Cardiac Magnetic Resonance—Detected Acute Myocardial Edema as Predictor of Favourable Prognosis: A Comprehensive Review
Abstract
1. Introduction
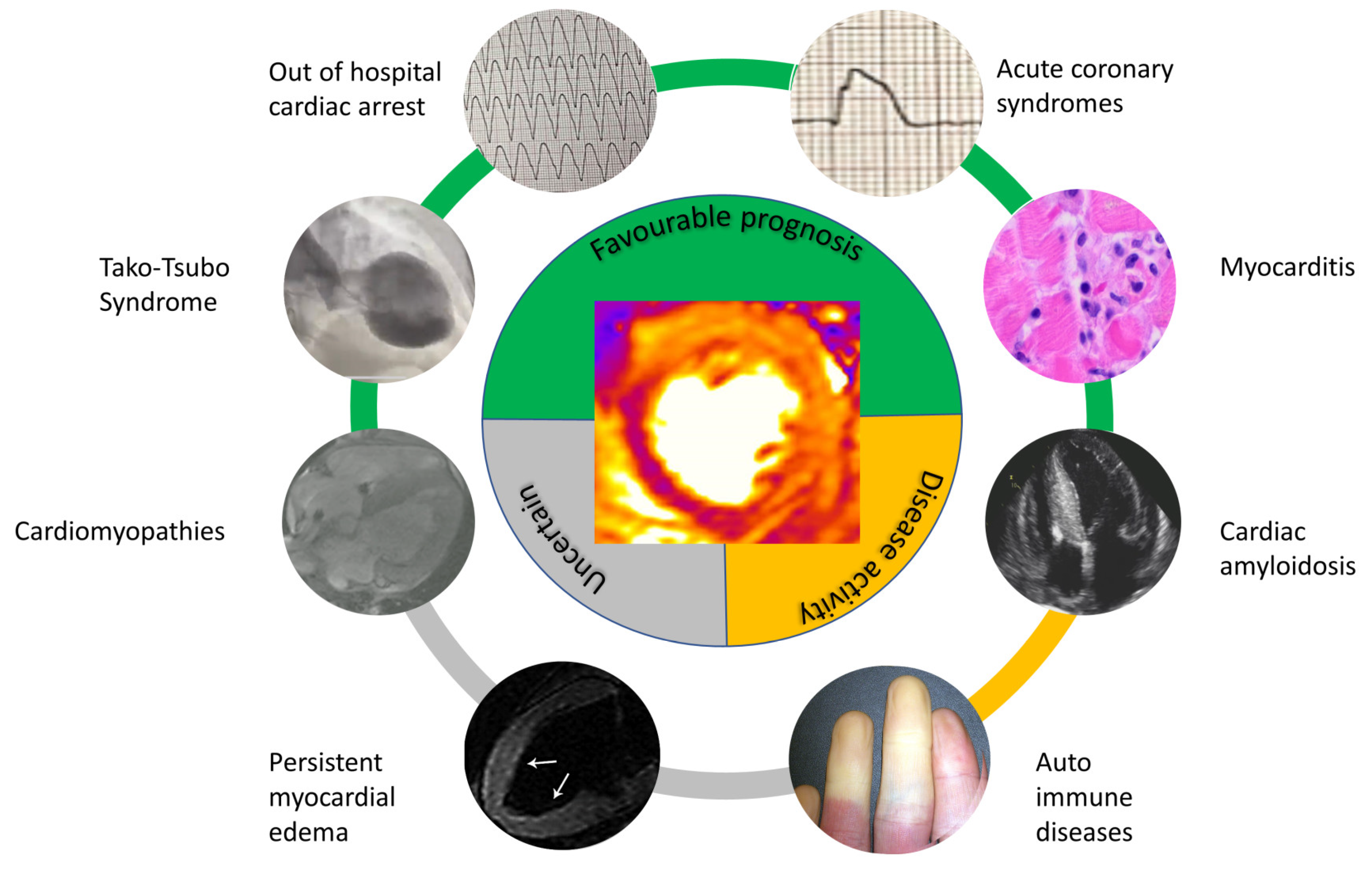
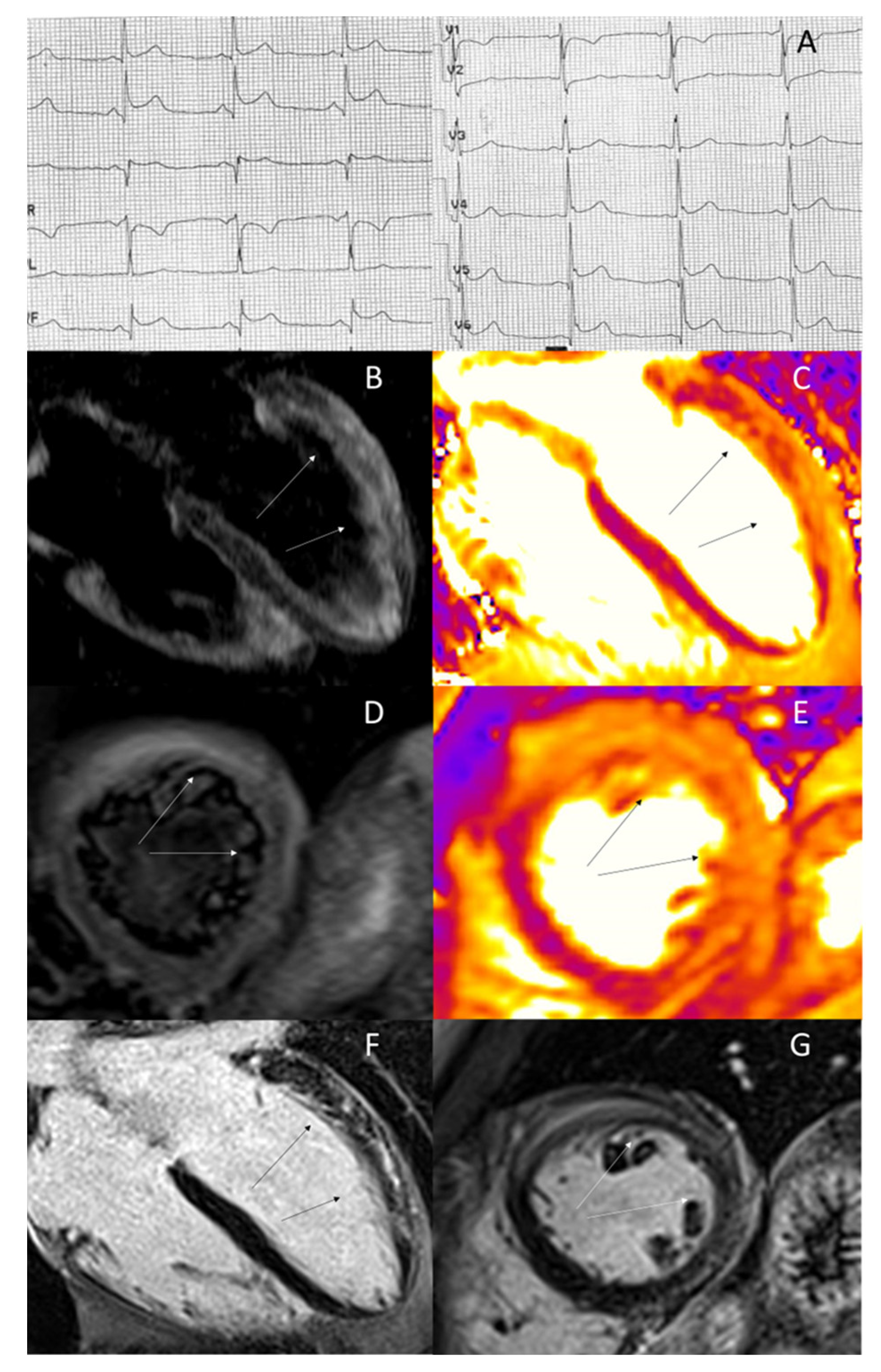
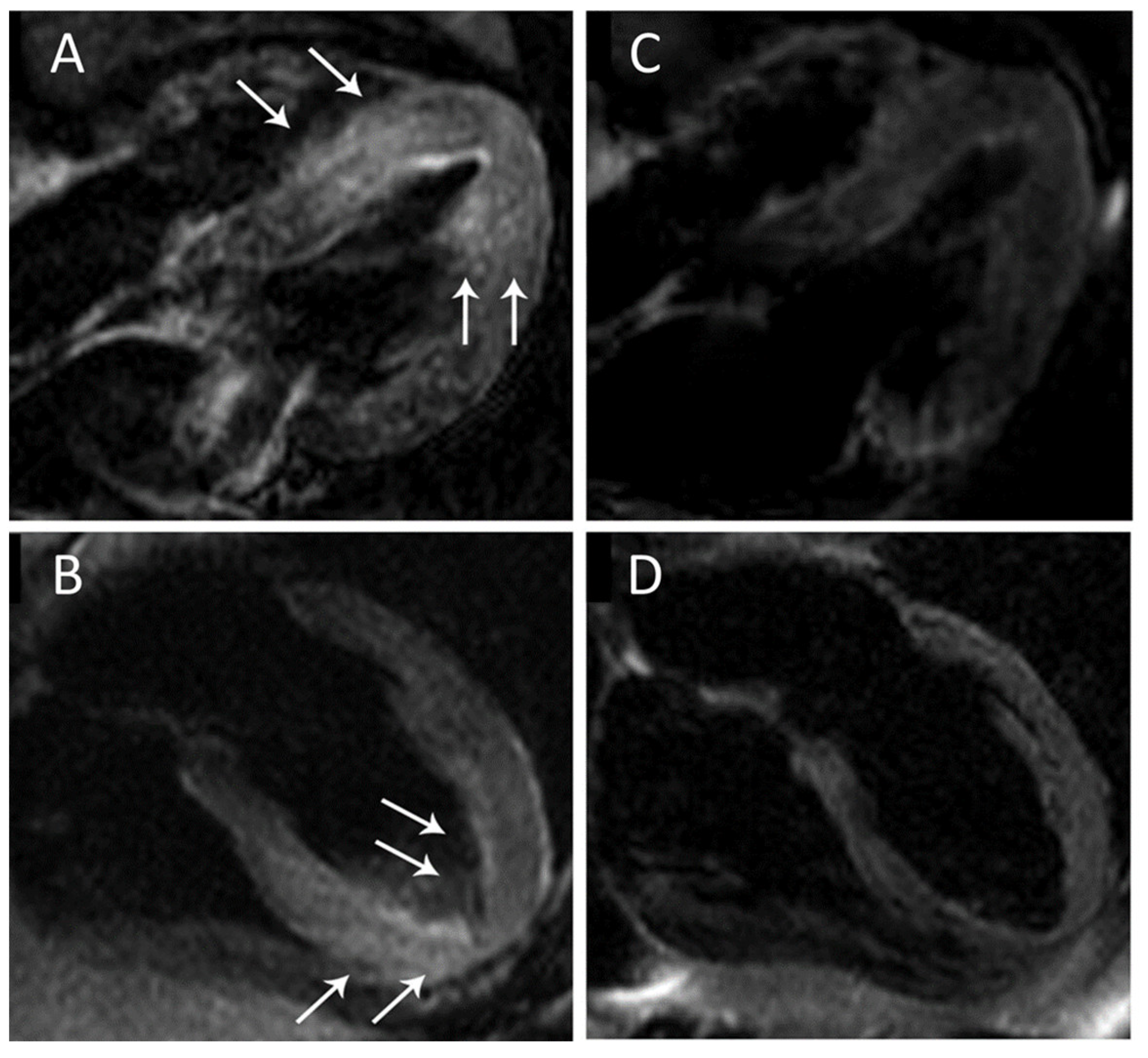
2. Acute Coronary Syndromes
3. Acute Myocarditis
4. Takotsubo Syndrome
5. Resuscitated Sudden Cardiac Death
6. Hypertrophic Cardiomyopathy
7. Other Cardiac Diseases
8. Gaps of Evidence
9. Future Perspectives
10. Conclusions
| Disease | Study | n | Time of Scanning | Prevalence | Prognostic Index | Outcome |
|---|---|---|---|---|---|---|
| Acute coronary syndromes | [12] | 208 | 1–4 days | 100% | MSI extension | Less MACE at 6 months |
| [13] | 92 | 3–5 days | 100% | MSI extension | Less re–AMI at 263 days | |
| Acute myocarditis | [26] | 388 | 2–6 days | 63% | LGE without edema | More MACE at 15 days |
| [28] | 187 | 1–7 days 6 months | 96% 16% | LGE without edema | More MACE at 7 years | |
| TakoTsubo syndrome | [39] | 199 | 2–4 days 6 months | 81% 0% | LVEF and isolated AME | Normalization of LVEF and AME resolution at 6 months |
| [40] | 20 | 3 days 3 months | 100% 0% | LVEF and isolated AME | Normalization of LVEF and AME resolution at 3 months | |
| OHCA | [47] | 44 | 1–7 days | 41% | Isolated AME | No arrhythmic event at 3 years |
| [34] | 101 | 8–22 days | 18% | Isolated AME | No appropriate ICD therapy at 47 months | |
| HCM | [54] | 65 | Time of diagnosis | 42% | Isolated AME | Higher risk of LV arrhythmias |
| [55] | 674 | Time of diagnosis | 42% | LGE with edema | More risk of CVE at 36 months. | |
| CA | [61] | 286 | Time of diagnosis | 100% AL 0% ATTR | Isolated AME | More risk of death at 23 months |
| Autoimmune disease | [56] | 78 | Time of diagnosis | 56% | Isolated AME | Reduction of AME after appropriate therapy |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corrado, D.; Marra, M.P.; Zorzi, A. Myocardial edema: Bonum et laudabile. Trends Cardiovasc. Med. 2022, in press. [Google Scholar] [CrossRef]
- Eitel, I.; Friedrich, M.G. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J. Cardiovasc. Magn. Reson. 2011, 13, 13. [Google Scholar] [CrossRef]
- Scallan, J.; Huxley, V.H.; Korthuis, R.J. Capillary Fluid Exchange: Regulation, Functions, and Pathology; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Garcia-Dorado, D.; Andres-Villarreal, M.; Ruiz-Meana, M.; Inserte, J.; Barba, I. Myocardial edema: A translational view. J. Mol. Cell Cardiol. 2012, 52, 931–939. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Montant, P.; Sigovan, M.; Revel, D.; Douek, P. MR imaging assessment of myocardial edema with T2 mapping. Diagn. Interv. Imaging 2015, 96, 885–890. [Google Scholar] [CrossRef]
- O’Brien, A.T.; Gil, K.E.; Varghese, J.; Simonetti, O.P.; Zareba, K.M. T2 mapping in myocardial disease: A comprehensive review. J. Cardiovasc. Magn. Reson. 2022, 24, 33. [Google Scholar] [CrossRef]
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovasc. Imaging 2016, 9, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Małek, Ł.A.; Śpiewak, M. Isolated myocardial edema in cardiac magnetic resonance—In search of a management strategy. Trends Cardiovasc. Med. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Dharmakumar, R.; Arai, A.E.; Berry, C.; Hausenloy, D.J. Cardiovascular Magnetic Resonance in Acute ST-Segment-Elevation Myocardial Infarction: Recent Advances, Controversies, and Future Directions. Circulation 2018, 137, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Baritussio, A.; Scatteia, A.; Bucciarelli-Ducci, C. Role of cardiovascular magnetic resonance in acute and chronic ischemic heart disease. Int. J. Cardiovasc. Imaging 2018, 34, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Desch, S.; Fuernau, G.; Hildebrand, L.; Gutberlet, M.; Schuler, G.; Thiele, H. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J. Am. Coll. Cardiol. 2010, 55, 2470–2479. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, G.; Wang, X.; Li, Y.; Zhao, Y.; Gu, X.; Xu, B.; Cui, J.; Wang, X.; Sun, Y.; et al. Prognostic significance of myocardial salvage assessed by cardiac magnetic resonance in reperfused ST-segment elevation myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 924428. [Google Scholar] [CrossRef] [PubMed]
- Dall’Armellina, E.; Karia, N.; Lindsay, A.C.; Karamitsos, T.D.; Ferreira, V.; Robson, M.D.; Kellman, P.; Francis, J.M.; Forfar, C.; Prendergast, B.D.; et al. Dynamic changes of edema and late gadolinium enhancement after acute myocardial infarction and their relationship to functional recovery and salvage index. Circ. Cardiovasc. Imaging 2011, 4, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jiménez, R.; Sánchez-González, J.; Agüero, J.; García-Prieto, J.; López-Martín, G.J.; García-Ruiz, J.M.; Molina-Iracheta, A.; Rosselló, X.; Fernández-Friera, L.; Pizarro, G.; et al. Myocardial edema after ischemia/reperfusion is not stable and follows a bimodal pattern: Imaging and histological tissue characterization. J. Am. Coll. Cardiol. 2015, 65, 315–323. [Google Scholar] [CrossRef]
- Fernández-Jiménez, R.; García-Prieto, J.; Sánchez-González, J.; Agüero, J.; López-Martín, G.J.; Galán-Arriola, C.; Molina-Iracheta, A.; Doohan, R.; Fuster, V.; Ibáñez, B. Pathophysiology Underlying the Bimodal Edema Phenomenon After Myocardial Ischemia/Reperfusion. J. Am. Coll. Cardiol. 2015, 66, 816–828. [Google Scholar] [CrossRef]
- Carrick, D.; Haig, C.; Ahmed, N.; Rauhalammi, S.; Clerfond, G.; Carberry, J.; Mordi, I.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; et al. Temporal Evolution of Myocardial Hemorrhage and Edema in Patients After Acute ST-Segment Elevation Myocardial Infarction: Pathophysiological Insights and Clinical Implications. J. Am. Heart Assoc. 2016, 5, e002834. [Google Scholar] [CrossRef]
- Jablonowski, R.; Engblom, H.; Kanski, M.; Nordlund, D.; Koul, S.; van der Pals, J.; Englund, E.; Heiberg, E.; Erlinge, D.; Carlsson, M.; et al. Contrast-Enhanced CMR Overestimates Early Myocardial Infarct Size: Mechanistic Insights Using ECV Measurements on Day 1 and Day 7. JACC Cardiovasc. Imaging 2015, 8, 1379–1389. [Google Scholar] [CrossRef]
- Bulluck, H.; Hammond-Haley, M.; Weinmann, S.; Martinez-Macias, R.; Hausenloy, D.J. Myocardial Infarct Size by CMR in Clinical Cardioprotection Studies: Insights from Randomized Controlled Trials. JACC Cardiovasc. Imaging 2017, 10, 230–240. [Google Scholar] [CrossRef]
- Gallagher, S.; Jones, D.A.; Anand, V.; Mohiddin, S. Diagnosis and management of patients with acute cardiac symptoms, troponin elevation and culprit-free angiograms. Heart 2012, 98, 974–981. [Google Scholar] [CrossRef]
- Tarantini, G.; Cacciavillani, L.; Corbetti, F.; Ramondo, A.; Marra, M.P.; Bacchiega, E.; Napodano, M.; Bilato, C.; Razzolini, R.; Iliceto, S. Duration of ischemia is a major determinant of transmurality and severe microvascular obstruction after primary angioplasty: A study performed with contrast-enhanced magnetic resonance. J. Am. Coll. Cardiol. 2005, 46, 1229–1235. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef]
- Pontone, G.; Di Bella, G.; Castelletti, S.; Maestrini, V.; Festa, P.; Ait-Ali, L.; Masci, P.G.; Monti, L.; di Giovine, G.; De Lazzari, M.; et al. Clinical recommendations of cardiac magnetic resonance, Part II: Inflammatory and congenital heart disease, cardiomyopathies and cardiac tumors: A position paper of the working group ‘Applicazioni della Risonanza Magnetica’ of the Italian Society of Cardiology. J. Cardiovasc. Med. 2017, 18, 209–222. [Google Scholar]
- Rajiah, P.; Kirsch, J.; Bolen, M.A.; Batlle, J.C.; Brown, R.K.J.; Francois, C.J.; Galizia, M.S.; Hanneman, K.; Inacio, J.R.; Johri, A.M.; et al. ACR Appropriateness Criteria® Nonischemic Myocardial Disease with Clinical Manifestations (Ischemic Cardiomyopathy Already Excluded). J. Am. Coll. Radiol. 2021, 18, S83–S105. [Google Scholar] [CrossRef]
- De Lazzari, M.; Zorzi, A.; Baritussio, A.; Siciliano, M.; Migliore, F.; Susana, A.; Giorgi, B.; Lacognata, C.; Iliceto, S.; Perazzolo Marra, M.; et al. Relationship between T-wave inversion and transmural myocardial edema as evidenced by cardiac magnetic resonance in patients with clinically suspected acute myocarditis: Clinical and prognostic implications. J. Electrocardiol. 2016, 49, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, Y.; Garot, J.; Hovasse, T.; Unterseeh, T.; Di Lena, C.; Boukefoussa, W.; Tawa, C.; Renard, C.; Limouzineau, I.; Duhamel, S.; et al. Clinical and Cardiovascular Magnetic Resonance Predictors of Early and Long-Term Clinical Outcome in Acute Myocarditis. Front. Cardiovasc. Med. 2022, 9, 886607. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.J.; McKenzie, S.C.; Taylor, A.J. Cardiac magnetic resonance imaging predicts recovery of left ventricular function in acute onset cardiomyopathy. Heart Lung Circ. 2012, 21, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Ghebru Habtemicael, Y.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Lanzillo, C.; Scatteia, A.; Di Roma, M.; Pontone, G.; et al. Prognostic Value of Repeating Cardiac Magnetic Resonance in Patients with Acute Myocarditis. J. Am. Coll. Cardiol. 2019, 74, 2439–2448. [Google Scholar] [CrossRef]
- Sanguineti, F.; Garot, P.; Mana, M.; O‘h-Ici, D.; Hovasse, T.; Unterseeh, T.; Louvard, Y.; Troussier, X.; Morice, M.C.; Garot, J. Cardiovascular magnetic resonance predictors of clinical outcome in patients with suspected acute myocarditis. J. Cardiovasc. Magn. Reson. 2015, 17, 78. [Google Scholar] [CrossRef]
- Grün, S.; Schumm, J.; Greulich, S.; Wagner, A.; Schneider, S.; Bruder, O.; Kispert, E.M.; Hill, S.; Ong, P.; Klingel, K.; et al. Long-term follow-up of biopsy-proven viral myocarditis: Predictors of mortality and incomplete recovery. J. Am. Coll. Cardiol. 2012, 59, 1604–1615. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Marcotte, F. Cardiac magnetic resonance assessment of myocarditis. Circ. Cardiovasc. Imaging 2013, 6, 833–839. [Google Scholar] [CrossRef]
- Georgiopoulos, G.; Figliozzi, S.; Sanguineti, F.; Aquaro, G.D.; di Bella, G.; Stamatelopoulos, K.; Chiribiri, A.; Garot, J.; Masci, P.G.; Ismail, T.F. Prognostic Impact of Late Gadolinium Enhancement by Cardiovascular Magnetic Resonance in Myocarditis: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2021, 14, e011492. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients with Acute Myocarditis: Multicenter Lombardy Registry. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Mattesi, G.; Baldi, E.; Toniolo, M.; Guerra, F.; Cauti, F.M.; Cipriani, A.; De Lazzari, M.; Muser, D.; Stronati, G.; et al. Prognostic Role of Myocardial Edema as Evidenced by Early Cardiac Magnetic Resonance in Survivors of Out-of-Hospital Cardiac Arrest: A Multicenter Study. J. Am. Heart Assoc. 2021, 10, e021861. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Behrendt, F.; Schindler, K.; Kivelitz, D.; Gutberlet, M.; Schuler, G.; Thiele, H. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur. Heart J. 2008, 29, 2651–2659. [Google Scholar] [CrossRef]
- Haghi, D.; Fluechter, S.; Suselbeck, T.; Kaden, J.J.; Borggrefe, M.; Papavassiliu, T. Cardiovascular magnetic resonance findings in typical versus atypical forms of the acute apical ballooning syndrome (Takotsubo cardiomyopathy). Int. J. Cardiol. 2007, 120, 205–211. [Google Scholar] [CrossRef]
- Dawson, D.K.; Neil, C.J.; Henning, A.; Cameron, D.; Jagpal, B.; Bruce, M.; Horowitz, J.; Frenneaux, M.P. Tako-Tsubo Cardiomyopathy: A Heart Stressed Out of Energy? JACC Cardiovasc. Imaging 2015, 8, 985–987. [Google Scholar] [CrossRef]
- Scally, C.; Ahearn, T.; Rudd, A.; Neil, C.J.; Srivanasan, J.; Jagpal, B.; Horowitz, J.; Frenneaux, M.; Dawson, D.K. Right Ventricular Involvement and Recovery After Acute Stress-Induced (Tako-tsubo) Cardiomyopathy. Am. J. Cardiol. 2016, 117, 775–780. [Google Scholar] [CrossRef]
- Eitel, I.; von Knobelsdorff-Brenkenhoff, F.; Bernhardt, P.; Carbone, I.; Muellerleile, K.; Aldrovandi, A.; Francone, M.; Desch, S.; Gutberlet, M.; Strohm, O.; et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA 2011, 306, 277–286. [Google Scholar] [CrossRef]
- Perazzolo Marra, M.; Zorzi, A.; Corbetti, F.; De Lazzari, M.; Migliore, F.; Tona, F.; Tarantini, G.; Iliceto, S.; Corrado, D. Apicobasal gradient of left ventricular myocardial edema underlies transient T-wave inversion and QT interval prolongation (Wellens’ ECG pattern) in Tako-Tsubo cardiomyopathy. Heart Rhythm 2013, 10, 70–77. [Google Scholar] [CrossRef]
- Ojha, V.; Khurana, R.; Ganga, K.P.; Kumar, S. Advanced cardiac magnetic resonance imaging in takotsubo cardiomyopathy. Br. J. Radiol. 2020, 93, 20200514. [Google Scholar] [CrossRef]
- Migliore, F.; Zorzi, A.; Marra, M.P.; Basso, C.; Corbetti, F.; De Lazzari, M.; Tarantini, G.; Buja, P.; Lacognata, C.; Thiene, G.; et al. Myocardial edema underlies dynamic T-wave inversion (Wellens’ ECG pattern) in patients with reversible left ventricular dysfunction. Heart Rhythm 2011, 8, 1629–1634. [Google Scholar] [CrossRef]
- Zorzi, A.; Perazzolo Marra, M.; Migliore, F.; De Lazzari, M.; Tarantini, G.; Iliceto, S.; Corrado, D. Relationship between repolarization abnormalities and myocardial edema in atypical Tako-Tsubo syndrome. J. Electrocardiol. 2013, 46, 348–351. [Google Scholar] [CrossRef]
- Migliore, F.; Zorzi, A.; Perazzolo Marra, M.; Iliceto, S.; Corrado, D. Myocardial edema as a substrate of electrocardiographic abnormalities and life-threatening arrhythmias in reversible ventricular dysfunction of takotsubo cardiomyopathy: Imaging evidence, presumed mechanisms, and implications for therapy. Heart Rhythm 2015, 12, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Migliore, F.; Zorzi, A.; Peruzza, F.; Perazzolo Marra, M.; Tarantini, G.; Iliceto, S.; Corrado, D. Incidence and management of life-threatening arrhythmias in Takotsubo syndrome. Int. J. Cardiol. 2013, 166, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Grand, J.; Meyer, M.A.S.; Wiberg, S.; Mogelvang, R.; Vejlstrup, N.; Schousboe, B.; Gjedsted, J.; Oestergaard, M.; Wanscher, M.; et al. Global myocardial edema in resuscitated out-of-hospital cardiac arrest patients assessed by cardiac magnetic resonance: A pilot study. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Susana, A.; De Lazzari, M.; Migliore, F.; Vescovo, G.; Scarpa, D.; Baritussio, A.; Tarantini, G.; Cacciavillani, L.; Giorgi, B.; et al. Diagnostic value and prognostic implications of early cardiac magnetic resonance in survivors of out-of-hospital cardiac arrest. Heart Rhythm 2018, 15, 1031–1041. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Todiere, G.; Barison, A.; Baritussio, A.; Cipriani, A.; Guaricci, A.I.; Pica, S.; Indolfi, C.; Pontone, G.; Dellegrottaglie, S.; Working Group on Cardiac Magnetic Resonance of the Italian Society of Cardiology. Acute clinical presentation of nonischemic cardiomyopathies: Early detection by cardiovascular magnetic resonance. J. Cardiovasc. Med. 2023, 24 (Suppl. S1), e36–e46. [Google Scholar] [CrossRef]
- Merlo, M.; Gagno, G.; Baritussio, A.; Bauce, B.; Biagini, E.; Canepa, M.; Cipriani, A.; Castelletti, S.; Dellegrottaglie, S.; Guaricci, A.I.; et al. Clinical application of CMR in cardiomyopathies: Evolving concepts and techniques: A position paper of myocardial and pericardial diseases and cardiac magnetic resonance working groups of Italian society of cardiology. Heart Fail. Rev. 2023, 28, 77–95. [Google Scholar] [CrossRef]
- Melacini, P.; Corbetti, F.; Calore, C.; Pescatore, V.; Smaniotto, G.; Pavei, A.; Bobbo, F.; Cacciavillani, L.; Iliceto, S. Cardiovascular magnetic resonance signs of ischemia in hypertrophic cardiomyopathy. Int. J. Cardiol. 2008, 128, 364–373. [Google Scholar] [CrossRef]
- Cecchi, F.; Olivotto, I.; Gistri, R.; Lorenzoni, R.; Chiriatti, G.; Camici, P.G. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N. Engl. J. Med. 2003, 349, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Todiere, G.; Barison, A.; Strata, E.; Marzilli, M.; Pingitore, A.; Lombardi, M. Myocardial blood flow and fibrosis in hypertrophic cardiomyopathy. J. Card. Fail. 2011, 17, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Todiere, G.; Pisciella, L.; Barison, A.; Del Franco, A.; Zachara, E.; Piaggi, P.; Re, F.; Pingitore, A.; Emdin, M.; Lombardi, M.; et al. Abnormal T2-STIR magnetic resonance in hypertrophic cardiomyopathy: A marker of advanced disease and electrical myocardial instability. PLoS ONE 2014, 9, e111366. [Google Scholar]
- Xu, Z.; Wang, J.; Cheng, W.; Wan, K.; Li, W.; Pu, L.; Xu, Y.; Sun, J.; Han, Y.; Chen, Y. Incremental significance of myocardial oedema for prognosis in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.; Markousis-Mavrogenis, G.; Koutsogeorgopoulou, L.; Dimitroulas, T.; Bratis, K.; Kitas, G.D.; Sfikakis, P.; Tektonidou, M.; Karabela, G.; Stavropoulos, E.; et al. Cardiovascular magnetic resonance imaging pattern at the time of diagnosis of treatment naïve patients with connective tissue diseases. Int. J. Cardiol. 2017, 236, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.; Gargani, L.; Pepe, A.; Monti, L.; Markousis-Mavrogenis, G.; De Santis, M.; De Marchi, D.; Koutsogeorgopoulou, L.; Karabela, G.; Stavropoulos, E.; et al. Cardiac magnetic resonance predicts ventricular arrhythmias in scleroderma: The Scleroderma Arrhythmia Clinical Utility Study (SAnCtUS). Rheumatology 2020, 59, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Malczuk, E.; Tłustochowicz, W.; Kramarz, E.; Kisiel, B.; Marczak, M.; Tłustochowicz, M.; Małek, Ł.A. Early Myocardial Changes in Patients with Rheumatoid Arthritis without Known Cardiovascular Diseases-A Comprehensive Cardiac Magnetic Resonance Study. Diagnostics 2021, 11, 2290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Corona-Villalobos, C.P.; Kiani, A.N.; Eng, J.; Kamel, I.R.; Zimmerman, S.L.; Petri, M. Myocardial T2 mapping by cardiovascular magnetic resonance reveals subclinical myocardial inflammation in patients with systemic lupus erythematosus. Int. J. Cardiovasc. Imaging 2015, 31, 389–397. [Google Scholar] [CrossRef]
- Ridouani, F.; Damy, T.; Tacher, V.; Derbel, H.; Legou, F.; Sifaoui, I.; Audureau, E.; Bodez, D.; Rahmouni, A.; Deux, J.F. Myocardial native T2 measurement to differentiate light-chain and transthyretin cardiac amyloidosis and assess prognosis. J. Cardiovasc. Magn. Reson. 2018, 20, 58. [Google Scholar] [CrossRef]
- Kotecha, T.; Martinez-Naharro, A.; Treibel, T.A.; Francis, R.; Nordin, S.; Abdel-Gadir, A.; Knight, D.S.; Zumbo, G.; Rosmini, S.; Maestrini, V.; et al. Myocardial Edema and Prognosis in Amyloidosis. J. Am. Coll. Cardiol. 2018, 71, 2919–2931. [Google Scholar] [CrossRef] [PubMed]
- Vermes, E.; Pantaléon, C.; Auvet, A.; Cazeneuve, N.; Machet, M.C.; Delhommais, A.; Bourguignon, T.; Aupart, M.; Brunereau, L. Cardiovascular magnetic resonance in heart transplant patients: Diagnostic value of quantitative tissue markers: T2 mapping and extracellular volume fraction, for acute rejection diagnosis. J. Cardiovasc. Magn. Reson. 2018, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- Bonnemains, L.; Villemin, T.; Escanye, J.M.; Hossu, G.; Odille, F.; Vanhuyse, F.; Felblinger, J.; Marie, P.Y. Diagnostic and prognostic value of MRI T2 quantification in heart transplant patients. Transpl. Int. 2014, 27, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Dorniak, K.; Stopczyńska, I.; van Heeswijk, R.B.; Żaczyńska-Buchowiecka, M.; Fijałkowska, J.; Glińska, A.; Gruchała, M.; Szurowska, E.; Dudziak, M.; Sabisz, A. Cardiac magnetic resonance imaging with T2 mapping for the monitoring of acute heart transplant rejection in patients with problematic endomyocardial biopsy: In anticipation of new recommendations. Kardiol. Pol. 2021, 79, 339–343. [Google Scholar] [CrossRef]
- Sierra-Galan, L.M.; Bhatia, M.; Alberto-Delgado, A.L.; Madrazo-Shiordia, J.; Salcido, C.; Santoyo, B.; Martinez, E.; Soto, M.E. Cardiac Magnetic Resonance in Rheumatology to Detect Cardiac Involvement Since Early and Pre-clinical Stages of the Autoimmune Diseases: A Narrative Review. Front. Cardiovasc. Med. 2022, 9, 870200. [Google Scholar] [CrossRef]
- Zhang, L.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.O.; Thuny, F.; Zlotoff, D.A.; Murphy, S.P.; Stone, J.R.; Golden, D.L.A.; et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur. Heart J. 2020, 41, 1733–1743. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Zhang, L.; Zafar, A.; Drobni, Z.D.; Mahmood, S.S.; Cabral, M.; Awadalla, M.; Nohria, A.; Zlotoff, D.A.; Thuny, F.; et al. Myocardial T1 and T2 Mapping by Magnetic Resonance in Patients with Immune Checkpoint Inhibitor-Associated Myocarditis. J. Am. Coll. Cardiol. 2021, 77, 1503–1516. [Google Scholar] [CrossRef]
- Guo, C.W.; Alexander, M.; Dib, Y.; Lau, P.K.H.; Weppler, A.M.; Au-Yeung, G.; Lee, B.; Khoo, C.; Mooney, D.; Joshi, S.B.; et al. A closer look at immune-mediated myocarditis in the era of combined checkpoint blockade and targeted therapies. Eur. J. Cancer 2020, 124, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, P.; Koch, A.; Schmidt, M.; Magedanz, A.; Lindhoff-Last, E.; Voigtländer, T.; Schmermund, A.; Mehta, R.H.; Eggebrecht, H. Clinical and cardiac magnetic resonance findings in post-COVID patients referred for suspected myocarditis. Clin. Res. Cardiol. 2021, 110, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Friedrich, M.G.; Leiner, T.; Elias, M.D.; Ferreira, V.M.; Fenski, M.; Flamm, S.D.; Fogel, M.; Garg, R.; Halushka, M.K.; et al. Cardiovascular Magnetic Resonance for Patients With COVID-19. JACC Cardiovasc. Imaging 2022, 15, 685–699. [Google Scholar] [CrossRef]
- Hu, C.; Huber, S.; Nguyen, V.; Baldassarre, L.; Mojibian, H.; Peters, D. Fat-saturated dark-blood cardiac T2 mapping in a single breath-hold. Magn. Reson. Imaging 2021, 81, 24–32. [Google Scholar] [CrossRef] [PubMed]
- De Gaspari, M.; Sinigiani, G.; De Michieli, L.; Della Barbera, M.; Rizzo, S.; Thiene, G.; Iliceto, S.; Perazzolo Marra, M.; Mele, D.; Basso, C.; et al. Relative apical sparing in cardiac amyloidosis is not always explained by an amyloid gradient. Eur. Heart J. Cardiovasc. Imaging 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Dorbala, S.; Vangala, D.; Bruyere, J., Jr.; Quarta, C.; Kruger, J.; Padera, R.; Foster, C.; Hanley, M.; Di Carli, M.F.; Falk, R. Coronary microvascular dysfunction is related to abnormalities in myocardial structure and function in cardiac amyloidosis. JACC Heart Fail. 2014, 2, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; De Michieli, L.; Porcari, A.; Licchelli, L.; Sinigiani, G.; Tini, G.; Zampieri, M.; Sessarego, E.; Argirò, A.; Fumagalli, C.; et al. Low QRS Voltages in Cardiac Amyloidosis: Clinical Correlates and Prognostic Value. JACC CardioOncol. 2022, 4, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Martini, N.; Sinigiani, G.; De Michieli, L.; Mussinelli, R.; Perazzolo Marra, M.; Iliceto, S.; Zorzi, A.; Perlini, S.; Corrado, D.; Cipriani, A. Electrocardiographic features and rhythm disorders in cardiac amyloidosis. Trends Cardiovasc. Med. 2023, in press. [Google Scholar] [CrossRef]
- Mekkaoui, C.; Reese, T.G.; Jackowski, M.P.; Bhat, H.; Sosnovik, D.E. Diffusion MRI in the heart. NMR Biomed. 2017, 30, e3426. [Google Scholar] [CrossRef]
- Sosnovik, D.E.; Mekkaoui, C.; Huang, S.; Chen, H.H.; Dai, G.; Stoeck, C.T.; Ngoy, S.; Guan, J.; Wang, R.; Kostis, W.J.; et al. Microstructural impact of ischemia and bone marrow-derived cell therapy revealed with diffusion tensor magnetic resonance imaging tractography of the heart in vivo. Circulation 2014, 129, 1731–1741. [Google Scholar] [CrossRef]
- Wu, M.T.; Su, M.Y.; Huang, Y.L.; Chiou, K.R.; Yang, P.; Pan, H.B.; Reese, T.G.; Wedeen, V.J.; Tseng, W.Y. Sequential changes of myocardial microstructure in patients postmyocardial infarction by diffusion-tensor cardiac MR: Correlation with left ventricular structure and function. Circ. Cardiovasc. Imaging 2009, 2, 32–40. [Google Scholar] [CrossRef]
- Wu, M.T.; Tseng, W.Y.; Su, M.Y.; Liu, C.P.; Chiou, K.R.; Wedeen, V.J.; Reese, T.G.; Yang, C.F. Diffusion tensor magnetic resonance imaging mapping the fiber architecture remodeling in human myocardium after infarction: Correlation with viability and wall motion. Circulation 2006, 114, 1036–1045. [Google Scholar] [CrossRef]
- Nguyen, C.; Fan, Z.; Xie, Y.; Dawkins, J.; Tseliou, E.; Bi, X.; Sharif, B.; Dharmakumar, R.; Marbán, E.; Li, D. In vivo contrast free chronic myocardial infarction characterization using diffusion-weighted cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2014, 16, 68. [Google Scholar] [CrossRef]
- Sosnovik, D.E.; Wang, R.; Dai, G.; Reese, T.G.; Wedeen, V.J. Diffusion MR tractography of the heart. J. Cardiovasc. Magn. Reson. 2009, 11, 47. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinigiani, G.; De Michieli, L.; De Conti, G.; Ricci, F.; De Lazzari, M.; Migliore, F.; Perazzolo Marra, M.; Zorzi, A.; Corrado, D.; Cipriani, A. Cardiac Magnetic Resonance—Detected Acute Myocardial Edema as Predictor of Favourable Prognosis: A Comprehensive Review. J. Cardiovasc. Dev. Dis. 2023, 10, 319. https://doi.org/10.3390/jcdd10080319
Sinigiani G, De Michieli L, De Conti G, Ricci F, De Lazzari M, Migliore F, Perazzolo Marra M, Zorzi A, Corrado D, Cipriani A. Cardiac Magnetic Resonance—Detected Acute Myocardial Edema as Predictor of Favourable Prognosis: A Comprehensive Review. Journal of Cardiovascular Development and Disease. 2023; 10(8):319. https://doi.org/10.3390/jcdd10080319
Chicago/Turabian StyleSinigiani, Giulio, Laura De Michieli, Giorgio De Conti, Fabrizio Ricci, Manuel De Lazzari, Federico Migliore, Martina Perazzolo Marra, Alessandro Zorzi, Domenico Corrado, and Alberto Cipriani. 2023. "Cardiac Magnetic Resonance—Detected Acute Myocardial Edema as Predictor of Favourable Prognosis: A Comprehensive Review" Journal of Cardiovascular Development and Disease 10, no. 8: 319. https://doi.org/10.3390/jcdd10080319
APA StyleSinigiani, G., De Michieli, L., De Conti, G., Ricci, F., De Lazzari, M., Migliore, F., Perazzolo Marra, M., Zorzi, A., Corrado, D., & Cipriani, A. (2023). Cardiac Magnetic Resonance—Detected Acute Myocardial Edema as Predictor of Favourable Prognosis: A Comprehensive Review. Journal of Cardiovascular Development and Disease, 10(8), 319. https://doi.org/10.3390/jcdd10080319








