Cardiac Structure and Cardiorespiratory Fitness in Young Male Japanese Rugby Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Echocardiography
2.3. CPET
2.4. Statistical Analysis
3. Results
3.1. Anthropometry and Cardiorespiratory Fitness
3.2. Echocardiographic Findings of LV Cavity, Wall, and Function
3.3. Echocardiographic Findings of the Atria and Aortic Root
3.4. Association of CPET Parameters with Echocardiographic Parameters
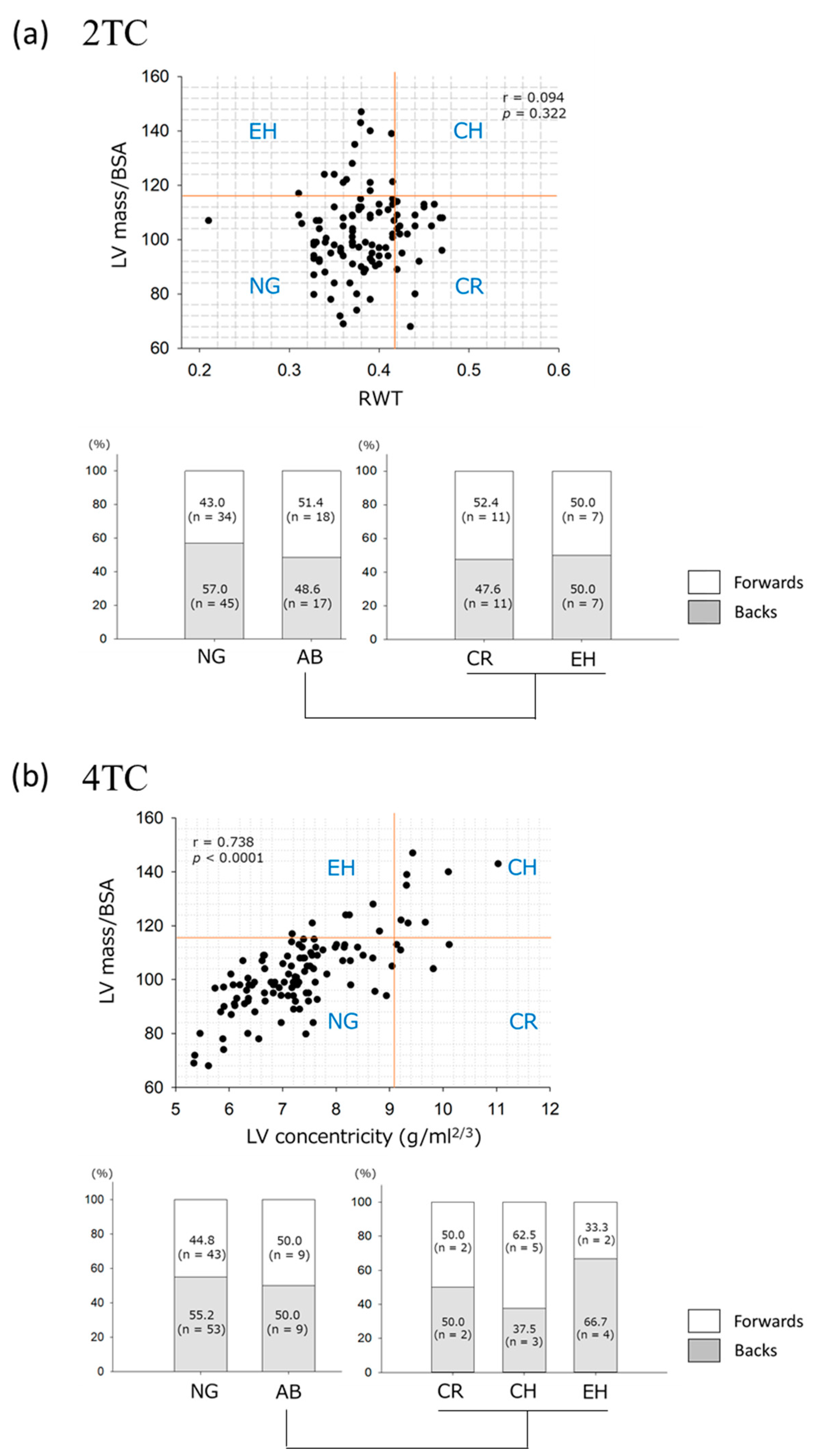
3.5. LV Geometry Assessed by the 2TC and 4TC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’Andrea, A.; D’Ascenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: Recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s heart. Eur. Heart J. 2018, 39, 1949–1969. [Google Scholar] [CrossRef] [PubMed]
- Gabbett, T.; King, T.; Jenkins, D. Applied physiology of rugby league. Sports Med. 2008, 38, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Landry, C.H.; Allan, K.S.; Connelly, K.A.; Cunningham, K.; Morrison, L.J.; Dorian, P.; Rescu Investigators. Sudden cardiac arrest during participation in competitive sports. N. Engl. J. Med. 2017, 377, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, L.; Kervio, G.; Corneloup, L.; Vincent, M.P.; Baudot, C.; Rebeyrol, J.L.; Merle, F.; Gencel, L.; Carré, F. Athlete’s heart patterns in elite rugby players: Effects of training specificities. Arch. Cardiovasc. Dis. 2013, 106, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Forsythe, L.; Somauroo, J.; Papadakis, M.; George, K.; Oxborough, D. Cardiac structure and function in elite Native Hawaiian and Pacific Islander Rugby Football League athletes: An exploratory study. Int. J. Cardiovasc. Imaging 2018, 34, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, L.; MacIver, D.H.; Johnson, C.; George, K.; Somauroo, J.; Papadakis, M.; Brown, B.; Qasem, M.; Oxborough, D. The relationship between left ventricular structure and function in the elite rugby football league athlete as determined by conventional echocardiography and myocardial strain imaging. Int. J. Cardiol. 2018, 261, 211–217. [Google Scholar] [CrossRef]
- Kay, S.; Moore, B.M.; Moore, L.; Seco, M.; Barnes, C.; Marshman, D.; Grieve, S.M.; Celermajer, D.S. Rugby Player’s aorta: Alarming prevalence of ascending aortic dilatation and effacement in elite rugby players. Heart Lung Circ. 2020, 29, 196–201. [Google Scholar] [CrossRef]
- Hainline, B.; Drezner, J.A.; Baggish, A.; Harmon, K.G.; Emery, M.S.; Myerburg, R.J.; Sanchez, E.; Molossi, S.; Parsons, J.T.; Thompson, P.D. Interassociation consensus statement on cardiovascular care of college student-athletes. J. Am. Coll. Cardiol. 2016, 67, 2981–2995. [Google Scholar] [CrossRef]
- Iso, Y.; Kitai, H.; Kyuno, E.; Tsunoda, F.; Nishinaka, N.; Funato, M.; Nishimura, E.; Akihiro, S.; Tanuma, H.; Yonechi, T.; et al. Prevalence and significance of sleep disordered breathing in adolescent athletes. ERJ Open Res. 2019, 5, 00029. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Braverman, A.C.; Harris, K.M.; Kovacs, R.J.; Maron, B.J. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task force 7: Aortic diseases, including Marfan syndrome: A scientific statement from the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2015, 66, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- Tsujiuchi, M.; Ebato, M.; Maezawa, H.; Ikeda, N.; Mizukami, T.; Nagumo, S.; Iso, Y.; Yamauchi, T.; Suzuki, H. The prognostic value of left atrial reservoir functional indices measured by three-dimensional speckle-tracking echocardiography for major cardiovascular events. Circ. J. 2021, 85, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, L.D.; Ryffel, C.P.; De Marchi, S.; Seiler, C.; Brugger, N.; Eser, P.; Wilhelm, M. Exercise-induced cardiac remodeling in non-elite endurance athletes: Comparison of 2-tiered and 4-tiered classification of left ventricular hypertrophy. PLoS ONE 2018, 13, e0193203. [Google Scholar] [CrossRef] [PubMed]
- Kyuno, E.; Iso, Y.; Tsujiuchi, M.; Maeda, A.; Miyazawa, R.; Kowaita, H.; Kitai, H.; Sato, T.; Ebato, M.; Sambe, T.; et al. Impact of exercise-based cardiac rehabilitation on the mid-term outcomes of patients after acute myocardial infarction treated with current acute-phase management and optimal medical therapy. Heart Lung Circ. 2021, 30, 1320–1328. [Google Scholar] [CrossRef]
- Itoh, H.; Ajisaka, R.; Koike, A.; Makita, S.; Omiya, K.; Kato, Y.; Adachi, H.; Nagayama, M.; Maeda, T.; Tajima, A.; et al. Heart rate and blood pressure response to ramp exercise and exercise capacity in relation to age, gender, and mode of exercise in a healthy population. J. Cardiol. 2013, 61, 71–78. [Google Scholar] [CrossRef]
- Hossri, C.A.; Souza, I.P.A.; de Oliveira, J.S.; Mastrocola, L.E. Assessment of oxygen-uptake efficiency slope in healthy children and children with heart disease: Generation of appropriate reference values for the OUES variable. Eur. J. Prev. Cardiol. 2019, 26, 177–184. [Google Scholar] [CrossRef]
- Bang, C.N.; Gerdts, E.; Aurigemma, G.P.; Boman, K.; de Simone, G.; Dahlöf, B.; Køber, L.; Wachtell, K.; Devereux, R.B. Four-group classification of left ventricular hypertrophy based on ventricular concentricity and dilatation identifies a low-risk subset of eccentric hypertrophy in hypertensive patients. Circ. Cardiovasc. Imaging 2014, 7, 422–429. [Google Scholar] [CrossRef]
- Daimon, M.; Watanabe, H.; Abe, Y.; Hirata, K.; Hozumi, T.; Ishii, K.; Ito, H.; Iwakura, K.; Izumi, C.; Matsuzaki, M.; et al. Normal values of echocardiographic parameters in relation to age in a healthy Japanese population: The JAMP study. Circ. J. 2008, 72, 1859–1866. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, K.H.; Rink, L.; Hornsby, K.; Park, H.; Park, J.H.; Yoon, H.J.; Ahn, Y.; Jeong, M.H.; Cho, J.G.; et al. University athletes and changes in cardiac geometry: Insight from the 2015 Gwangju Summer Universiade. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 407–416. [Google Scholar] [CrossRef]
- Pelliccia, A.; Spataro, A.; Caselli, G.; Maron, B.J. Absence of left ventricular wall thickening in athletes engaged in intense power training. Am. J. Cardiol. 1993, 72, 1048–1054. [Google Scholar] [CrossRef]
- Colosio, A.L.; Pedrinolla, A.; Da Lozzo, G.; Pogliaghi, S. Heart rate-index estimates oxygen uptake, energy expenditure and aerobic fitness in rugby players. J. Sports Sci. Med. 2018, 17, 633–639. [Google Scholar] [PubMed]
- Scott, A.C.; Roe, N.; Coats, A.J.; Piepoli, M.F. Aerobic exercise physiology in a professional rugby union team. Int. J. Cardiol. 2003, 87, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, M.U.; Maw, G.J.; Jenkins, D.; Reaburn, P. Heart rate, blood lactate and kinematic data of elite colts (under-19) rugby union players during competition. J. Sports Sci. 1998, 16, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, M.; Tager, I.B. Oxygen uptake efficiency slope: An index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J. Am. Coll. Cardiol. 2000, 36, 194–201. [Google Scholar] [CrossRef]
- Hutchinson, P.L.; Cureton, K.J.; Outz, H.; Wilson, G. Relationship of cardiac size to maximal oxygen uptake and body size in men and women. Int. J. Sports Med. 1991, 12, 369–373. [Google Scholar] [CrossRef]
- Wieling, W.; Borghols, E.A.; Hollander, A.P.; Danner, S.A.; Dunning, A.J. Echocardiographic dimensions and maximal oxygen uptake in oarsmen during training. Br. Heart J. 1981, 46, 190–195. [Google Scholar] [CrossRef]
- Bekaert, I.; Pannier, J.L.; Van de Weghe, C.; Van Durme, J.P.; Clement, D.L.; Pannier, R. Non-invasive evaluation of cardiac function in professional cyclists. Br. Heart J. 1981, 45, 213–218. [Google Scholar] [CrossRef]
- Riley-Hagan, M.; Peshock, R.M.; Stray-Gundersen, J.; Katz, J.; Ryschon, T.W.; Mitchell, J.H. Left ventricular dimensions and mass using magnetic resonance imaging in female endurance athletes. Am. J. Cardiol. 1992, 69, 1067–1074. [Google Scholar] [CrossRef]
- Yamazaki, H.; Onishi, S.; Katsukawa, F.; Ishida, H.; Kinoshita, N. Peak aerobic performance and left ventricular morphological characteristics in university students. Clin. J. Sport Med. 2000, 10, 286–290. [Google Scholar] [CrossRef]
- Garg, S.; de Lemos, J.A.; Ayers, C.; Khouri, M.G.; Pandey, A.; Berry, J.D.; Peshock, R.M.; Drazner, M.H. Association of a 4-tiered classification of LV hypertrophy with adverse CV outcomes in the general population. JACC Cardiovasc. Imaging 2015, 8, 1034–1041. [Google Scholar] [CrossRef]

| All (n = 114) | Forwards (n = 52) | Backs (n = 62) | p-Value | |
|---|---|---|---|---|
| Age (years) | 18.4 ± 0.6 | 18.4 ± 0.5 | 18.5 ± 0.6 | 0.266 |
| Ht (cm) | 173.3 ± 6.0 | 175.0 ± 5.7 | 171.9 ± 6.0 | 0.005 |
| Wt (kg) | 83.1 ± 12.5 | 92.9 ± 10.5 | 74.9 ± 7.0 | <0.001 |
| BMI (kg/m2) | 27.6 ± 4.0 | 30.4 ± 4.0 | 25.3 ± 2.1 | <0.001 |
| BSA (m2) | 2.0 ± 0.2 | 2.1 ± 0.1 | 1.9 ± 0.1 | <0.001 |
| Appendicular muscle mass index (kg/m2) | 9.4 ± 0.8 | 9.8 ± 0.7 | 9.1 ± 0.6 | <0.001 |
| Fat mass index (kg/m2) | 5.8 ± 3.0 | 7.8 ± 3.2 | 4.1 ± 1.4 | <0.001 |
| % Bodyfat (%) | 20.0 ± 7.7 | 24.8 ± 7.9 | 16.0 ± 4.8 | <0.001 |
| All (n = 110) | Forwards (n = 50) | Backs (n = 60) | p-Value | ||
|---|---|---|---|---|---|
| HR (bpm) | at rest | 71.4 ± 8.0 | 71.6 ± 8.0 | 71.3 ± 8.0 | 0.866 |
| at peak | 175.6 ± 15.1 | 175.6 ± 15.9 | 175.6 ± 14.6 | 0.989 | |
| SBP (mmHg) | at rest | 127.9 ± 15.0 | 133.5 ± 16.3 | 123.4 ± 12.1 | <0.001 |
| at peak | 194.0 ± 23.4 | 195.1 ± 25.2 | 193.0 ± 22.0 | 0.646 | |
| VO2 (mL/min/kg) | at rest | 4.3 ± 0.6 | 4.1 ± 0.5 | 4.5 ± 0.6 | 0.001 |
| at peak | 42.4 ± 8.0 | 36.0 ± 7.8 | 45.2 ± 7.2 | <0.001 | |
| OUES | 3851 ± 603 | 3978 ± 580 | 3746 ± 606 | 0.044 | |
| OUES/kg | 47.2 ± 8.8 | 43.5 ± 8.0 | 50.4 ± 8.2 | <0.001 | |
| O2 pulse at peak (mL/beat) | 19.5 ± 2.8 | 20.2 ± 2.8 | 19.0 ± 2.7 | 0.029 |
| All (n = 114) | Forwards (n = 52) | Backs (n = 62) | p-Value | |
|---|---|---|---|---|
| LVEDD (mm) | 53.0 ± 3.3 | 54.0 ± 3.2 | 52.1 ± 3.1 | 0.001 |
| LVEDD/BSA (mm/m2) | 26.6 ± 2.0 | 25.6 ± 1.7 | 27.4 ± 1.9 | <0.001 |
| LVESD (mm) | 33.2 ± 3.2 | 33.8 ± 3.1 | 32.8 ± 3.2 | 0.098 |
| LVESD/BSA (mm/m2) | 16.7 ± 1.8 | 16.0 ± 1.7 | 17.2 ± 1.7 | <0.001 |
| IVSth (mm) | 10.3 ± 0.8 | 10.4 ± 0.9 | 10.1 ± 0.8 | 0.068 |
| PWth (mm) | 10.1 ± 0.9 | 10.4 ± 1.0 | 9.9 ± 0.8 | 0.002 |
| RWT | 0.38 ± 0.04 | 0.39 ± 0.04 | 0.38 ± 0.04 | 0.864 |
| LVEDV (mL) | 150.2 ± 26.4 | 157.6 ± 27.6 | 143.9 ± 23.8 | 0.005 |
| LVEDV/BSA (mL/m2) | 75.0 ± 11.4 | 74.5 ± 11.9 | 75.5 ± 11.1 | 0.633 |
| LVESV (mL) | 53.9 ± 10.8 | 56.6 ± 12.2 | 51.6 ± 8.9 | 0.012 |
| LVESV/BSA (mL/m2) | 27.0 ± 5.0 | 26.7 ± 5.4 | 27.1 ± 4.7 | 0.690 |
| LVM (g) | 206.7 ± 34.9 | 221.1 ± 37.2 | 194.6 ± 27.8 | <0.001 |
| LVM/BSA (g/m2) | 102.4 ± 14.5 | 103.4 ± 16.2 | 101.6 ± 12.9 | 0.496 |
| LAD (mm) | 36.5 ± 3.9 | 38.1 ± 3.7 | 35.1 ± 3.5 | <0.001 |
| RAD (mm) | 41.3 ± 4.9 | 41.7 ± 4.7 | 40.9 ± 5.0 | 0.374 |
| LAV/BSA (mL/m2) | 28.4 ± 6.4 | 28.8 ± 7.0 | 28.0 ± 5.9 | 0.512 |
| AOD (mm) | 30.9 ± 2.6 | 31.2 ± 2.3 | 30.6 ± 2.8 | 0.281 |
| AOD/BSA (mm/m2) | 15.5 ± 1.6 | 14.8 ± 1.3 | 16.1 ± 1.6 | <0.001 |
| LVEF (%) | 64.4 ± 4.3 | 64.4 ± 4.6 | 64.3 ± 4.1 | 0.940 |
| E/A | 2.3 ± 0.5 | 2.2 ± 0.6 | 2.3 ± 0.5 | 0.642 |
| E/e’ | 6.3 ± 1.3 | 6.6 ± 1.4 | 6.1 ± 1.3 | 0.053 |
| GLS (%) | −17.8 ± 1.4 | −17.6 ± 1.3 | −17.9 ± 1.4 | 0.248 |
| Peak VO2 (mL/min/kg) | OUES/kg | O2 Pulse at Peak (mL/beat) | ||||
|---|---|---|---|---|---|---|
| Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | |
| LVEDD/BSA | 0.319 | <0.001 | 0.398 | <0.001 | −0.160 | 0.096 |
| LVESD/BSA | 0.263 | 0.006 | 0.353 | <0.001 | −0.095 | 0.328 |
| LVEDV/BSA | 0.182 | 0.057 | 0.198 | 0.038 | 0.230 | 0.016 |
| LVESV/BSA | 0.208 | 0.029 | 0.141 | 0.142 | 0.171 | 0.076 |
| LVM/BSA | 0.144 | 0.133 | 0.104 | 0.281 | 0.211 | 0.028 |
| LAV/BSA | −0.091 | 0.342 | −0.051 | 0.596 | 0.079 | 0.413 |
| AOD/BSA | 0.263 | 0.006 | 0.293 | 0.002 | −0.114 | 0.238 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iso, Y.; Kitai, H.; Ichimori, K.; Kubota, M.; Tsujiuchi, M.; Nagumo, S.; Toshida, T.; Yonechi, T.; Ebato, M.; Suzuki, H. Cardiac Structure and Cardiorespiratory Fitness in Young Male Japanese Rugby Athletes. J. Cardiovasc. Dev. Dis. 2023, 10, 12. https://doi.org/10.3390/jcdd10010012
Iso Y, Kitai H, Ichimori K, Kubota M, Tsujiuchi M, Nagumo S, Toshida T, Yonechi T, Ebato M, Suzuki H. Cardiac Structure and Cardiorespiratory Fitness in Young Male Japanese Rugby Athletes. Journal of Cardiovascular Development and Disease. 2023; 10(1):12. https://doi.org/10.3390/jcdd10010012
Chicago/Turabian StyleIso, Yoshitaka, Hitomi Kitai, Keiko Ichimori, Megumi Kubota, Miki Tsujiuchi, Sakura Nagumo, Tsutomu Toshida, Toru Yonechi, Mio Ebato, and Hiroshi Suzuki. 2023. "Cardiac Structure and Cardiorespiratory Fitness in Young Male Japanese Rugby Athletes" Journal of Cardiovascular Development and Disease 10, no. 1: 12. https://doi.org/10.3390/jcdd10010012
APA StyleIso, Y., Kitai, H., Ichimori, K., Kubota, M., Tsujiuchi, M., Nagumo, S., Toshida, T., Yonechi, T., Ebato, M., & Suzuki, H. (2023). Cardiac Structure and Cardiorespiratory Fitness in Young Male Japanese Rugby Athletes. Journal of Cardiovascular Development and Disease, 10(1), 12. https://doi.org/10.3390/jcdd10010012





