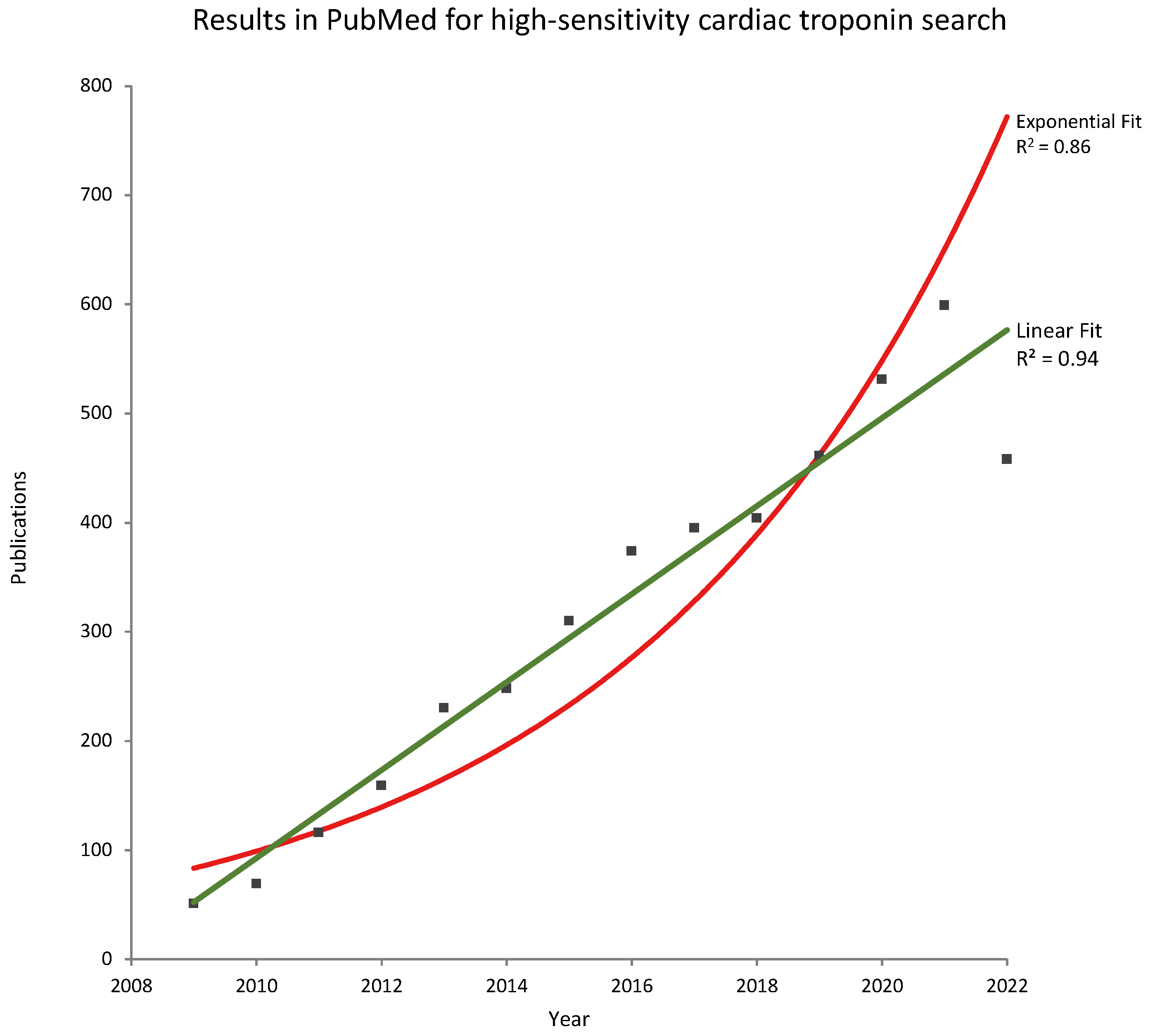
High-Sensitivity Cardiac Troponin Publications during the COVID-19 Pandemic (2020–2022)
Funding
Conflicts of Interest
References
- Kavsak, P.A.; MacRae, A.R.; Yerna, M.J.; Jaffe, A.S. Analytic and clinical utility of a next-generation, highly sensitive cardiac troponin I assay for early detection of myocardial injury. Clin. Chem. 2009, 55, 573–577. [Google Scholar] [CrossRef]
- Reichlin, T.; Hochholzer, W.; Bassetti, S.; Steuer, S.; Stelzig, C.; Hartwiger, S.; Biedert, S.; Schaub, N.; Buerge, C.; Potocki, M.; et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N. Engl. J. Med. 2009, 361, 858–867. [Google Scholar] [CrossRef]
- Kavsak, P.A. Approaching 2020 acuity for high-sensitivity cardiac troponin assays in Clinical Biochemistry. Clin. Biochem. 2020, 78, 1–3. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M. Clinical Chemistry and Laboratory Medicine: Enjoying the present and assessing the future. Clin. Chem. Lab. Med. 2022, 60, 1313–1315. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Hammarsten, O.; Worster, A.; Smith, S.W.; Apple, F.S. Cardiac Troponin Testing in Patients with COVID-19: A Strategy for Testing and Reporting Results. Clin. Chem. 2021, 67, 107–113. [Google Scholar] [CrossRef]
- Mohamed Ali, A.; Wasim, D.; Larsen, T.H.; Bogale, N.; Bleie, Ø.; Saeed, S. Acute Myocardial Infarction Due to Microvascular Obstruction in a Young Woman Who Recently Recovered from COVID-19 Infection. J. Cardiovasc. Dev. Dis. 2021, 8, 66. [Google Scholar] [CrossRef]
- Bularga, A.; Oskoui, E.; Fujisawa, T.; Jenks, S.; Sutherland, R.; Apple, F.S.; Hammarsten, O.; Mills, N.L. Macrotroponin Complex as a Cause for Cardiac Troponin Increase after COVID-19 Vaccination and Infection. Clin. Chem. 2022, 68, 1015–1019. [Google Scholar] [CrossRef]
- Harley, K.; Bissonnette, S.; Inzitari, R.; Schulz, K.; Apple, F.S.; Kavsak, P.A.; Gunsolus, I.L. Independent and combined effects of biotin and hemolysis on high-sensitivity cardiac troponin assays. Clin. Chem. Lab. Med. 2021, 59, 1431–1443. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Clark, L.; Martin, J.; Mark, C.T.; Paré, G.; Mondoux, S.; Chetty, V.T.; Ainsworth, C.; Worster, A. Acute Phase Response and Non-Reproducible Elevated Concentrations with a High-Sensitivity Cardiac Troponin I Assay. J. Clin. Med. 2021, 10, 1014. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Mondoux, S.E.; Martin, J.; Hewitt, M.K.; Clark, L.; Caruso, N.; Mark, C.T.; Chetty, V.T.; Ainsworth, C.; Worster, A. Disagreement between Cardiac Troponin Tests Yielding a Higher Incidence of Myocardial Injury in the Emergency Setting. J. Cardiovasc. Dev. Dis. 2021, 8, 31. [Google Scholar] [CrossRef]
- Lafrenière, M.A.; Tandon, V.; Ainsworth, C.; Nouri, K.; Mondoux, S.E.; Worster, A.; Kavsak, P.A. Storage conditions, sample integrity, interferences, and a decision tool for investigating unusual high-sensitivity cardiac troponin results. Clin. Biochem. 2022, in press. [CrossRef] [PubMed]
- Lam, L.; Tse, R.; Gladding, P.; Kyle, C. Effect of Macrotroponin in a Cohort of Community Patients with Elevated Cardiac Troponin. Clin. Chem. 2022, 68, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Chiang, C.H.; Chiang, C.H.; Pickering, J.W.; Stoyanov, K.M.; Chew, D.P.; Neumann, J.T.; Ojeda, F.; Sörensen, N.A.; Su, K.Y.; Kavsak, P.; et al. Performance of the European Society of Cardiology 0/1-Hour, 0/2-Hour, and 0/3-Hour Algorithms for Rapid Triage of Acute Myocardial Infarction: An International Collaborative Meta-analysis. Ann. Intern. Med. 2022, 175, 101–113. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Hewitt, M.K.; Mondoux, S.E.; Cerasuolo, J.O.; Ma, J.; Clayton, N.; McQueen, M.; Griffith, L.E.; Perez, R.; Seow, H.; et al. Diagnostic Performance of Serial High-Sensitivity Cardiac Troponin Measurements in the Emergency Setting. J. Cardiovasc. Dev. Dis. 2021, 8, 97. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Mondoux, S.E.; Hewitt, M.K.; Ainsworth, C.; Hill, S.; Worster, A. Can the Addition of NT-proBNP and Glucose Measurements Improve the Prognostication of High-Sensitivity Cardiac Troponin Measurements for Patients with Suspected Acute Coronary Syndrome? J. Cardiovasc. Dev. Dis. 2021, 8, 106. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Cerasuolo, J.O.; Ko, D.T.; Ma, J.; Sherbino, J.; Mondoux, S.E.; Perez, R.; Seow, H.; Worster, A. High-Sensitivity Cardiac Troponin I vs a Clinical Chemistry Score for Predicting All-Cause Mortality in an Emergency Department Population. CJC Open 2020, 2, 296–302. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Cerasuolo, J.O.; Hewitt, M.K.; Mondoux, S.E.; Perez, R.; Seow, H.; Ainsworth, C.; Ma, J.; Worster, A.; Ko, D.T. Identifying very low risk patients for future myocardial infarction or death. Can. J. Cardiol. 2022, in press. [CrossRef]
- Lu, P.; Lu, X.; Li, B.; Wang, C.; Wang, X.; Ji, Y.; Liu, Z.; Li, X.; Yi, C.; Song, M.; et al. High-Sensitivity Cardiac Troponin T in Prediction and Diagnosis of Early Postoperative Hypoxemia after Off-Pump Coronary Artery Bypass Grafting. J. Cardiovasc. Dev. Dis. 2022, 9, 416. [Google Scholar] [CrossRef]
- Devereaux, P.J.; Lamy, A.; Chan, M.T.V.; Allard, R.V.; Lomivorotov, V.V.; Landoni, G.; Zheng, H.; Paparella, D.; McGillion, M.H.; Belley-Côté, E.P.; et al. High-Sensitivity Troponin I after Cardiac Surgery and 30-Day Mortality. N. Engl. J. Med. 2022, 386, 827–836. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [PubMed]
- Zhang, X.; Sun, Y.; Zhang, Y.; Fang, F.; Liu, J.; Xia, Y.; Liu, Y. Cardiac Biomarkers for the Detection and Management of Cancer Therapy-Related Cardiovascular Toxicity. J. Cardiovasc. Dev. Dis. 2022, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Morfino, P.; Aimo, A.; Castiglione, V.; Vergaro, G.; Emdin, M.; Clerico, A. Biomarkers of HFpEF: Natriuretic Peptides, High-Sensitivity Troponins and Beyond. J. Cardiovasc. Dev. Dis. 2022, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Graziano, F.; Juhasz, V.; Brunetti, G.; Cipriani, A.; Szabo, L.; Merkely, B.; Corrado, D.; D’Ascenzi, F.; Vago, H.; Zorzi, A. May Strenuous Endurance Sports Activity Damage the Cardiovascular System of Healthy Athletes? A Narrative Review. J. Cardiovasc. Dev. Dis. 2022, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Costache, A.-D.; Leon-Constantin, M.-M.; Roca, M.; Maștaleru, A.; Anghel, R.-C.; Zota, I.-M.; Drugescu, A.; Costache, I.-I.; Chetran, A.; Moisă, Ș.-M.; et al. Cardiac Biomarkers in Sports Cardiology. J. Cardiovasc. Dev. Dis. 2022, 9, 453. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavsak, P.A. High-Sensitivity Cardiac Troponin Publications during the COVID-19 Pandemic (2020–2022). J. Cardiovasc. Dev. Dis. 2023, 10, 5. https://doi.org/10.3390/jcdd10010005
Kavsak PA. High-Sensitivity Cardiac Troponin Publications during the COVID-19 Pandemic (2020–2022). Journal of Cardiovascular Development and Disease. 2023; 10(1):5. https://doi.org/10.3390/jcdd10010005
Chicago/Turabian StyleKavsak, Peter A. 2023. "High-Sensitivity Cardiac Troponin Publications during the COVID-19 Pandemic (2020–2022)" Journal of Cardiovascular Development and Disease 10, no. 1: 5. https://doi.org/10.3390/jcdd10010005
APA StyleKavsak, P. A. (2023). High-Sensitivity Cardiac Troponin Publications during the COVID-19 Pandemic (2020–2022). Journal of Cardiovascular Development and Disease, 10(1), 5. https://doi.org/10.3390/jcdd10010005




