Sternal Wound Reconstruction Following Deep Sternal Wound Infection: Past, Present and Future: A Literature Review
Abstract
1. Introduction
2. Past Treatment Options
2.1. Prophylaxis and Infection Management
Prophylactic Antibiotics and Effective Infection Control Strategies Are Essential in Reducing Postoperative DSWI Rates
3. Current Paradigms in Management
3.1. Medical and Surgical Treatment
3.2. Sternal Fixation
3.3. Flap Reconstruction
3.3.1. Advantages and Disadvantages of Sternal Wound Reconstruction
3.3.2. Comparison on Types of Flaps/Reconstruction Techniques
4. Discussion
4.1. Future Direction for Mediastinitis Treatment Using Sternal Wound Reconstruction
4.2. Surgical Techniques
4.2.1. Allogenic Flaps
4.2.2. Breast Flaps
4.2.3. Cerclage Wiring
4.2.4. Episternal Fixating Devices
4.3. Biomaterials for Sternal Wound Reconstruction
4.4. Minimally Invasive Techniques for Sternal Wound Reconstruction
4.5. Antibiotic-Loaded Biomaterials for Infection Prevention
4.6. Gene Therapy for Tissue Regeneration
4.7. Psychological Impact on Patients
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phoon, P.H.Y.; Hwang, N.C. Deep Sternal Wound Infection: Diagnosis, Treatment and Prevention. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1602–1613. [Google Scholar] [CrossRef] [PubMed]
- Chello, C.; Lusini, M.; Nenna, A.; Nappi, F.; Spadaccio, C.; Satriano, U.M.; Cardetta, F.; Mastroianni, C.; Chello, M. Deep Sternal Wound Infection (DSWI) and Mediastinitis After Cardiac Surgery: Current Approaches and Future Trends in Prevention and Management. Surg. Technol. Int. 2020, 36, 212–216. [Google Scholar] [PubMed]
- Spindler, N.; Moter, A.; Wiessner, A.; Gradistanac, T.; Borger, M.; Rodloff, A.C.; Langer, F.; Kikhney, J. Fluorescence in situ Hybridization (FISH) in the Microbiological Diagnostic of Deep Sternal Wound Infection (DSWI). Infect. Drug Resist. 2021, 14, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Perezgrovas-Olaria, R.; Audisio, K.; Cancelli, G.; Rahouma, M.; Ibrahim, M.; Soletti, G.J.; Chadow, D.; Demetres, M.; Girardi, L.N.; Gaudino, N. Deep Sternal Wound Infection and Mortality in Cardiac Surgery: A Meta-analysis. Ann. Thorac. Surg. 2023, 115, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Mauermann, W.J.; Sampathkumar, P.; Thompson, R.L. Sternal wound infections. Best Pract. Res. Clin. Anaesthesiol. 2008, 22, 423–436. [Google Scholar] [CrossRef]
- Shumacker, H.B. Continuous antibiotic irrigation in the treatment of infection. Arch. Surg. 1963, 86, 384. [Google Scholar] [CrossRef]
- Bryant, L.R.; Spencer, F.C.; Trinkle, J.K. Treatment of median sternotomy infection by mediastinal irrigation with an antibiotic solution. Ann. Surg. 1969, 169, 914–920. [Google Scholar] [CrossRef]
- Calvat, S.; Trouillet, J.-L.; Natal, P.; Vuagnat, A.; Chastre, J.; Gibert, C. Closed drainage using redon catheters for local treatment of poststernotomy mediastinitis. Ann. Thorac. Surg. 1996, 61, 195–201. [Google Scholar] [CrossRef]
- Barois, A.; Grosbuis, S.; Simon, N.; Combes, A.; Bourda, J.L.; Chapuis, C.; Goulon, M. Treatment of mediastinitis in children after cardiac surgery. Intensiv. Care Med. 1978, 4, 35–39. [Google Scholar] [CrossRef]
- Prevosti, L.G.; Subramainian, V.A.; Rothaus, K.O.; Dineen, P. A comparison of the open and closed methods in the initial treatment of sternal wound infections. J. Cardiovasc. Surg. 1989, 30, 757–763. [Google Scholar]
- Durandy, Y.; Batisse, A.; Bowel, P.; Dibie, A.; Lemoine, G.; Lecompte, Y. Mediastinal infection after cardiac operation. J. Thorac. Cardiovasc. Surg. 1989, 97, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Catarino, P.A.; Chamberlain, M.H.; Wright, N.C.; Black, E.; Campbell, K.; Robson, D.; Pillai, R.G. Highpressure suction drainage via a polyurethane foam in the management of poststernotomy mediastinitis. Ann. Thorac. Surg. 2000, 70, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.; Tam, D.Y.; Yu, M.; Yanagawa, B.; Gaudino, M.; Lam, T.; Fremes, S.E. Wire Cerclage Versus Cable Closure After Sternotomy for Dehiscence and DSWI: A Systematic Review and Meta-Analysis. Innovations 2020, 15, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.A.; Culliford, A.T.; Krieger, K.H.; Kloth, D.; Press, R.; Baumann, F.G.; Spencer, F.C. A survey of 77 major infectious complications of median sternotomy: A review of 7,949 consecutive operative procedures. Ann. Thorac. Surg. 1985, 40, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, M.; Mekontso-Dessap, A.; Houël, R.; Giroud, E.; Hillion, M.-L.; Loisance, D.Y. Closed drainage using redon catheters for poststernotomy mediastinitis: Results and risk factors for adverse outcome. Ann. Thorac. Surg. 2001, 71, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Anderson, E.; Harper, J.G. Overview and Management of Sternal Wound Infection. Semin. Plast. Surg. 2011, 25, 25–33. [Google Scholar] [CrossRef]
- Cimochowski, G.E.; Harostock, M.D.; Brown, R.; Bernardi, M.; Alonzo, N.; Coyle, K. Intranasal mupirocin reduces sternal wound infection after open heart surgery in diabetics and nondiabetics. Ann. Thorac. Surg. 2001, 71, 1572–1578; discussion 1578–1579. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, Y.; Jiao, G.; Jing, Z.; Bu, F.; Zhang, J.; Wei, L.; Rong, X.; Li, M. The combined application of antibiotic-loaded bone cement and vacuum sealing drainage for sternal reconstruction in the treatment of deep sternal wound infection. J. Cardiothorac. Surg. 2022, 26, 209. [Google Scholar] [CrossRef]
- Tam, D.Y.; Nedadur, R.; Yu, M.; Yanagawa, B.; Fremes, S.E.; Friedrich, J.O. Rigid Plate Fixation Versus Wire Cerclage for Sternotomy After Cardiac Surgery: A Meta-Analysis. Ann. Thorac. Surg. 2018, 106, 298–304. [Google Scholar] [CrossRef]
- Allen, K.B.; Thourani, V.H.; Naka, Y.; Grubb, K.J.; Grehan, J.; Patel, N.; Guy, T.S.; Landolfo, K.; Gerdisch, M.; Bonnell, M.; et al. Randomized, multicenter trial comparing sternotomy closure with rigid plate fixation to wire cerclage. J. Thorac. Cardiovasc. Surg. 2017, 153, 888–896.e1. [Google Scholar] [CrossRef]
- Morykwas, M.J.; Argenta, L.C.; Shelton-Brown, E.I.; McGuirt, W. Vacuum-assisted closure: A new method for wound control and treatment: Animal studies and basic foundation. Ann. Plast. Surg. 1997, 38, 553–562. [Google Scholar] [CrossRef]
- Wackenfors, A.; Gustafsson, R.; Sjögren, J.; Algotsson, L.; Ingemansson, R.; Malmsjö, M. Blood flow responses in the peristernal thoracic wall during vacuum-assisted closure therapy. Ann. Thorac. Surg. 2005, 79, 1724–1730; discussion 1730–1731. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.V.; Hayes, P.; McCarthy, M. Vacuum assisted closure: A review of development and current applications. Eur. J. Vasc. Endovasc. Surg. 2005, 29, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Domkowski, P.W.; Smith, M.L.; Gonyon, D.L.; Drye, C.; Wooten, M.K.; Levin, L.; Wolfe, W.G. Evaluation of vacuum-assisted closure in the treatment of poststernotomy mediastinitis. J. Thorac. Cardiovasc. Surg. 2003, 126, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Jurkiewicz, M.J.; Bostwick, J.; Wood, R.; Bried, J.T.; Culbertson, J.; Robert, H.; Felmont, E.; Grant, C.; Foad, N. Management of the infected median sternotomy wound with muscle flaps. The Emory 20-year experience. Ann. Surg. 1997, 225, 766–776; discussion 776–778. [Google Scholar] [CrossRef]
- Rand, R.P.; Cochran, R.P.; Aziz, S.; Hofer, B.O.; Allen, M.D.; Verrier, E.D.; Kunzelman, K.S. Prospective trial of catheter irrigation and muscle flaps for sternal wound infection. Ann. Thorac. Surg. 1998, 65, 1046–1049. [Google Scholar] [CrossRef]
- Hugo, N.E.; Sultan, M.R.; Ascherman, J.A.; Patsis, M.C.; Smith, C.R.; Rose, E.A. Single-stage management of 74 consecutive sternal wound complications with pectoralis major myocutaneous advancement flaps. Plast Reconstr. Surg. 1994, 93, 1433–1441. [Google Scholar] [CrossRef]
- Yasuura, K.; Okamoto, H.; Morita, S.; Ogawa, Y.; Sawazaki, M.; Seki, A.; Masumoto, H.; Matsuura, A.; Maseki, T.; Torii, S. Results of omental flap transposition for deep sternal wound infection after cardiovascular surgery. Ann. Surg. 1998, 227, 455–459. [Google Scholar] [CrossRef]
- Levy, A.S.; Ascherman, J.A. Sternal Wound Reconstruction Made Simple. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2488. [Google Scholar] [CrossRef]
- Tang, G.H.; Maganti, M.; Weisel, R.D.; Borger, M.A. Prevention and management of deep sternal wound infection. Semin. Thorac. Cardiovasc. Surg. 2004, 16, 62–69. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, B.; Qu, C.; Liu, L.; Song, Y. Analysis of Risk Factors for Sternal Wound Infection After Off-Pump Coronary Artery Bypass Grafting. Infect. Drug Resist. 2022, 15, 5249–5256. [Google Scholar] [CrossRef]
- Chen, C.; Gao, Y.; Zhao, D.; Ma, Z.; Su, Y.; Mo, R. Deep sternal wound infection and pectoralis major muscle flap reconstruction: A single-center 20-year retrospective study. Front. Surg. 2022, 9, 870044. [Google Scholar] [CrossRef] [PubMed]
- Biancari, F.; Gatti, G.; Rosato, S.; Mariscalco, G.; Pappalardo, A.; Onorati, F.; Faggian, G.; Salsano, G.; Santini, F.; Ruggieri, V.G.; et al. Preoperative risk stratification of deep sternal wound infection after coronary surgery. Infect Control. Hosp. Epidemiol. 2020, 41, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Koulaxouzidis, G.; Orhun, A.; Stavrakis, T.; Witzel, C. Second intercostal internal mammary artery perforator (IMAP) fasciocutaneous flap as an alternative choice for the treatment of deep sternal wound infections (DSWI). J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Miyata, H.; Motomura, N.; Ono, M.; Takamoto, S.; Harii KOura, N.; Hirabayashi, S.; Kyo, S. Deep sternal wound infection after cardiac surgery. J. Cardiothorac. Surg. 2013, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Sears, E.D.; Wu, L.; Waljee, J.F.; Momoh, A.O.; Zhong, L.; Chung, K.C. The Impact of Deep Sternal Wound Infection on Mortality and Resource Utilization: A Population-based Study. World J. Surg. 2016, 40, 2673–2680. [Google Scholar] [CrossRef]
- Hever, P.; Singh, P.; Eiben, I.; Eiben, P.; Nikkhah, D. The management of deep sternal wound infection: Literature review and reconstructive algorithm. JPRAS Open 2021, 28, 77–89. [Google Scholar] [CrossRef]
- Lo Torto, F.; Turriziani, G.; Donato, C.; Marcasciano, M.; Redi, U.; Greco, M.; Ribuffo, D. Deep sternal wound infection following cardiac surgery: A comparison of the monolateral with the bilateral pectoralis major flaps. Int. Wound J. 2020, 17, 683–691. [Google Scholar] [CrossRef]
- Ren, G.H.; Xiang, D.Y.; Wu, X.H.; Chen, Y.B.; Li, R. A neglected problem in the utilization of free anterolateral thigh flap toward reconstructing complicated wounds of extremities: The obliteration of deep dead space. J. Orthop. Surg. Res. 2020, 15, 483. [Google Scholar] [CrossRef]
- Wingerden, J.J.V.; Lapid, O.; Boonstra, P.W.; de Mol, B.A.J.M. Muscle flaps or omental flap in the management of deep sternal wound infection. Interact. Cardiovasc. Thorac. Surg. 2011, 13, 179–188. [Google Scholar] [CrossRef]
- Cancelli, G.; Alzghari, T.; Dimagli, A.; Audisio, K.; Dabsha, A.; Harik, L.; Olaria, R.P.; Soletti, G.J.; Demetres, M.G.; Gaudino, M. Mortality after sternal reconstruction with pectoralis major flap vs omental flap for postsurgical mediastinitis: A systematic review and meta-analysis. J. Card. Surg. 2022, 37, 5263–5268. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, J.; Yang, H.; Pan, Y.; Chen, L. Bilateral partial pectoralis major muscle turnover flaps for the management of deep sternal wound infection following cardiac surgery. J. Thorac. Dis. 2020, 12, 6010–6015. [Google Scholar] [CrossRef] [PubMed]
- Netscher, D.T.; Eladoumikdachi, F.; Goodman, C.M. Rectus Abdominis Muscle Flaps Used Successfully for Median Sternotomy Wounds After Ipsilateral Internal Mammary Artery Ligation. Ann. Plast. Surg. 2001, 47, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, V. Omentum a powerful biological source in regenerative surgery. Regen. Ther. 2019, 11, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Abolhoda, A.; Bui, T.D.; Milliken, J.C.; Wirth, G.A. Pedicled latissimus dorsi muscle flap: Routine use in high-risk thoracic surgery. Tex. Heart Inst. J. 2009, 36, 298–302. [Google Scholar] [PubMed]
- Murthy, V.; Gopinath, K.A. Reconstruction of Groin Defects Following Radical Inguinal Lymphadenectomy: An Evidence Based Review. Indian J. Surg. Oncol. 2012, 3, 130–138. [Google Scholar] [CrossRef]
- George, R.; Krishnamurthy, A. Microsurgical free flaps: Controversies in maxillofacial reconstruction. Ann. Maxillofac. Surg. 2013, 3, 72. [Google Scholar] [CrossRef]
- Mahajan, R.K.; Srinivasan, K.; Bhamre, A.; Singh, M.; Kumar, P.; Tambotra, A. A retrospective analysis of latissimus dorsi–serratus anterior chimeric flap reconstruction in 47 patients with extensive lower extremity trauma. Indian J. Plast. Surg. 2018, 51, 24–32. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, K.; Qin, S.; He, R.; Jiang, S.; Qi, X.; Wen, B. Impact of internal mammary artery perforator propeller flaps combined with radiotherapy in the treatment of large chest keloids: Our experience. Front. Surg. 2023, 10, 1136496. [Google Scholar] [CrossRef]
- Piwnica-Worms, W.; Azoury, S.C.; Kozak, G.; Nathan, S.; Stranix, J.T.; Colen, D.; Othman, S.; Vallabhajosyula, P.; Serletti, J.; Kovach, S. Flap Reconstruction for Deep Sternal Wound Infections: Factors Influencing Morbidity and Mortality. Ann. Thorac. Surg. 2020, 109, 1584–1590. [Google Scholar] [CrossRef]
- Preminger, B.A.; Yaghoobzadeh, Y.; Ascherman, J.A. Management of sternal wounds by limited debridement and partial pectoralis major myocutaneous advancement flaps in 25 patients: A less invasive approach. Ann. Plast. Surg. 2014, 72, 446–450. [Google Scholar] [CrossRef]
- Ascherman, J.A.; Hugo, N.E.; Sultan, M.R.; Patsis, M.C.; Smith, C.R. Single stage treatment of sternal wound complications in heart transplant recepients in whom pectoralis major myocutaneous advancement flaps were used. J. Thorac. Cardiovasc. Surg. 1995, 110, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Dosios, T.; Papadopoulos, O.; Mantas, D.; Georgiiou, P.; Asimacopoulos, P. Pedicled myocutaneous and muscle flaps in the management of complicated cardiothoracic problems. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2003, 37, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Backer, C.L.; Pensler, J.M.; Tobin, G.R.; Mavroudis, C. Vascularised muscle flaps for life threatening mediastinal wounds in children. Ann. Thorac. Surg. 1994, 57, 797. [Google Scholar] [CrossRef]
- Davison, S.P.; Clemens, M.W.; Armstrong, D.; Newton, E.D.; Swartz, W. Sternotomy wounds. Plast. Reconstr. Surg. 2007, 120, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Parissis, H.; Al-Alao, B.; Soo, A.; Orr, D.; Young, V. Risk analysis and outcome of deep mediastinal wound infections with specific emphasis to omental transposition. J. Cardiothorac. Surg. 2011, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Kuntscher, M.V.; Mansouri, S.; Noack, N.; Hartmann, B. Verstality of vertical rectus abdominis musculocutaneous flaps. Microsurgery 2006, 26, 363–369. [Google Scholar] [CrossRef]
- Little, S.C. Latissimus Myocutaneous Flap: Overview, Anatomy, Contraindications. J. Cardiothorac. Surg. 2011, 6, 111. [Google Scholar]
- Denewer, A.T. Myomammary Flap of Pectoralis Major Muscle for Breast Reconstruction: New Technique. World J. Surg. 1997, 21, 57–61. [Google Scholar] [CrossRef]
- Kim, W.K.; Kim, J.B.; Jung, S.-H.; Choo, S.J.; Chung, C.H.; Lee, J.W. Titanium Plate Fixation for a Dehisced Sternum Following Coronary Artery Bypass Grafting: A Case Report. Korean J. Thorac. Cardiovasc. Surg. 2012, 45, 127–130. [Google Scholar] [CrossRef]
- Yu, C.; Yu, C.; Yao, W.; Chen, Y.; Lee, A.; Liu, Y.; Tu, C.; Huang, W.; Tung, K.; Tsai, M. Efficacy and safety of pectoralis muscle flap combined rectus abdominis muscle sheath fasciocutaneous flap for reconstruction of sternal infection. Int. Wound J. 2022, 19, 1829–1837. [Google Scholar] [CrossRef]
- Sun, L.; Chen, X.; Ma, K.; Chen, R.; Mao, Y.; Chao, R.; Wang, H.; Yu, B.; Wang, J.; Zhang, S. Novel Titanium Implant: A 3D Multifunction Architecture with Charge-Trapping and Piezoelectric Self-Stimulation. Adv. Health Mater. 2023, 12, e2202620. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, L.; Jabbour, G.; Centofanti, P.; Giordano, S.; Abdelnour, E.; Gonzalez, M.; Raffoul, W.; di Summa, P.G. Deep sternal wound infections: Evidence for prevention, treatment, and reconstructive surgery. Arch. Plast. Surg. 2019, 46, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Matsen, C.B.; Mehrara, B.; Eaton, A.; Capko, D.; Berg, A.; Stempel, M.; Van Zee, K.J.; Pusic, A.; King, T.A.; Cody, H.S.; et al. Skin Flap Necrosis After Mastectomy with Reconstruction: A Prospective Study. Ann. Surg. Oncol. 2016, 23, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Mohammadyari, F.; Parvin, S.; Khorvash, M.; Amini, A.; Behzadi, A.; HajEbrahimi, R.; Kasaei, F.; Olangian-Tehrani, S. Acellular dermal matrix in reconstructive surgery: Applications, benefits, and cost. Front. Transplant. 2023, 2, 1133806. [Google Scholar] [CrossRef]
- Sharma, S.; Rai, V.K.; Narang, R.K.; Markandeywar, T.S. Collagen-based formulations for wound healing: A literature review. Life Sci. 2021, 290, 120096. [Google Scholar] [CrossRef]
- Guo, X.; Schaudinn, C.; Blume-Peytavi, U.; Vogt, A.; Rancan, F. Effects of Adipose-Derived Stem Cells and Their Conditioned Medium in a Human Ex Vivo Wound Model. Cells 2022, 11, 1198. [Google Scholar] [CrossRef]
- Hämäläinen, E.; Laurikka, J.; Huhtala, H.; Järvinen, O. Vacuum assistance therapy as compared to early reconstructive treatment in deep sternal wound infection. Scand. J. Surg. 2020, 110, 248–253. [Google Scholar] [CrossRef]
- De Martino, A.; Del Re, F.; Falcetta, G.; Morganti, R.; Ravenni, G.; Bortolotti, U. Sternal Wound Complications: Results of Routine Use of Negative Pressure Wound Therapy. Braz. J. Cardiovasc. Surg. 2020, 35, 50–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, W.; Zheng, L.; Hu, J.; Nie, L.; Zeng, H.; Tan, X.; Jiang, Y.; Li, Y.; Zhao, T.; et al. Efficient bone regeneration of BMP9- stimulated human periodontal ligament stem cells (hPDLSCs) in decellularized bone matrix (DBM) constructs to model maxillofacial intrabony defect repair. Stem Cell Res. Ther. 2022, 13, 535. [Google Scholar] [CrossRef]
- Loop, F.D.; Lytle, B.W.; Cosgrove, D.M.; Mahfood, S.; McHenry, M.C.; Goormastic, M.; Stewart, R.W.; Golding, L.A.; Taylor, P.C. Sternal wound complications after isolated coronary artery bypass grafting: Early and late mortality, morbidity, and cost of care. Ann. Thorac. Surg. 1990, 49, 179–187. [Google Scholar] [CrossRef]
- Eklund, A.M.; Lyytikäinen, O.; Klemets, P.; Huotari, K.; Anttila, V.-J.; Werkkala, K.A.; Valtonen, M. Mediastinitis after more than 10,000 cardiac surgical procedures. Ann. Thorac. Surg. 2006, 82, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Graf, K.; Ott, E.; Vonberg, R.-P.; Kuehn, C.; Haverich, A.; Chaberny, I.F. Economic aspects of deep sternal wound infections. Eur. J. Cardio-Thorac. Surg. 2010, 37, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Ngaage, M.; Agius, M. The Psychology of Scars: A Mini-Review. Psychiatr. Danub. 2018, 30 (Suppl. S7), 633–638. [Google Scholar] [PubMed]
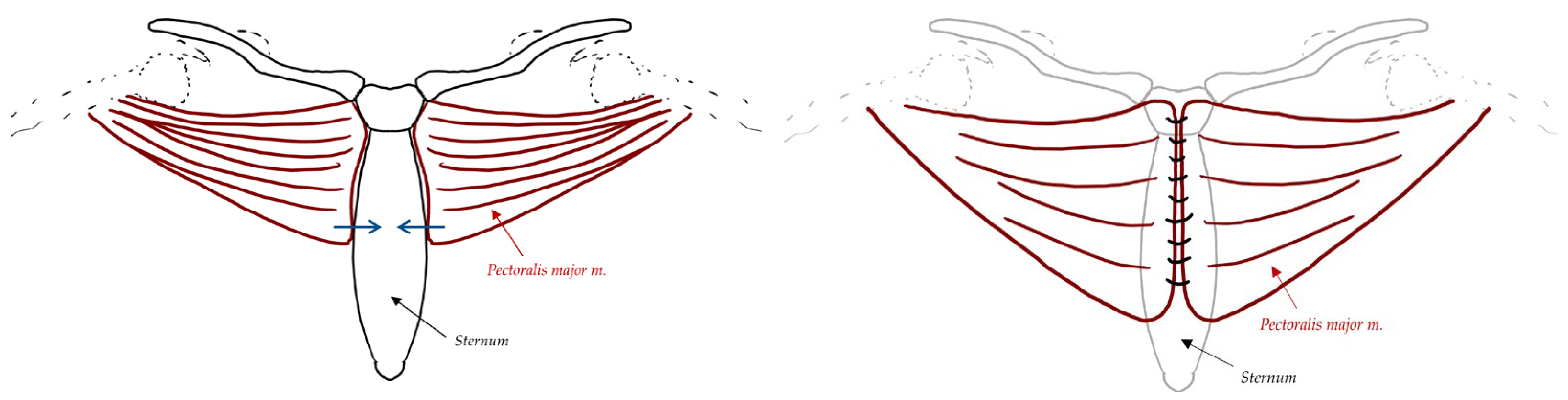
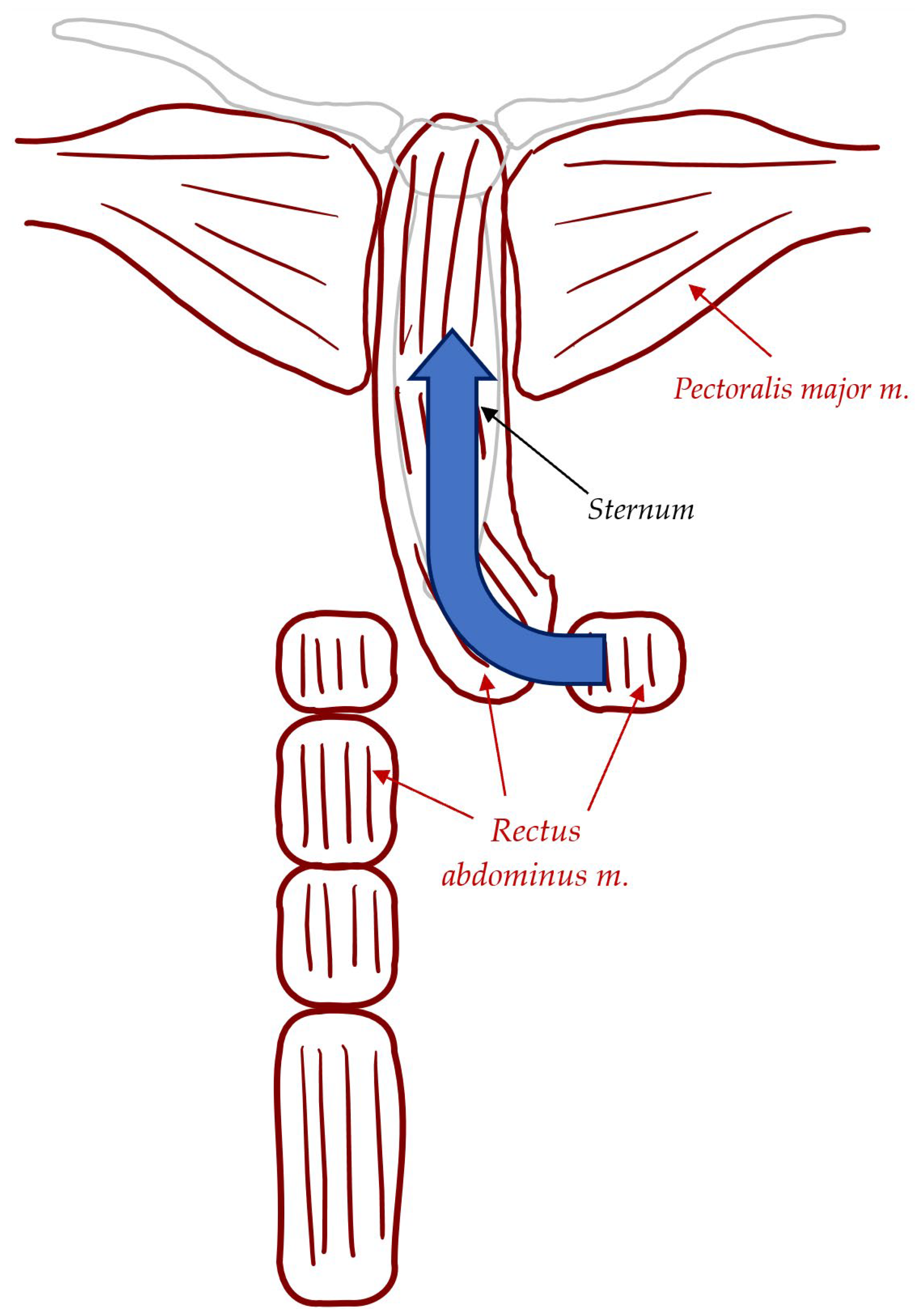


| Reconstruction Methods for DSWI | Description |
|---|---|
| Pectoralis Major Myocutaneous Flaps | Flaps elevated beneath the pectoralis major muscle from central to lateral direction. Dissection stops between midclavicular and anterior axillary lines, inferior to the clavicle superiorly, and deep to anterior rectus sheath to xiphoid process inferiorly. Closed suction drains placed beside each flap. Flaps sutured together with interrupted sutures (Vicryl or polysorb) with pectoralis fascia and rectus sheath in the same closure layer, Limited coverage over xiphoid and inferior portion of the wound, which can be overcome by bringing anterior rectus sheath in continuity with the pectoralis major flap |
| Rectus Abdominis Muscle Flaps | Harvested when pectoralis major muscles are compromised, and a patent ipsilateral IMA is present. Pedicled flap based on the superior epigastric artery (SEA). Divided at the inferior most portion and rotated superiorly. Provides more stable coverage but may lead to complications like abdominal wall weakness, bulge, or hernia due to requiring a second donor site for the flap. |
| Omentum Flap | Secondary option for extensive loss of chest wall soft tissue and inadequate skin for closure, particularly for lower third infections. Harvesting via laparotomy. Provides less tissue bulk and no extra support to chest wall stability. May require creation of abdominal fascia or diaphragm opening for the omentum flap to reach the sternum, possibly needing skin grafting. |
| Cerclage Wiring | Stainless steel wires used to stabilise the sternum in primary closure, particularly in low-risk patients without sternal instability. Simple and cost-effective. |
| Episternal Fixating Devices (Plates) | Titanium plates providing mechanical stability in cases of sternal instability or complex reconstructions. Superior fixation for high-risk patients or those with prior sternotomies. |
| Advantages | Disadvantages |
|---|---|
| Improved wound healing | Complexity and expertise required |
| Promotes better wound healing | Limited availability of experienced plastic surgeons |
| Reduced risk of infection | Prolonged and time-consuming surgery |
| Faster healing and decreased complications | Increased risk of intraoperative complications |
| Improved aesthetic outcomes | Risk of flap necrosis, infection, hematoma, seroma formation, and wound dehiscence |
| Enhanced patient satisfaction with appearance | Limited availability of specialised plastic surgeons |
| Restoration of functional integrity | Delays in treatment due to limited options |
| Flap/Reconstruction Technique | Advantages | Disadvantages | Reported Success Rates |
|---|---|---|---|
| Pectoralis Major Flaps | -Provides a good vascular supply unaffected by internal mammary artery harvesting and is relatively simple to harvest [29]. -Offers coverage of exposed vital structures [29]. -Provides ribcage stability without the need for osteosynthesis [50,51]. -Preserves strength in at least one arm, with the possibility of preserving the contralateral muscle group in case of surgical failure [52]. -Demonstrates resistance to wound infection [53,54]. | -May cause long-term functional impairment. -Loss of skeletal continuity of the chest wall may be more disabling than the loss of pectoral muscle function. | A study from Fujian Medical University involving 11 patients undergoing sternal wound reconstruction with this method reported no bleeding or secondary thoracotomies, with drains removed within 7 days and sutures removed within 14 days post-op [42]. Only one patient experienced secondary healing, with no subcutaneous hematoma or bleeding requiring further surgery. Six-month follow-up showed no postoperative complications, including pain, abnormal upper limb movement, or chest wall deformities [42]. Another study at Linköping University Hospital reviewed outcomes of 43 flaps; 37 were successful, with 3 failed procedures associated with higher BMI (31.1 versus 27.8), older age (78.6 versus 74.4), and more pre-existing conditions [52]. Postoperative complications were recorded in 49% of patients, including bleeding, infection, wound dehiscence, fistulation, and skin necrosis [52]. |
| Rectus Abdominis Flaps | -Superior to pectoralis major flaps for covering the inferior sternum [55]. -Can maintain a viable flap even with ipsilateral IMA ligation [43]. -Capable of transferring large skin areas with varying thickness and amounts of underlying muscle [50]. | -May require arterial and/or venous re-charging. -May be less effective than pectoral flaps in certain cases [56]. -Associated with an increased risk of surgical site infection (SSI). In one study, all 5 patients undergoing VRAM (vertical rectus abdominis myocutaneous) flap reconstructions experienced postoperative complications [50]. | A case report involving 5 male chronic smokers (age range: 42–74, mean age: 58) demonstrated 100% muscle survival. All patients experienced good healing and infection-free wounds [43]. |
| Omental Grafts | -Large size and bulk help fill dead spaces. -Rich vascular and lymphatic networks, with large pedicles, make them particularly useful in managing infection-related sternal wound complications. | -May be prone to malignancies originating from a primary cancer. -Associated with higher rates of reoperation (18%), SSI (17%), skin necrosis (4.7%), flap necrosis (3.8%), dehiscence (3.8%), hematoma (2.8%), and mortality (2%) [50]. | In a study of laparoscopic omental harvests, 7 out of 9 procedures were used for reconstruction of infection-related sternal wounds, and 2 for the repair of intrathoracic viscera. The study showed excellent early outcomes, with no late flap failures, an 8.3% mortality rate, and a mean hospital stay of 59 days for patients with DSWIs who underwent omental transposition [56,57]. |
| Technique | Pros | Cons |
|---|---|---|
| Plates (Titanium) | Stable fixation, reduced recovery time | Expensive, requires specialised tools |
| Cerclage Wiring | Simple, cost-effective, widely used | Risk of sternal instability, infection |
| VAC Therapy | Promotes blood flow, reduces infection | Requires specialised equipment |
| Muscle Flaps | Effective in high-risk patients | Complex surgery, cosmetic concerns |
| Allografts (Emerging) | Promising tissue regeneration | Experimental, lack of long-term data |
| Gene Therapies (Emerging) | Potential for revolutionary healing | Expensive, not yet widely available |
| Future Directions for Mediastinitis Treatment Using Sternal Wound Reconstruction | Examples |
|---|---|
| Surgical Techniques | Pectoralis muscle flaps, omental flaps, titanium plates, three-dimensional-printed titanium implant, allogenic flaps, breast flaps |
| Biomaterials for Sternal Wound Reconstruction | Acellular dermal matrix (ADM), collagen based biomaterials, adipose-derived stem cells (ASCs) |
| Minimally Invasive Techniques for Sternal Wound Reconstruction | Closed-chest vacuum-assisted closure (VAC) system |
| Antibiotic-loaded Biomaterials for Infection Prevention | Antibiotic-loaded bone cement, antibiotic loaded ADM |
| Gene Therapy for Tissue Regeneration | Adenovirus-mediated delivery of bone morphogenetic protein-2 (BMP-2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khashkhusha, A.; Butt, S.; Abdelghaffar, M.; Wang, W.; Rajananthanan, A.; Roy, S.; Khurshid, B.N.; Zeinah, M.; Harky, A. Sternal Wound Reconstruction Following Deep Sternal Wound Infection: Past, Present and Future: A Literature Review. J. Cardiovasc. Dev. Dis. 2024, 11, 361. https://doi.org/10.3390/jcdd11110361
Khashkhusha A, Butt S, Abdelghaffar M, Wang W, Rajananthanan A, Roy S, Khurshid BN, Zeinah M, Harky A. Sternal Wound Reconstruction Following Deep Sternal Wound Infection: Past, Present and Future: A Literature Review. Journal of Cardiovascular Development and Disease. 2024; 11(11):361. https://doi.org/10.3390/jcdd11110361
Chicago/Turabian StyleKhashkhusha, Arwa, Sundas Butt, Mariam Abdelghaffar, William Wang, Asveny Rajananthanan, Sakshi Roy, Bakht Noor Khurshid, Mohamed Zeinah, and Amer Harky. 2024. "Sternal Wound Reconstruction Following Deep Sternal Wound Infection: Past, Present and Future: A Literature Review" Journal of Cardiovascular Development and Disease 11, no. 11: 361. https://doi.org/10.3390/jcdd11110361
APA StyleKhashkhusha, A., Butt, S., Abdelghaffar, M., Wang, W., Rajananthanan, A., Roy, S., Khurshid, B. N., Zeinah, M., & Harky, A. (2024). Sternal Wound Reconstruction Following Deep Sternal Wound Infection: Past, Present and Future: A Literature Review. Journal of Cardiovascular Development and Disease, 11(11), 361. https://doi.org/10.3390/jcdd11110361






