Intravenous Treprostinil in Severe Inoperable Chronic Thromboembolic Pulmonary Hypertension Using Implantable Pumps—Single-Center Experience over More Than a Decade
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin-Suarez, S.; Loforte, A.; Cavalli, G.G.; Gliozzi, G.; Botta, L.; Mariani, C.; Orioli, V.; Votano, D.; Costantino, A.; Santamaria, V.; et al. Therapeutic alternatives in chronic thromboembolic pulmonary hypertension: From pulmonary endarterectomy to balloon pulmonary angioplasty to medical therapy. State of the art from a multidisciplinary team. Ann. Cardiothorac. Surg. 2022, 11, 120–127. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Galié, N.; Humbert, M.; Vachiéry, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.-L.; Barbera, J.A.; Beghetti, M.; Corris, P.; Gaine, S.; Gibbs, J.S.; et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2009, 30, 2493–2537. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.-A.; D’Armini, A.M.; Grimminger, F.; Hoeper, M.M.; Jansa, P.; Kim, N.H.; Mayer, E.; Simonneau, G.; Wilkins, M.R.; Fritsch, A.; et al. Riociguat for the Treatment of Chronic Thromboembolic Pulmonary Hypertension. N. Engl. J. Med. 2013, 369, 319–329. [Google Scholar] [CrossRef]
- Sadushi-Kolici, R.; Jansa, P.; Kopeć, G.; Torbicki, A.; Skoro-Sajer, N.; Campean, I.-A.; Halank, M.; Simkova, I.; Karlocai, K.; Steringer-Mascherbauer, R.; et al. Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPH): A double-blind, phase 3, randomised controlled trial. Lancet Respir. Med. 2019, 7, 239–248. [Google Scholar] [CrossRef]
- Laliberte, K.; Arneson, C.; Jeffs, R.; Hunt, T.; Wade, M. Pharmacokinetics and Steady-State Bioequivalence of Treprostinil Sodium (Remodulin®) Administered by the Intravenous and Subcutaneous Route to Normal Volunteers. J. Cardiovasc. Pharmacol. 2004, 44, 209–214. [Google Scholar] [CrossRef]
- Simonneau, G.; Barst, R.J.; Galie, N.; Naeije, R.; Rich, S.; Bourge, R.C.; Keogh, A.; Oudiz, R.; Frost, A.; Blackburn, S.D.; et al. Continuous Subcutaneous Infusion of Treprostinil, a Prostacyclin Analogue, in Patients with Pulmonary Arterial Hypertension: A double-blind, randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2002, 165, 800–804. [Google Scholar] [CrossRef]
- Barst, R.J.; Galie, N.; Naeije, R.; Simonneau, G.; Jeffs, R.; Arneson, C.; Rubin, L.J. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur. Respir. J. 2006, 28, 1195–1203. [Google Scholar] [CrossRef]
- Sadushi-Koliçi, R.; Skoro-Sajer, N.; Zimmer, D.; Bonderman, D.; Schemper, M.; Klepetko, W.; Glatz, J.; Jakowitsch, J.; Lang, I.M. Long-term treatment, tolerability, and survival with sub-cutaneous treprostinil for severe pulmonary hypertension. J. Heart Lung Transpl. 2012, 31, 735–743. [Google Scholar] [CrossRef]
- Tapson, V.F.; Gomberg-Maitland, M.; McLaughlin, V.V.; Benza, R.L.; Widlitz, A.C.; Krichman, A.; Barst, R.J. Safety and Efficacy of IV Treprostinil for Pulmonary Arterial Hypertension: A prospective, multicenter, open-label, 12-week trial. Chest 2006, 129, 683–688. [Google Scholar] [CrossRef]
- Hiremath, J.; Thanikachalam, S.; Parikh, K.; Shanmugasundaram, S.; Bangera, S.; Shapiro, L.; Pott, G.B.; Vnencak-Jones, C.L.; Arneson, C.; Wade, M.; et al. Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: A placebo-controlled trial. J. Heart Lung Transpl. 2010, 29, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Kallen, A.J.; Lederman, E.; Balaji, A.; Trevino, I.; Petersen, E.E.; Shoulson, R.; Saiman, L.; Horn, E.M.; Gomberg-Maitland, M.; Barst, R.J.; et al. Bloodstream Infections in Patients Given Treatment with Intravenous Prostanoids. Infect. Control Hosp. Epidemiol. 2008, 29, 342–349. [Google Scholar] [CrossRef]
- Kaw, R.; Pasupuleti, V.; Deshpande, A.; Hamieh, T.; Walker, E.; Minai, O.A. Pulmonary hypertension: An important predictor of outcomes in patients undergoing non-cardiac surgery. Respir. Med. 2011, 105, 619–624. [Google Scholar] [CrossRef]
- Steringer-Mascherbauer, R.; Lummersdorfer, M.; Fuegger, R.; Sigmund, E.; Huber, C.; Engleder, D.; Froeschl, U.; Aichinger, J. Long-term experience with implantable infusion pumps for intravenous treprostinil in pulmonary arterial hypertension—Procedural safety and system-related complications. Pulm. Circ. 2020, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Steringer-Mascherbauer, R.; Maria, L.; Reinhold, F.; Elisabeth, S.; Charlotte, H.; Dagmar, E.; Uwe, F.; Josef, A. Rapid Switch From Subcutaneous to Intravenous Treprostinil in Precapillary Pulmonary Hypertension by Pump Implantation. J. Cardiovasc. Pharmacol. 2021, 77, 38–42. [Google Scholar] [CrossRef]
- Jaïs, X.; D’Armini, A.M.; Jansa, P.; Torbicki, A.; Delcroix, M.; Ghofrani, H.A.; Hoeper, M.M.; Lang, I.M.; Mayer, E.; Pepke-Zaba, J.; et al. Bosentan for Treatment of Inoperable Chronic Thromboembolic Pulmonary Hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a Randomized, Placebo-Controlled Trial. J. Am. Coll. Cardiol. 2008, 52, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- Reichenberger, F.; Voswinckel, R.; Enke, B.; Rutsch, M.; El Fechtali, E.; Schmehl, T.; Olschewski, H.; Schermuly, R.; Weissmann, N.; Ghofrani, H.A.; et al. Long-term treatment with sildenafil in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2007, 30, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Bresser, P.; Fedullo, P.; Auger, W.; Channick; Robbins, I.; Kerr, K.; Jamieson, S.; Rubin, L. Continuous intravenous epoprostenol for chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2004, 23, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Skoro-Sajer, N.; Bonderman, D.; Wiesbauer, F.; Harja, E.; Jakowitsch, J.; Klepetko, W.; Kneussl, M.P.; Lang, I.M. Treprostinil for severe inoperable chronic thromboembolic pulmonary hypertension. J. Thromb. Haemost. 2007, 5, 483–489. [Google Scholar] [CrossRef]
- Verso, M.; Agnelli, G. Venous Thromboembolism Associated with Long-Term Use of Central Venous Catheters in Cancer Patients. J. Clin. Oncol. 2003, 21, 3665–3675. [Google Scholar] [CrossRef] [PubMed]
- Jevnikar, M.; Montani, D.; Savale, L.; Seferian, A.; Jutant, E.-M.; Boucly, A.; Preda, M.; Weatherald, J.; Bulifon, S.; Parent, F.; et al. Chronic thromboembolic pulmonary hypertension and totally implantable central venous access systems. Eur. Respir. J. 2020, 57, 2002208. [Google Scholar] [CrossRef]
- Cabrol, S.; Souza, R.; Jais, X.; Fadel, E.; Ali, R.H.S.; Humbert, M.; Dartevelle, P.; Simonneau, G.; Sitbon, O. Intravenous Epoprostenol in Inoperable Chronic Thromboembolic Pulmonary Hypertension. J. Heart Lung Transpl. 2007, 26, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.J.; Harutyunova, S.; Bollmann, T.; Classen, S.; Gall, H.; Gerhardt, F.; Grimminger, F.; Grimminger, J.; Grünig, E.; Guth, S.; et al. Long-term safety and outcome of intravenous treprostinil via an implanted pump in pulmonary hypertension. J. Heart Lung Transpl. 2018, 37, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
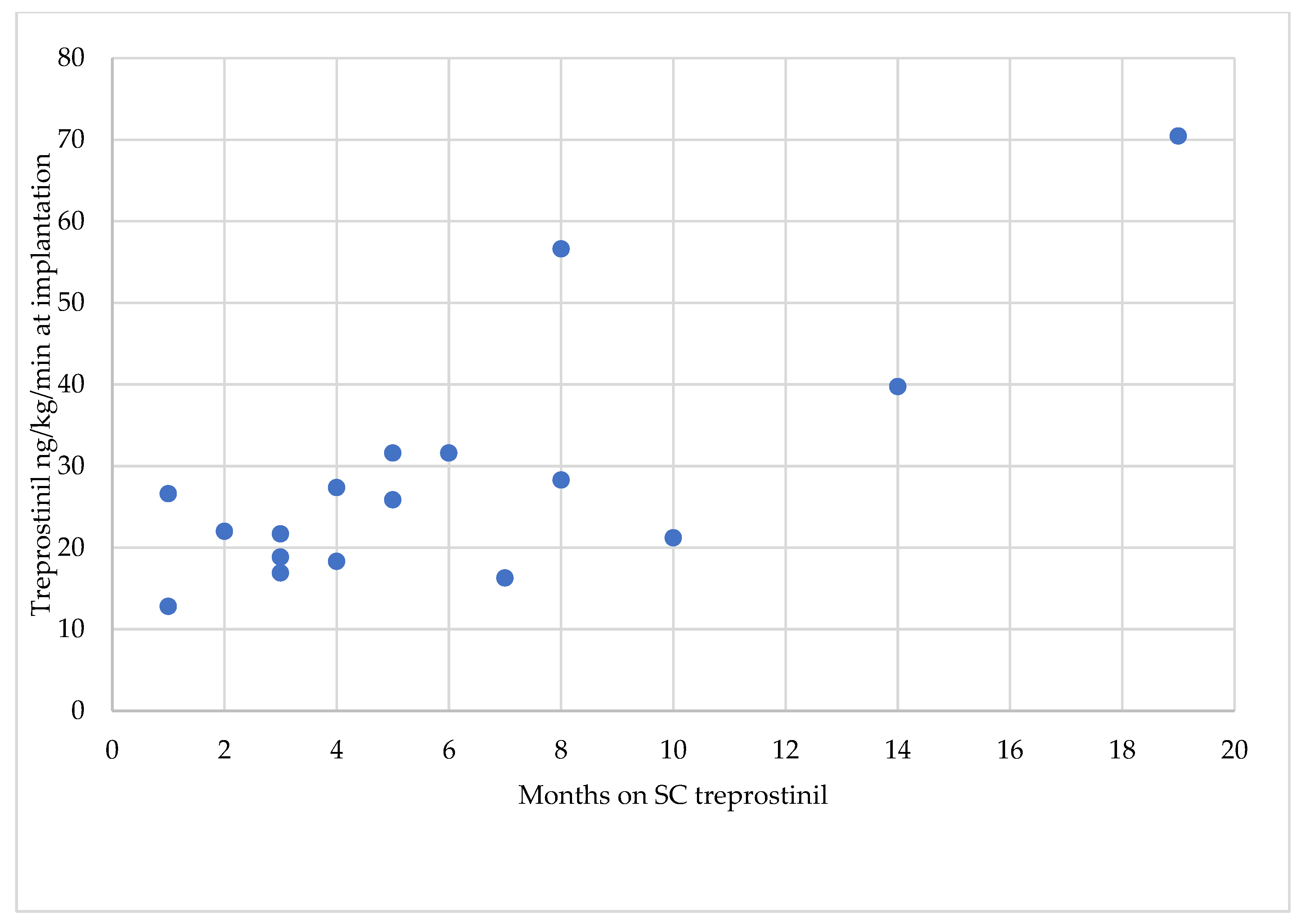
| Treprostinil Treatment Initiation Timepoint | IIP Implantation Timepoint | ||||||
|---|---|---|---|---|---|---|---|
| Patient N° | Sex | mPAP [mmHg] | PVR [WU] | WHO FC | Age [Years] | Duration of SC Treprostinil Therapy [Months] | Treprostinil Dose [ng/kg/min] |
| 1 | f | 47 | 4 | III | 56 | 5 | 31.6 |
| 2 | m | 37 | 13.5 | IV | 74 | 14 | 39.74 |
| 3 | f | 63 | 17 | III | 43 | 4 | 18.3 |
| 4th | f | 46 | 4 | III | 80 | 8 | 28.28 |
| 5 | m | 44 | 5 | IV | 67 | 5 | 25.85 |
| 6 | f | 44 | 4 | III | 78 | 3 | 18.85 |
| 7 | m | 34 | 6 | III | 82 | 10 | 21.21 |
| 8 | f | 36 | 6 | III | 75 | 1 | 26.61 |
| 9 | f | 45 | 11 | III | 72 | 4 | 27.36 |
| 10 | m | 45 | 12 | III | 75 | 8 | 56.6 |
| 11 | f | 59 | 11 | III | 53 | 1 | 12.8 |
| 12 | m | 61 | 19.6 | III | 82 | 19 | 70.45 |
| 13 | m | 57 | 11 | III | 82 | 3 | 21.69 |
| 14 | f | 50 | 7.5 | III | 73 | 2 | 22 |
| 15 | f | 45 | 8 | III | 73 | 3 | 16.89 |
| 16 | m | 42 | 6 | III | 60 | 7 | 16.28 |
| 17 | f | 48 | 4 | III | 82 | 6 | 31.6 |
| Complications | Count | Per 1000 Patient Days |
|---|---|---|
| Total | 13 | 0.8 |
| Procedure related | 1 | 0.1 |
| Postoperative hospital stay | 1 | 0.1 |
| Device related | 12 | 0.7 |
| Planned | ||
| Pump flow rate (planned change) | 4 | 0.2 |
| Related implantation | 1 | 0.1 |
| Unplanned | ||
| Connector dislocation | 2 | 0.1 |
| Pump/catheter problem without pump change | 2 | 0.1 |
| Pump/catheter problem with pump change | 2 | 0.1 |
| Explantation | 1 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steringer-Mascherbauer, R.; Huber, C.; Fröschl, U.; Engleder, D.; Függer, R.; Lummersdorfer, M.; Lenhard, R.; Martinek, M. Intravenous Treprostinil in Severe Inoperable Chronic Thromboembolic Pulmonary Hypertension Using Implantable Pumps—Single-Center Experience over More Than a Decade. J. Cardiovasc. Dev. Dis. 2023, 10, 318. https://doi.org/10.3390/jcdd10080318
Steringer-Mascherbauer R, Huber C, Fröschl U, Engleder D, Függer R, Lummersdorfer M, Lenhard R, Martinek M. Intravenous Treprostinil in Severe Inoperable Chronic Thromboembolic Pulmonary Hypertension Using Implantable Pumps—Single-Center Experience over More Than a Decade. Journal of Cardiovascular Development and Disease. 2023; 10(8):318. https://doi.org/10.3390/jcdd10080318
Chicago/Turabian StyleSteringer-Mascherbauer, Regina, Charlotte Huber, Uwe Fröschl, Dagmar Engleder, Reinhold Függer, Maria Lummersdorfer, Ralf Lenhard, and Martin Martinek. 2023. "Intravenous Treprostinil in Severe Inoperable Chronic Thromboembolic Pulmonary Hypertension Using Implantable Pumps—Single-Center Experience over More Than a Decade" Journal of Cardiovascular Development and Disease 10, no. 8: 318. https://doi.org/10.3390/jcdd10080318
APA StyleSteringer-Mascherbauer, R., Huber, C., Fröschl, U., Engleder, D., Függer, R., Lummersdorfer, M., Lenhard, R., & Martinek, M. (2023). Intravenous Treprostinil in Severe Inoperable Chronic Thromboembolic Pulmonary Hypertension Using Implantable Pumps—Single-Center Experience over More Than a Decade. Journal of Cardiovascular Development and Disease, 10(8), 318. https://doi.org/10.3390/jcdd10080318







