, F.; Fici, A.; Tupputi, S.; Solitro, G.; Colavolpe, L.; Marengo, S.; Mumoli, N. Heart at Hand: The Role of Point-of-Care Cardiac Ultrasound in Internal Medicine. J. Cardiovasc. Dev. Dis. 2025, 12, 379. https://doi.org/10.3390/jcdd12100379
Tarantini P, Cei F, Longhi F, Fici A, Tupputi S, Solitro G, Colavolpe L, Marengo S, Mumoli N. Heart at Hand: The Role of Point-of-Care Cardiac Ultrasound in Internal Medicine. Journal of Cardiovascular Development and Disease. 2025; 12(10):379. https://doi.org/10.3390/jcdd12100379
Chicago/Turabian StyleTarantini, Piero, Francesco Cei, Fabiola Longhi, Aldo Fici, Salvatore Tupputi, Gino Solitro, Lucia Colavolpe, Stefania Marengo, and Nicola Mumoli. 2025. "Heart at Hand: The Role of Point-of-Care Cardiac Ultrasound in Internal Medicine" Journal of Cardiovascular Development and Disease 12, no. 10: 379. https://doi.org/10.3390/jcdd12100379
APA StyleTarantini, P., Cei, F., Longhi, F., Fici, A., Tupputi, S., Solitro, G., Colavolpe, L., Marengo, S., & Mumoli, N. (2025). Heart at Hand: The Role of Point-of-Care Cardiac Ultrasound in Internal Medicine. Journal of Cardiovascular Development and Disease, 12(10), 379. https://doi.org/10.3390/jcdd12100379





